Amorphous Silicon Carbide Platform for Next Generation Penetrating Neural Interface Designs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thin Film Deposition and Array Fabrication
2.2. Buckling and Insertion Mechanics
2.3. Surgery and a-SiC Implantation
2.4. In Vivo Recording and Analysis
3. Results and Discussions
3.1. Insertion of Ultrathin Shanks into Cortex
3.1.1. PEG-Stabilized Shanks
3.1.2. Bundled Shanks
3.1.3. Reduction of Effective Shank Length
3.1.4. Insertion of Individual Shanks
3.2. Neural Recording
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jun, J.J.; Steinmetz, N.A.; Siegle, J.H.; Denman, D.J.; Bauza, M.; Barbarits, B.; Lee, A.K.; Anastassiou, C.A.; Andrei, A.; Aydın, Ç.; et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 2017, 551, 232–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire, M.A.M.; Morya, E.; Faber, J.; Santos, J.R.; Guimaraes, J.S.; Lemos, N.A.M.; Sameshima, K.; Pereira, A.; Ribeiro, S.; Nicolelis, M.A.L. Comprehensive Analysis of Tissue Preservation and Recording Quality from Chronic Multielectrode Implants. PLoS ONE 2011, 6, e27554. [Google Scholar] [CrossRef] [PubMed]
- McCreery, D.B.; Yuen, T.G.H.; Agnew, W.F.; Bullara, L.A. Stimulation with chronically implanted microelectrodes in the cochlear nucleus of the cat: Histologic and physiologic effects. Hear. Res. 1992, 62, 42–56. [Google Scholar] [CrossRef]
- Guitchounts, G.; Markowitz, J.E.; Liberti, W.A.; Gardner, T.J. A carbon-fiber electrode array for long-term neural recording. J. Neural Eng. 2013, 10, 046016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.R.; Na, K.; Zhang, H.; Kozai, T.D.Y.; Kotov, N.A.; Yoon, E.; Chestek, C.A. Insertion of linear 8.4 μm diameter 16 channel carbon fiber electrode arrays for single unit recordings. J. Neural Eng. 2015, 12, 046009. [Google Scholar] [CrossRef] [PubMed]
- Deku, F.; Joshi-Imre, A.; Mertiri, A.; Gardner, T.J.; Cogan, S.F. Electrodeposited Iridium Oxide on Carbon Fiber Ultramicroelectrodes for Neural Recording and Stimulation. J. Electrochem. Soc. 2018, 165, D375–D380. [Google Scholar] [CrossRef]
- Gillis, W.F.; Lissandrello, C.A.; Shen, J.; Pearre, B.W.; Mertiri, A.; Deku, F.; Cogan, S.; Holinski, B.J.; Chew, D.J.; White, A.E.; et al. Carbon fiber on polyimide ultra-microelectrodes. J. Neural Eng. 2018, 15, 016010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seto, J.Y.W. The electrical properties of polycrystalline silicon films. J. Appl. Phys. 1975, 46, 5247–5254. [Google Scholar] [CrossRef]
- Batey, J.; Tierney, E. Low-temperature deposition of high-quality silicon dioxide by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 1986, 60. [Google Scholar] [CrossRef]
- Yang, T.-C.; Saraswat, K.C. Effect of physical stress on the degradation of thin SiO/sub 2/ films under electrical stress. IEEE Trans. Electron. Devices 2000, 47, 746–755. [Google Scholar] [CrossRef]
- Sheikholeslami, A.; Parhami, F.; Puchner, H.; Selberherr, S. Planarization of Silicon Dioxide and Silicon Nitride Passivation Layers. J. Phys. Conf. Ser. 2007, 61, 1051–1055. [Google Scholar] [CrossRef] [Green Version]
- Hetke, J.F.; Lund, J.L.; Najafi, K.; Wise, K.D.; Anderson, D.J. Silicon Ribbon Cables for Chronically Implantable Microelectrode Arrays. IEEE Trans. Biomed. Eng. 1994, 41, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Barrese, J.C.; Aceros, J.; Donoghue, J.P. Scanning electron microscopy of chronically implanted intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2016, 13, 026003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrese, J.C.; Rao, N.; Paroo, K.; Triebwasser, C.; Vargas-Irwin, C.; Franquemont, L.; Donoghue, J.P. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2013, 10, 066014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, S.R.; Cogan, S.F.; Ehrlich, J.; Plante, T.D.; McCreery, D.B.; Troyk, P.R. Electrical performance of penetrating microelectrodes chronically implanted in cat cortex. IEEE Trans. Biomed. Eng. 2013, 60, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for neural implants. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Metz, S.; Bertsch, A.; Bertrand, D.; Renaud, P. Flexible polyimide probes with microelectrodes and embedded microfluidic channels for simultaneous drug delivery and multi-channel monitoring of bioelectric activity. Biosens. Bioelectron. 2004, 19, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Rousche, P.J.; Pellinen, D.S.; Pivin, D.P.; Williams, J.C.; Vetter, R.J.; Kipke, D.R. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans. Biomed. Eng. 2001, 48, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kuo, J.T.W.; Hara, S.A.; Lee, C.D.; Yu, L.; Gutierrez, C.A.; Hoang, T.Q.; Pikov, V.; Meng, E. 3D Parylene sheath neural probe for chronic recordings. J. Neural Eng. 2013, 10, 045002. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Ziegler, D.; Yoshida, Y.; Mabuchi, K.; Suzuki, T. Parylene flexible neural probes integrated with microfluidic channels. Lab Chip 2005, 5, 519. [Google Scholar] [CrossRef] [PubMed]
- Altuna, A.; Bellistri, E.; Cid, E.; Aivar, P.; Gal, B.; Berganzo, J.; Gabriel, G.; Guimerà, A.; Villa, R.; Fernández, L.J.; et al. SU-8 based microprobes for simultaneous neural depth recording and drug delivery in the brain. Lab Chip 2013, 13, 1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, G.; DeWeerth, S.P. PDMS-based conformable microelectrode arrays with selectable novel 3-D microelectrode geometries for surface stimulation and recording. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 2009, 1623–1626. [Google Scholar] [CrossRef]
- Ware, T.; Simon, D.; Rennaker, R.L.; Voit, W. Smart Polymers for Neural Interfaces. Polym. Rev. 2013, 53, 108–129. [Google Scholar] [CrossRef]
- Simon, D.M.; Charkhkar, H.; St. John, C.; Rajendran, S.; Kang, T.; Reit, R.; Arreaga-Salas, D.; McHail, D.G.; Knaack, G.L.; Sloan, A.; et al. Design and demonstration of an intracortical probe technology with tunable modulus. J. Biomed. Mater. Res. Part A 2017, 105, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Luan, L.; Zhao, Z.; Li, X.; Zhu, H.; Potnis, O.; Xie, C. Nanofabricated Ultraflexible Electrode Arrays for High-Density Intracortical Recording. Adv. Sci. 2018, 5, 1700625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalakrishnaiah, S.K.; Joseph, K.; Hofmann, U.G. Microfluidic drive for flexible brain implants. Curr. Dir. Biomed. Eng. 2017, 3, 675–678. [Google Scholar] [CrossRef] [Green Version]
- Kozai, T.D.Y.; Kipke, D.R. Insertion shuttle with carboxyl terminated self-assembled monolayer coatings for implanting flexible polymer neural probes in the brain. J. Neurosci. Methods 2009, 184, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoffstall, A.J.; Srinivasan, S.; Willis, M.; Stiller, A.M.; Ecker, M.; Voit, W.E.; Pancrazio, J.J.; Capadona, J.R. A Mosquito Inspired Strategy to Implant Microprobes into the Brain. Sci. Rep. 2018, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.; Wang, S.; Singh, S.; Damodaran, V.B.; Kaplan, H.M.; Kohn, J.; Shreiber, D.I.; Zahn, J.D. Coating flexible probes with an ultra fast degrading polymer to aid in tissue insertion. Biomed. Microdevices 2015, 17, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewitus, D.; Smith, K.L.; Shain, W.; Kohn, J. Ultrafast resorbing polymers for use as carriers for cortical neural probes. Acta Biomater. 2011, 7, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Lind, G.; Linsmeier, C.E.; Thelin, J.; Schouenborg, J. Gelatine-embedded electrodes—A novel biocompatible vehicle allowing implantation of highly flexible microelectrodes. J. Neural Eng. 2010, 7, 046005. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, A.; Descamps, E.; Bergaud, C. A review on mechanical considerations for chronically-implanted neural probes. J. Neural Eng. 2018, 15, 031001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohal, H.S.; Clowry, G.J.; Jackson, A.; O’Neill, A.; Baker, S.N. Mechanical Flexibility Reduces the Foreign Body Response to Long-Term Implanted Microelectrodes in Rabbit Cortex. PLoS ONE 2016, 11, e0165606. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.P.; Kipke, D.R. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 2007, 28, 3594–3607. [Google Scholar] [CrossRef] [PubMed]
- Deku, F.; Cohen, Y.; Joshi-Imre, A.; Kanneganti, A.; Gardner, T.J.; Cogan, S.F. Amorphous silicon carbide ultramicroelectrode arrays for neural stimulation and recording. J. Neural Eng. 2018, 15, 016007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, R.G.; Zhang, J.; Jones, D.G.; Myers, D.R.; Jog, A.V.; Jamshidi, B.; Wijesundara, M.B.J.; Maboudian, R.; Pisano, A.P. Silicon carbide coated MEMS strain sensor for harsh environment applications. Proceeding of the 2007 IEEE 20th International Conference on Micro Electro Mechanical Systems (MEMS), Hyogo, Japan, 21–25 January 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 643–646. [Google Scholar]
- Cogan, S.F.; Edell, D.J.; Guzelian, A.A.; Liu, Y.P.; Edell, R. Plasma-enhanced chemical vapor deposited silicon carbide as an implantable dielectric coating. J. Biomed. Mater. Res. A 2003, 67, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Knaack, G.L.; Charkhkar, H.; Cogan, S.F.; Pancrazio, J.J. Amorphous Silicon Carbide for Neural Interface Applications. Silicon Carbide Biotechnol. 2016, 249–260. [Google Scholar] [CrossRef]
- Knaack, G.L.; McHail, D.G.; Borda, G.; Koo, B.S.; Peixoto, N.; Cogan, S.F.; Dumas, T.C.; Pancrazio, J.J. In vivo Characterization of Amorphous Silicon Carbide as a Biomaterial for Chronic Neural Interfaces. Front. Neurosci. 2016, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, C.; Chen, B.; Poenar, D.P.; Lee, Y.Y. PECVD amorphous silicon carbide membranes for cell culturing. Sensors Actuators, B Chem. 2008, 129, 404–411. [Google Scholar] [CrossRef]
- Frewin, C.L.; Locke, C.; Saddow, S.E.; Weeber, E.J. Single-crystal cubic silicon carbide: An in vivo biocompatible semiconductor for brain machine interface devices. Proceeding of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 2957–2960. [Google Scholar]
- Oliveros, A.; Guiseppi-Elie, A.; Saddow, S.E. Silicon carbide: a versatile material for biosensor applications. Biomed. Microdevices 2013, 15, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Botia, C.A.; Luna, L.E.; Chamanzar, M.; Carraro, C.; Sabes, P.N.; Maboudian, R.; Maharbiz, M.M. Fabrication of all-silicon carbide neural interfaces. Proceeding of the 2017 8th International IEEE/EMBS Conference on Neural Engineering (NER), Shanghai, China, 25–28 May 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 170–173. [Google Scholar]
- Bernardin, E.; Frewin, C.; Everly, R.; Ul Hassan, J.; Saddow, S.; Bernardin, E.K.; Frewin, C.L.; Everly, R.; Ul Hassan, J.; Saddow, S.E. Demonstration of a Robust All-Silicon-Carbide Intracortical Neural Interface. Micromachines 2018, 9, 412. [Google Scholar] [CrossRef]
- Frewin, C.L.; Bernardin, E.E.; Deku, F.; Everly, R.; Hassan, J.; Pancrazio, J.J.; Saddow, S.E. Silicon Carbide as a Robust Neural Interface. ECS Trans. 2016, 75, 39–45. [Google Scholar] [CrossRef]
- Pancrazio, J.J.; Deku, F.; Ghazavi, A.; Stiller, A.M.; Rihani, R.; Frewin, C.L.; Varner, V.D.; Gardner, T.J.; Cogan, S.F. Thinking Small: Progress on Microscale Neurostimulation Technology. Neuromodulation Technol. Neural Interface 2017, 20, 745–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Khakani, M.A.; Chaker, M.; Jean, A.; Boily, S.; Kieffer, J.C.; O’Hern, M.E.; Ravet, M.F.; Rousseaux, F. Hardness and Young’s modulus of amorphous a-SiC thin films determined by nanoindentation and bulge tests. J. Mater. Res. 1994, 9, 96–103. [Google Scholar] [CrossRef]
- Cros, B.; Gat, E.; Saurel, J. Characterization of the elastic properties of amorphous silicon carbide thin films by acoustic microscopy. J. Non. Cryst. Solids 1997, 209, 273–282. [Google Scholar] [CrossRef]
- Xue, K.; Niu, L.-S.; Shi, H.-J. Mechanical Properties of Amorphous Silicon Carbide. Silicon Carbide IntechOpen 2011. [Google Scholar] [CrossRef] [Green Version]
- Jensen, W.; Yoshida, K.; Hofmann, U.G. In-vivo implant mechanics of flexible, silicon-based ACREO microelectrode arrays in rat cerebral cortex. IEEE Trans. Biomed. Eng. 2006, 53, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Wester, B.A.; Lee, R.H.; LaPlaca, M.C. Development and characterization of in vivo flexible electrodes compatible with large tissue displacements. J. Neural Eng. 2009, 6, 024002. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.A.; Abkes, B.A.; Ollendieck, M.C.; Noh, M.D.; Ritter, C.; Gillies, G.T. Measurement of the force required to move a neurosurgical probe through in vivo human brain tissue. IEEE Trans. Biomed. Eng. 1999, 46, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Molloy, J.A.; Ritter, R.C.; Grady, M.S.; Howard, M.A.; Quate, E.G.; Gillies, G.T. Experimental determination of the force required for insertion of a thermoseed into deep brain tissues. Ann. Biomed. Eng. 1990, 18, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.X.; He, J. Monitoring Insertion Force and Electrode Impedance during Implantation of Microwire Electrodes. Proceeding of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; IEEE: Piscataway, NJ, USA, 2006; pp. 7333–7336. [Google Scholar]
- Sridharan, A.; Nguyen, J.K.; Capadona, J.R.; Muthuswamy, J. Compliant intracortical implants reduce strains and strain rates in brain tissue in vivo. J. Neural Eng. 2015, 12, 036002. [Google Scholar] [CrossRef] [PubMed]
- Welkenhuysen, M.; Andrei, A.; Ameye, L.; Eberle, W.; Nuttin, B. Effect of Insertion Speed on Tissue Response and Insertion Mechanics of a Chronically Implanted Silicon-Based Neural Probe. IEEE Trans. Biomed. Eng. 2011, 58, 3250–3259. [Google Scholar] [CrossRef] [PubMed]
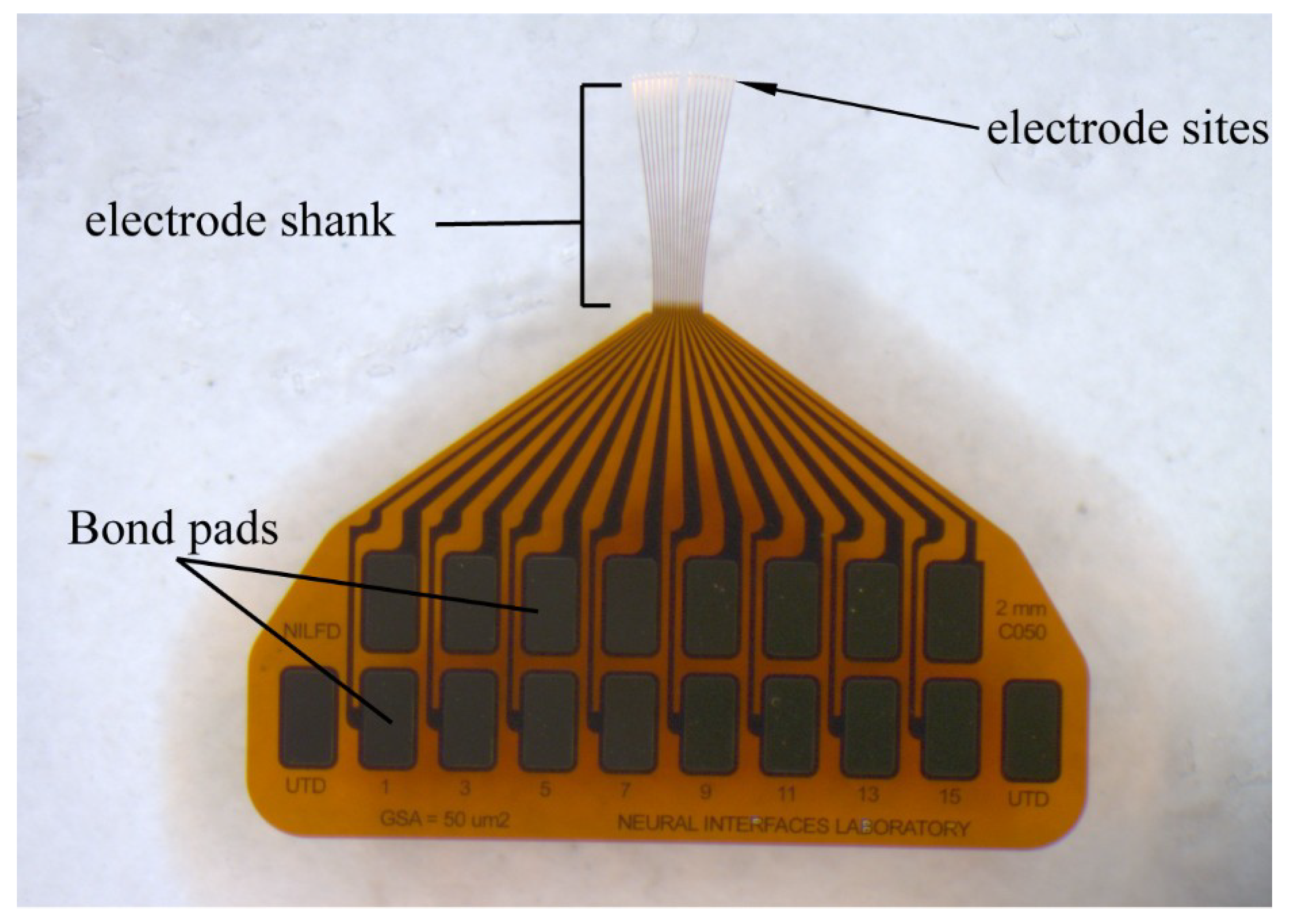

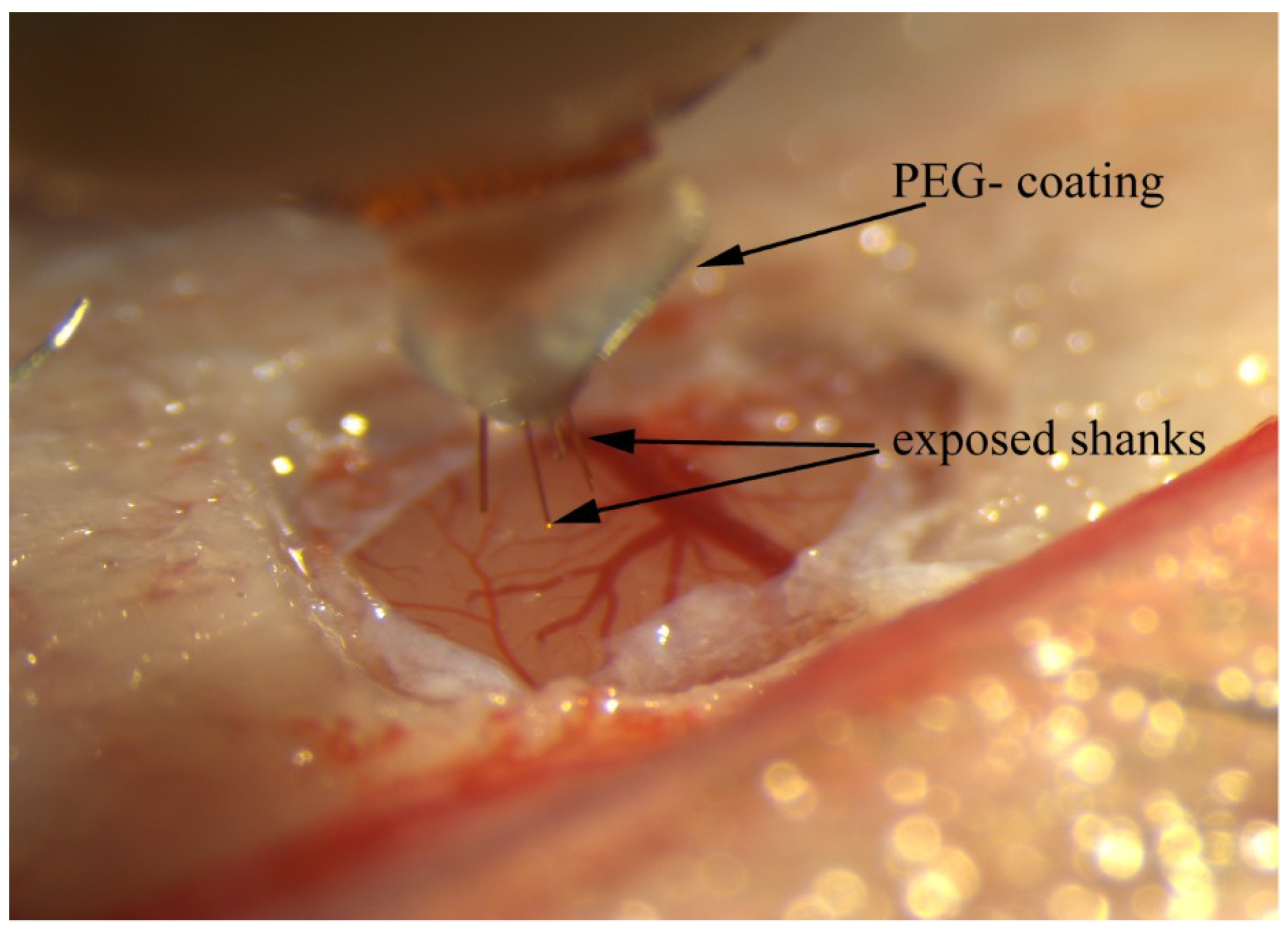




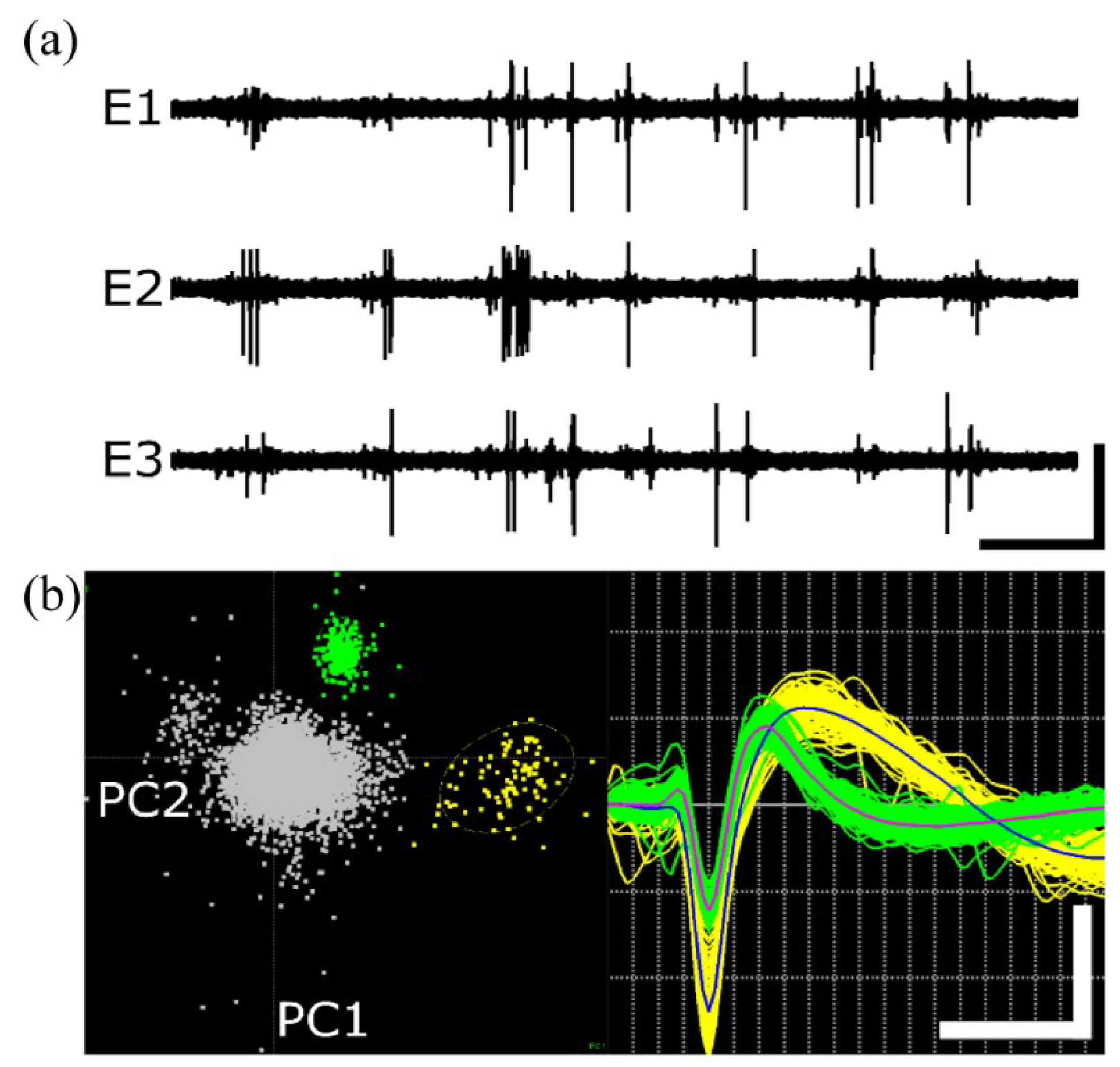
| Array # | AEY (%) | # of Units | Mean Vpp (μV) | RMS Noise (μV) | SNR |
|---|---|---|---|---|---|
| Array 1 | 75 | 16 | 179.0 ± 19.8 | 10.2 ± 1.8 | 25.6 ± 2.9 |
| Array 2 | 25 | 4 | 287.7 ± 64.4 | 8.8 ± 0.2 | 30.8 ± 6.8 |
| Array 3 | 31.3 | 7 | 118.5 ± 12.2 | 7.8 ± 0.4 | 16.7 ± 1.7 |
| Cumulative | 43.75% | 27 | 179.4 ± 18.4 | 8.9 ± 0.6 | 24.1 ± 2.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deku, F.; Frewin, C.L.; Stiller, A.; Cohen, Y.; Aqeel, S.; Joshi-Imre, A.; Black, B.; Gardner, T.J.; Pancrazio, J.J.; Cogan, S.F. Amorphous Silicon Carbide Platform for Next Generation Penetrating Neural Interface Designs. Micromachines 2018, 9, 480. https://doi.org/10.3390/mi9100480
Deku F, Frewin CL, Stiller A, Cohen Y, Aqeel S, Joshi-Imre A, Black B, Gardner TJ, Pancrazio JJ, Cogan SF. Amorphous Silicon Carbide Platform for Next Generation Penetrating Neural Interface Designs. Micromachines. 2018; 9(10):480. https://doi.org/10.3390/mi9100480
Chicago/Turabian StyleDeku, Felix, Christopher L. Frewin, Allison Stiller, Yarden Cohen, Saher Aqeel, Alexandra Joshi-Imre, Bryan Black, Timothy J. Gardner, Joseph J. Pancrazio, and Stuart F. Cogan. 2018. "Amorphous Silicon Carbide Platform for Next Generation Penetrating Neural Interface Designs" Micromachines 9, no. 10: 480. https://doi.org/10.3390/mi9100480
APA StyleDeku, F., Frewin, C. L., Stiller, A., Cohen, Y., Aqeel, S., Joshi-Imre, A., Black, B., Gardner, T. J., Pancrazio, J. J., & Cogan, S. F. (2018). Amorphous Silicon Carbide Platform for Next Generation Penetrating Neural Interface Designs. Micromachines, 9(10), 480. https://doi.org/10.3390/mi9100480







