Red Blood Cell Responses during a Long-Standing Load in a Microfluidic Constriction
Abstract
:1. Introduction
2. Related Works
3. Experimental System and Procedure
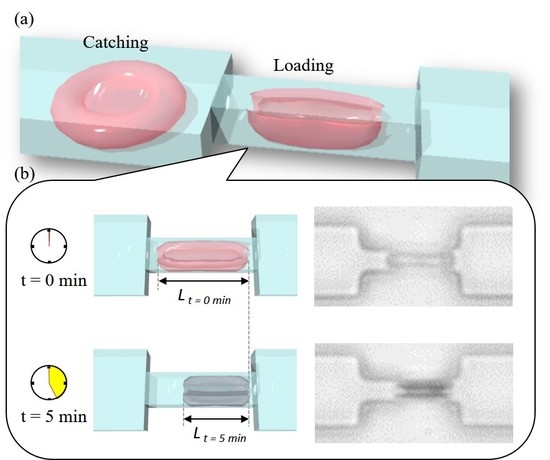
3.1. Cell Manipulation System
3.2. Design and Fabrication of PDMS Chip
3.3. RBC Sample Preparation and Control Sequence
4. Experimental Results
5. Discussion
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glenister, F.; Coppel, R.; Cowman, A.F.; Mohandas, N.; Cooke, B.M. Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood 2002, 99, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.T. Single cell mechanics study of the human disease malaria. J. Biomech. Sci. Eng. 2006, 1, 82–92. [Google Scholar] [CrossRef]
- Tomaiuolo, G. Biomechanical properties of red blood cells in health and disease towards microfluidics. Biomicrofluidics 2014, 8, 51501. [Google Scholar] [CrossRef] [PubMed]
- Mokken, F.; Kedaria, M.; Henny, C. The clinical importance of erythrocyte deformability, a hemorrheological parameter. Ann. Hematol. 1992, 64, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Vlahovska, P.; Karniadakis, G. Continuum- and particle-based modeling of shapes and dynamics of red blood cells in health and disease. Soft Matter 2013, 9, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.B.; Eggleton, C.D.; Neeves, K.B.; Marr, D.W.M. Measuring cell mechanics by optical alignment compression cytometry. Lab Chip 2013, 13, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Henry, T.K.; Lee, S.A.; Ying, Y.; Lindgren, A.G.; Yang, O.O.; Rao, J.; Clark, A.T.; Di Carlo, D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl. Acad. Sci. USA 2012, 109, 7630–7635. [Google Scholar] [CrossRef] [PubMed]
- Otto, O.; Rosendahl, P.; Mietke, A.; Golfier, S.; Herold, C.; Klaue, D.; Girardo, S.; Pagliara, S.; Ekpenyong, A.; Jacobi, A.; et al. Real-time deformability cytometry: On-the-fly cell mechanical phenotyping. Nat. Methods 2015, 12, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.D.; Sakuma, S.; Arai, F.; Taniguchi, T.; Ohtani, T.; Sakata, Y.; Kaneko, M. Geometrical alignment for improving cell evaluation in a microchannel with application on multiple myeloma red blood cells. RSC Adv. 2014, 4, 45050–45058. [Google Scholar] [CrossRef]
- Salehyar, S.; Zhu, Q. Deformation and internal stress in a red blood cell as it is driven through a slit by an incoming flow. Soft Matter 2016, 12, 3156–3164. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Dimitrakopoulos, P. Transient dynamics of an elastic capsule in a microfluidic constriction. Soft Matter 2013, 19, 8844–8855. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.M. Shape memory of human red blood cells. Biophys. J. 2004, 86, 3304–3313. [Google Scholar] [CrossRef]
- Markle, D.R.; Evans, E.A.; Hochmuth, R.M. Force relaxation and permanent deformation of erythrocyte membrane. Biophys. J. 1983, 42, 91–98. [Google Scholar] [CrossRef]
- Baez, S.D.; Kaul, M.E.; Nagel, R.L. Microvascular determinants of blood flow behavior and HbSS erythrocyte plugging in microcirculation. Blood Cells 1982, 8, 127–137. [Google Scholar] [PubMed]
- Wiewiora, M.; Sosada, K.; Wylezol, M.; Slowinska, L.; Zurawinski, W. Red blood cell aggregation and deformability among patients qualified for bariatric surgery. Obes. Surg. 2007, 17, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Cahn, A.; Livshits, L.; Srulevich, A.; Raz, I.; Yedgar, S.; Barshtein, G. Diabetic foot disease is associated with reduced erythrocyte deformability. Int. Wound J. 2015, 13, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Dondorp, A.M.; Kager, P.A.; Vreeken, J.; White, N.J. Abnormal blood flow and red blood cell deformability in severe malaria. Parasitol. Today 2000, 16, 228–232. [Google Scholar] [CrossRef]
- Pivkin, I.V.; Peng, Z.; Karniadakis, G.E.; Buffet, P.A.; Dao, M.; Suresh, S. Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc. Natl. Acad. Sci. USA 2016, 113, 7804–7809. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.; Fabry, M.E.; Nagel, R.L. The unique red cell heterogeneity of SC disease: Crystal formation, dense reticulocytes, and unusual morphology. Blood 1991, 78, 2104–2112. [Google Scholar] [PubMed]
- Zheng, Y.; Nguyen, J.; Wei, Y.; Sun, Y. Recent advances in microfluidic techniques for single-cell biophysical characterization. Lab Chip 2013, 13, 2464–2483. [Google Scholar] [CrossRef] [PubMed]
- Reich, E.; Goldberg, I.H. Actinomycin and nucleic acid function. Prog. Nucleic Acid Res. Mol. Boil. 1964, 3, 183–234. [Google Scholar]
- Tözeren, A.; Skalak, R.; Sung, K.L.; Chien, S. Influence of physicochemical factors on rheology of human neutrophils. Biophys. J. 1982, 39, 23–32. [Google Scholar] [CrossRef]
- Brandao, M.M.; Fontes, A.; Barjas-Castro, M.L.; Barbosa, L.C.; Costa, F.F.; Cesar, C.L.; Saad, S.T.O. Optical tweezers for measuring red blood cell elasticity: Application to the study of drug response in sickle cell disease. Eur. J. Haematol. 2003, 70, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Dulińska, I.; Targosz, M.; Strojny, W.; Lekka, M.; Czuba, P.; Balwierz, W.; Szymoński, M. Stiffness of normal and pathological erythrocytes studied by means of atomic force microscopy. J. Biochem. Biophys. Methods 2006, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Kaneko, M.; Kawahara, T.; Yamanishi, Y.; Arai, F. High speed cell stiffness evaluation toward 100% reliability. In Proceedings of the 2011 IEEE SENSORS, Limerick, Ireland, 28–31 October 2011; pp. 962–965. [Google Scholar]
- Hirose, Y.; Tadakuma, K.; Higashimori, M.; Arai, T.; Kaneko, M.; Iitsuka, R.; Yamanishi, Y.; Arai, F. A new evaluation toward high speed cell sorter. In Proceedings of the 2010 IEEE International Conference Robotics and Automation (ICRA), Anchorage, MD, USA, 3–7 May 2010; pp. 4113–4118. [Google Scholar]
- Tsai, C.D.; Sakuma, S.; Arai, F.; Kaneko, M. A new dimensionless index for evaluating cell stiffness-based deformability in microchannel. IEEE Trans. Biomed. Eng. 2014, 61, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shojaei-Baghini, E.; Azad, A.; Wang, C.; Sun, Y. High-throughput biophysical measurement of human red blood cells. Lab Chip 2012, 13, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Kuroda, K.; Tsai, C.D.; Fukui, W.; Arai, F.; Kaneko, M. Red blood cell fatigue evaluation based on close-encountering point between extensibility and recoverability. Lab Chip 2014, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Murakami, R.; Sakuma, S.; Tsai, C.D.; Gutsmann, T.; Brandenburg, K.; Ito, H.; Murakami, R.; Sakuma, S.; Tsai, C.D.; et al. Mechanical diagnosis of human erythrocytes by ultra-high speed manipulation unraveled critical time window for global cytoskeletal remodeling. Sci. Rep. 2017, 7, 43134. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Tsai, C.D.; Ito, H.; Tanaka, M.; Sakuma, S.; Arai, F.; Kaneko, M. Catch, Load and Launch toward On-Chip Active Cell Evaluation. In Proceedings of the 2016 IEEE International Conference Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016; pp. 1713–1718. [Google Scholar]
- Horade, M.; Tsai, C.D.; Ito, H.; Tanaka, M.; Kaneko, M. Chameleon effect of RBC under loading in microfluidic channel. In Proceedings of the 19th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS16), Dublin, Ireland, 9–13 October 2016; pp. 311–312. [Google Scholar]
- Tsai, C.D.; Horade, M.; Ito, H.; Tanaka, M.; Kaneko, M. High-resolution cell manipulation for longstanding load on red blood cells. In Proceedings of the IEEE International Conference on Mechatronics and Automation (ICMA2016), Harbin, China, 7–10 August 2016; pp. 914–919. [Google Scholar]
- Horade, M.; Kojima, M.; Kamiyama, K.; Kurata, T.; Mae, Y.; Arai, T. Development of an optimum end-effector with a nano-scale uneven surface for non-adhesion cell manipulation using a micromanipulator. J. Micromech. Microeng. 2015, 25, 5002. [Google Scholar] [CrossRef]
- Monzawa, T.; Kaneko, M.; Tsai, C.D.; Sakuma, S.; Arai, F. On-chip actuation transmitter for enhancing the dynamic response of cell manipulation using a macro-scale pump. Biomicrofluidics 2015, 9, 014114. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.D.; Tanaka, J.; Kaneko, M.; Horade, M.; Ito, H.; Taniguchi, T.; Ohtani, T.; Sakata, Y. An on-chip RBC deformability checker significantly improves velocity-deformation correlation. Micromachines 2016, 7, 176–185. [Google Scholar] [CrossRef]
- Evans, E.A.; Waugh, R.; Melnik, L. Elastic area compressibility modulus of red cell membrane. Biophys. J. 1976, 16, 585–595. [Google Scholar] [CrossRef]









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horade, M.; Tsai, C.-H.D.; Ito, H.; Kaneko, M. Red Blood Cell Responses during a Long-Standing Load in a Microfluidic Constriction. Micromachines 2017, 8, 100. https://doi.org/10.3390/mi8040100
Horade M, Tsai C-HD, Ito H, Kaneko M. Red Blood Cell Responses during a Long-Standing Load in a Microfluidic Constriction. Micromachines. 2017; 8(4):100. https://doi.org/10.3390/mi8040100
Chicago/Turabian StyleHorade, Mitsuhiro, Chia-Hung Dylan Tsai, Hiroaki Ito, and Makoto Kaneko. 2017. "Red Blood Cell Responses during a Long-Standing Load in a Microfluidic Constriction" Micromachines 8, no. 4: 100. https://doi.org/10.3390/mi8040100
APA StyleHorade, M., Tsai, C.-H. D., Ito, H., & Kaneko, M. (2017). Red Blood Cell Responses during a Long-Standing Load in a Microfluidic Constriction. Micromachines, 8(4), 100. https://doi.org/10.3390/mi8040100