Hollow Hydrogel Microfiber Encapsulating Microorganisms for Mass-Cultivation in Open Systems
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Microfluidic Formation of Hollow Hydrogel Microfiber
2.3. Culture of Bacteria in Hollow Microfiber
2.4. Double-Network Hollow Hydrogel Microfiber
3. Results and Discussion
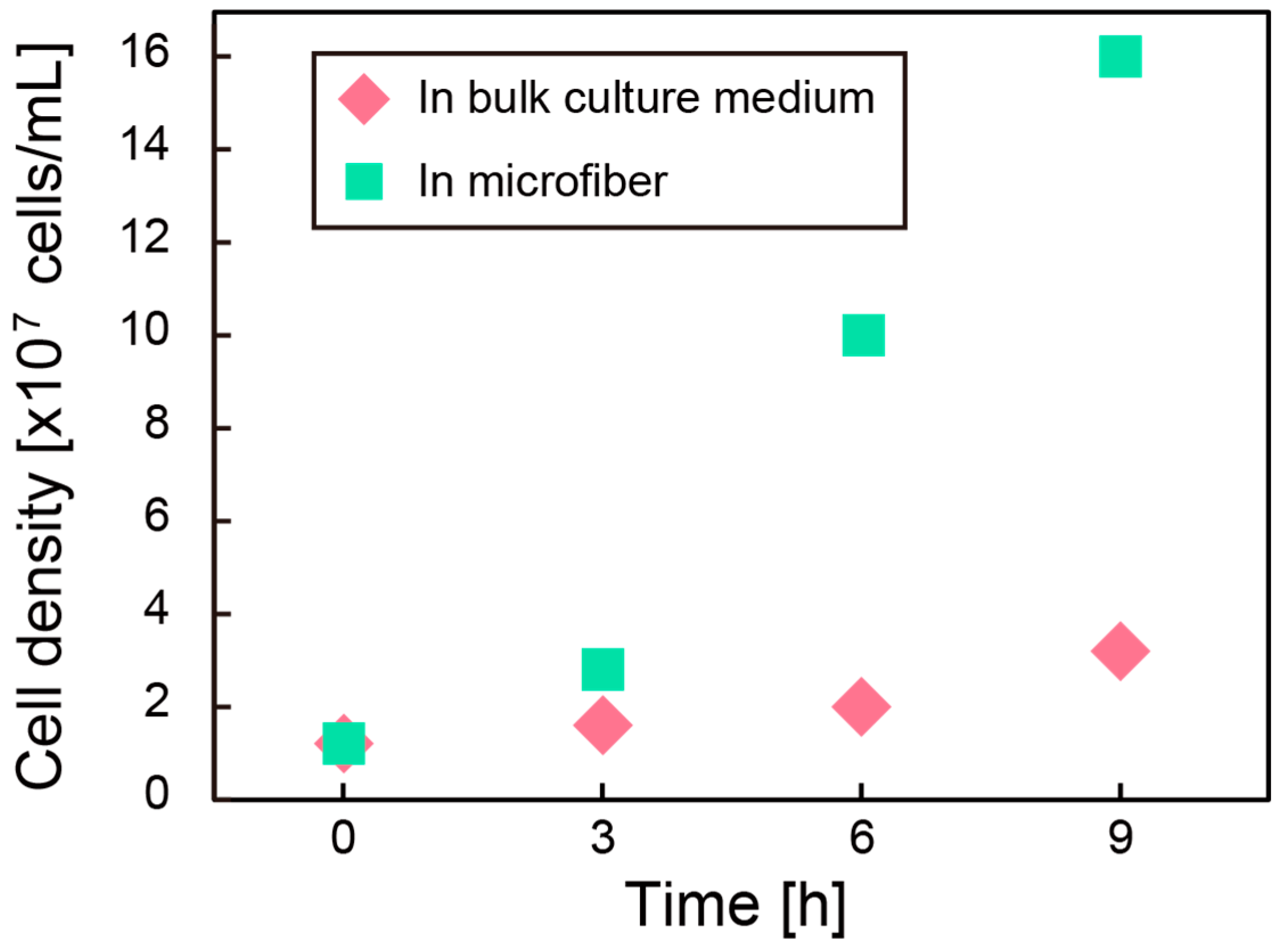
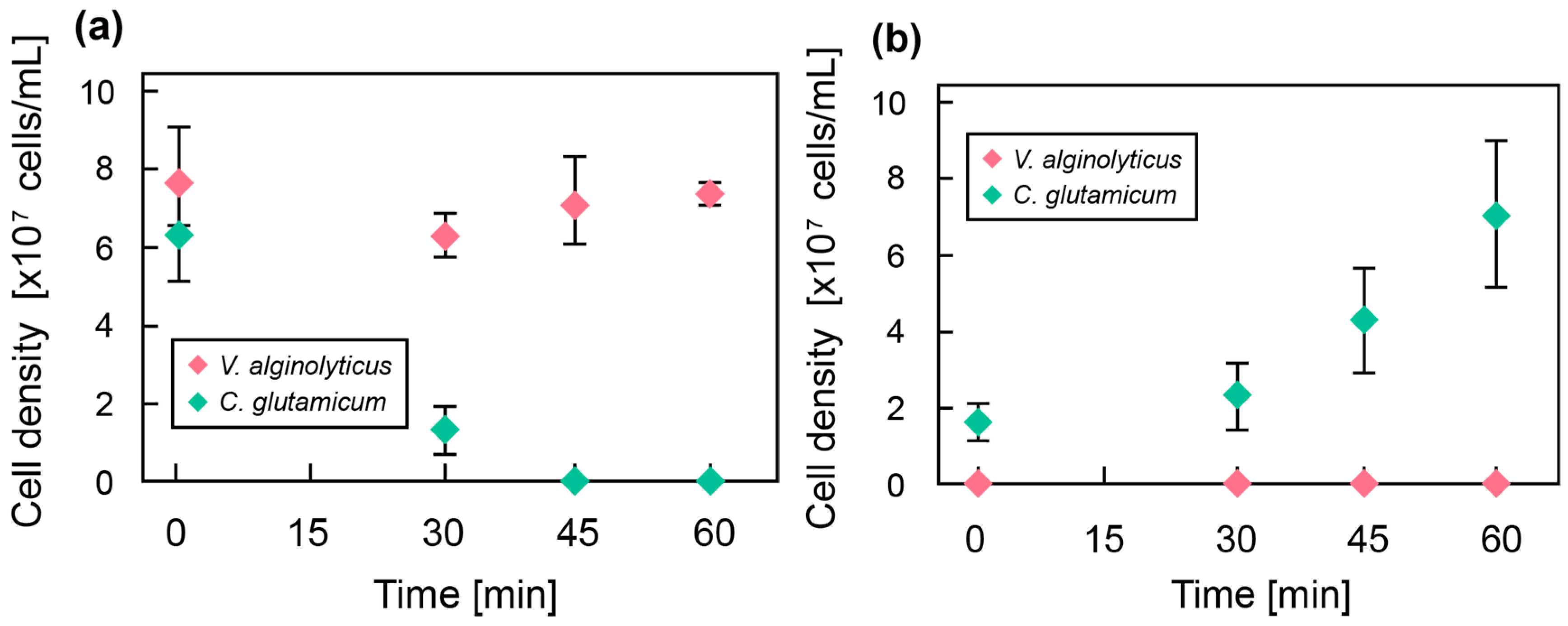
3.1. Culture of Bacteria in Hollow Microfiber
3.2. Double-Network Hollow Hydrogel Microfiber
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Najafpour, G. Biochemical Engineering and Biotechnology; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Goeddel, D.V.; Kleid, D.G.; Bolivar, F.; Heyneker, H.L.; Yansura, D.G.; Crea, R.; Hirose, T.; Kraszewski, A.; Itakura, K.; Riggs, A.D. Expression in Escherichia-coli of chemically synthesized genes for human insulin. Proc. Natl. Acad. Sci. USA 1979, 76, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Goeddel, D.V.; Heyneker, H.L.; Hozumi, T.; Arentzen, R.; Itakura, K.; Yansura, D.G.; Ross, M.J.; Miozzari, G.; Crea, R.; Seeburg, P.H. Direct expression in Escherichia-coli of a DNA-sequence coding for human growth-hormone. Nature 1979, 281, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.V.; Hinman, M.; Kothakota, S.; Fournier, M.J. Expression and purification of a spider silk protein: A new strategy for producing repetitive proteins. Protein Expr. Purif. 1996, 7, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Elvin, C.M.; Carr, A.G.; Huson, M.G.; Maxwell, J.M.; Pearson, R.D.; Vuocolo, T.; Liyou, N.E.; Wong, D.C.C.; Merritt, D.J.; Dixon, N.E. Synthesis and properties of crosslinked recombinant pro-resilin. Nature 2005, 437, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Lundquist, T.J.; Woertz, I.C.; Quinn, N.; Benemann, J.R. A realistic technology and engineering assessment of algae biofuel production. Energy Biosci. Inst. 2010, 1–178. [Google Scholar]
- Luque, R. Algal biofuels: The eternal promise? Energy Environ. Sci. 2010, 3, 254–257. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Chen, L.; Wang, J.F.; Liu, T.Z. The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresour. Technol. 2013, 128, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garrido, I.; Canavate, J.P. Assessing chemical compounds for controlling predator ciliates in outdoor mass cultures of the green algae Dunaliella salina. Aquac. Eng. 2001, 24, 107–114. [Google Scholar] [CrossRef]
- Xu, C.Y.; Wu, K.Y.; Van Ginkel, S.W.; Igou, T.; Lee, H.J.; Bhargava, A.; Johnston, R.; Snell, T.; Chen, Y.S. The use of the schizonticidal agent quinine sulfate to prevent pond crashes for algal-biofuel production. Int. J. Mol. Sci. 2015, 16, 27450–27456. [Google Scholar] [CrossRef] [PubMed]
- Van Ginkel, S.W.; Bidwell, M.; Igou, T.; Gijon-Felix, R.; Salvi, E.; De Oliveira, S.H.R.; Duarte, L.H.K.; Steiner, D.; Hu, Z.X.; Johnston, R.; et al. The prevention of saltwater algal pond contamination using the electron transport chain disruptor, rotenone. Algal Res. 2016, 18, 209–212. [Google Scholar] [CrossRef]
- Lincoln, E.P.; Hall, T.W.; Koopman, B. Zooplankton control in mass algal cultures. Aquaculture 1983, 32, 331–337. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae-a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Huntley, M.E.; Redalje, D.G. CO2 mitigation and renewable oil from photosynthetic microbes: A new appraisal. Mitig. Adapt. Strateg. Glob. Chang. 2007, 12, 573–608. [Google Scholar] [CrossRef]
- Ogawa, M.; Higashi, K.; Miki, N. Development of hydrogel microtubes for microbe culture in open environment. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 25–29 August 2015; pp. 5896–5899. [Google Scholar]
- Li, R.H.; Altreuter, D.H.; Gentile, F.T. Transport characterization of hydrogel matrices for cell encapsulation. Biotechnol. Bioeng. 1996, 50, 365–373. [Google Scholar] [CrossRef]
- Jeong, W.; Kim, J.; Kim, S.; Lee, S.; Mensing, G.; Beebe, D.J. Hydrodynamic microfabrication via “on the fly” photopolymerization of microscale fibers and tubes. Lab Chip 2004, 4, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Park, J.Y.; Lee, J.Y.; Park, H.; Park, Y.D.; Lee, K.B.; Whang, C.M.; Lee, S.H. “On the fly” continuous generation of alginate fibers using a microfluidic device. Langmuir 2007, 23, 9104–9108. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Shin, S.J.; Park, Y.; Lee, S.H. Synthesis of cell-laden alginate hollow fibers using microfluidic chips and microvascularized tissue-engineering applications. Small 2009, 5, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.H.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z.G. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashi, K.; Ogawa, M.; Fujimoto, K.; Onoe, H.; Miki, N. Hollow Hydrogel Microfiber Encapsulating Microorganisms for Mass-Cultivation in Open Systems. Micromachines 2017, 8, 176. https://doi.org/10.3390/mi8060176
Higashi K, Ogawa M, Fujimoto K, Onoe H, Miki N. Hollow Hydrogel Microfiber Encapsulating Microorganisms for Mass-Cultivation in Open Systems. Micromachines. 2017; 8(6):176. https://doi.org/10.3390/mi8060176
Chicago/Turabian StyleHigashi, Kazuhiko, Miho Ogawa, Kazuma Fujimoto, Hiroaki Onoe, and Norihisa Miki. 2017. "Hollow Hydrogel Microfiber Encapsulating Microorganisms for Mass-Cultivation in Open Systems" Micromachines 8, no. 6: 176. https://doi.org/10.3390/mi8060176
APA StyleHigashi, K., Ogawa, M., Fujimoto, K., Onoe, H., & Miki, N. (2017). Hollow Hydrogel Microfiber Encapsulating Microorganisms for Mass-Cultivation in Open Systems. Micromachines, 8(6), 176. https://doi.org/10.3390/mi8060176






