Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems
Abstract
:1. Introduction
1.1. History and Types of Parylene
1.2. Thin-Film Parylene Device Microfabrication
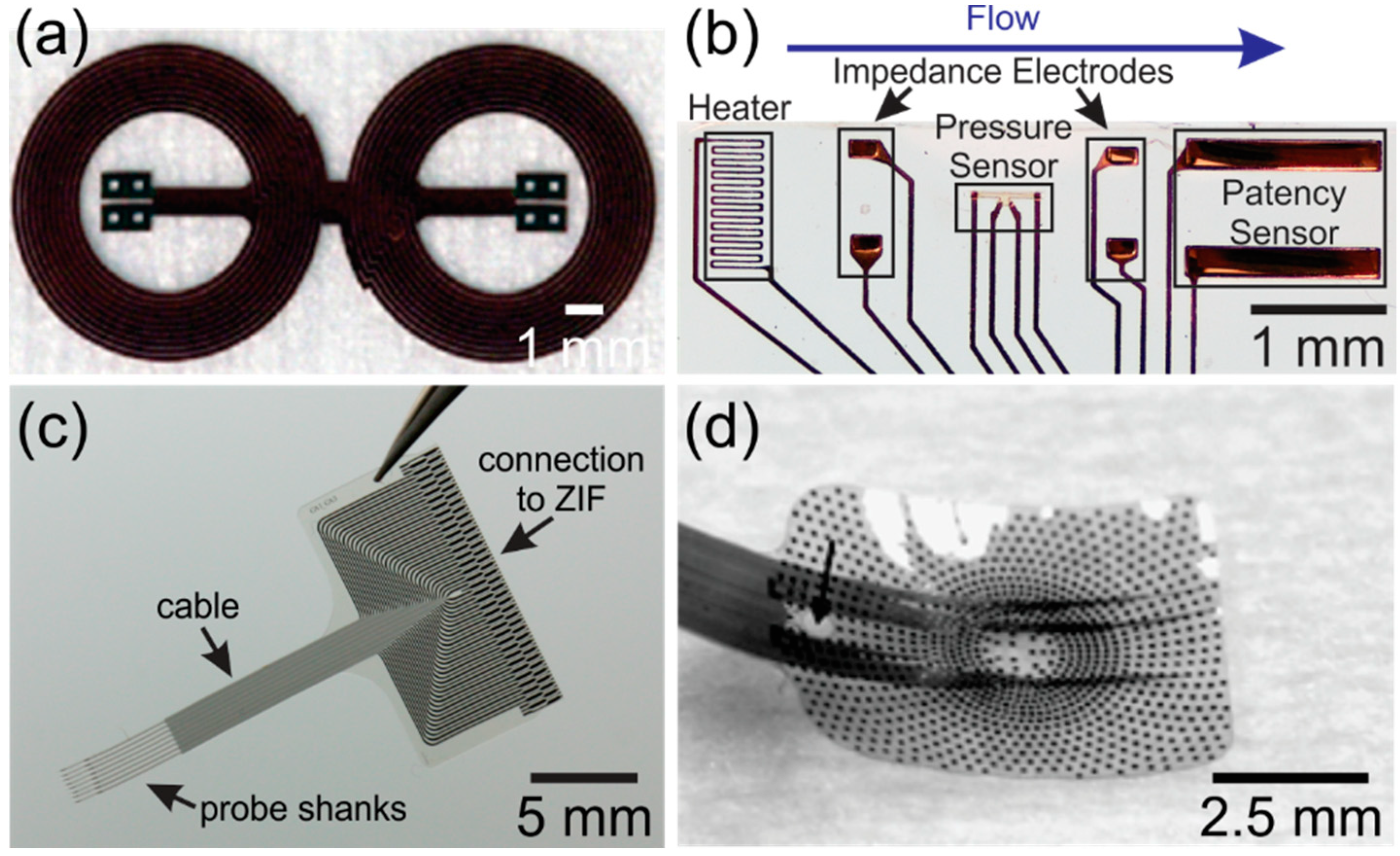
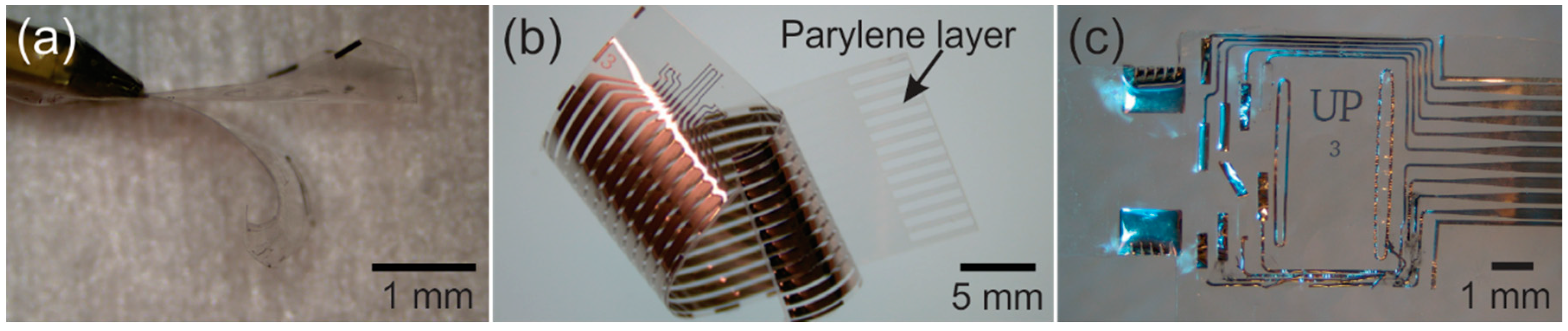
1.3. State-of-the-Art Parylene-Based Devices
2. Challenges
2.1. Thermal Budget
2.2. Water Diffusion through Parylene Films
2.2.1. Water Permeability
2.2.2. Ion Permeability
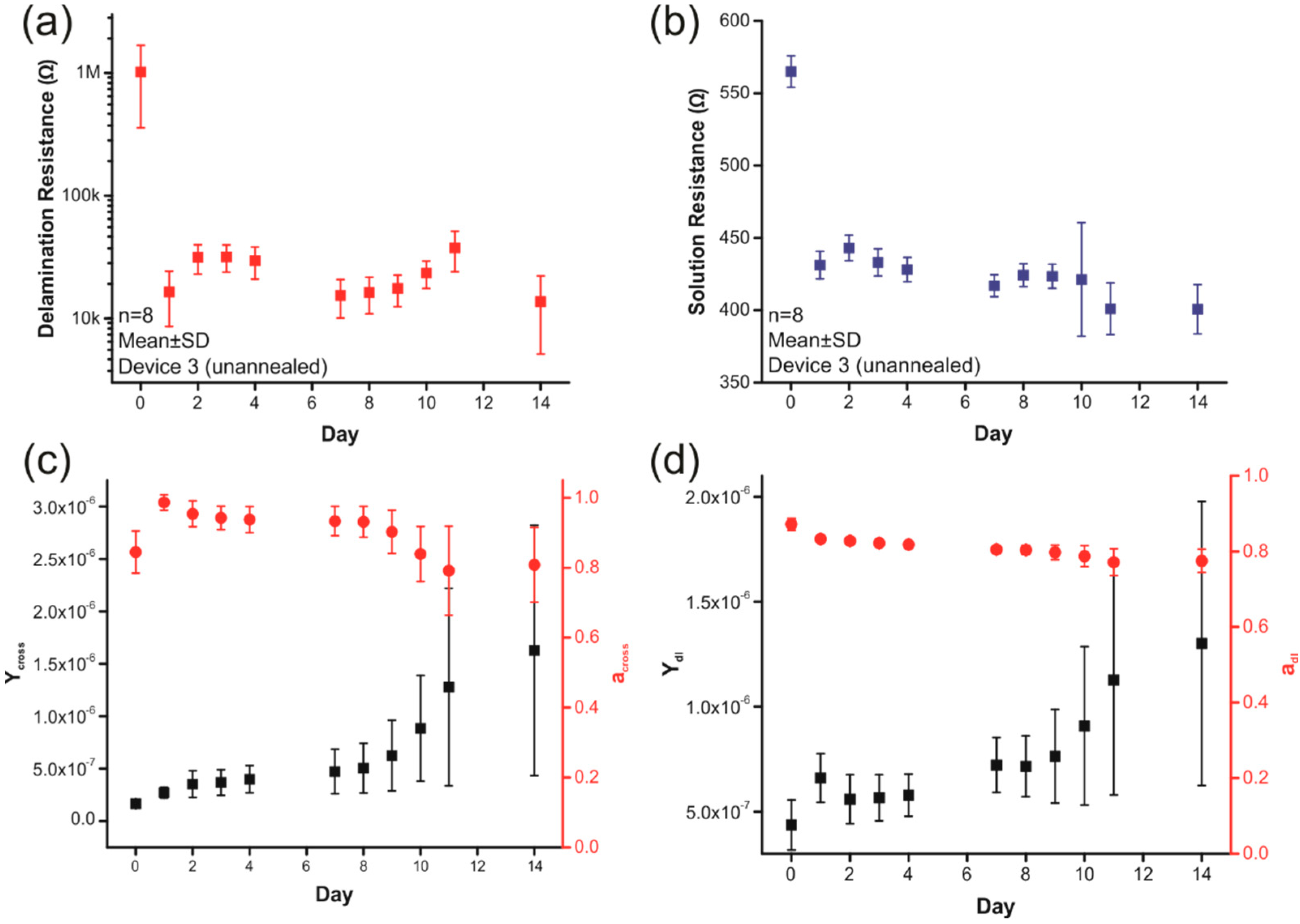
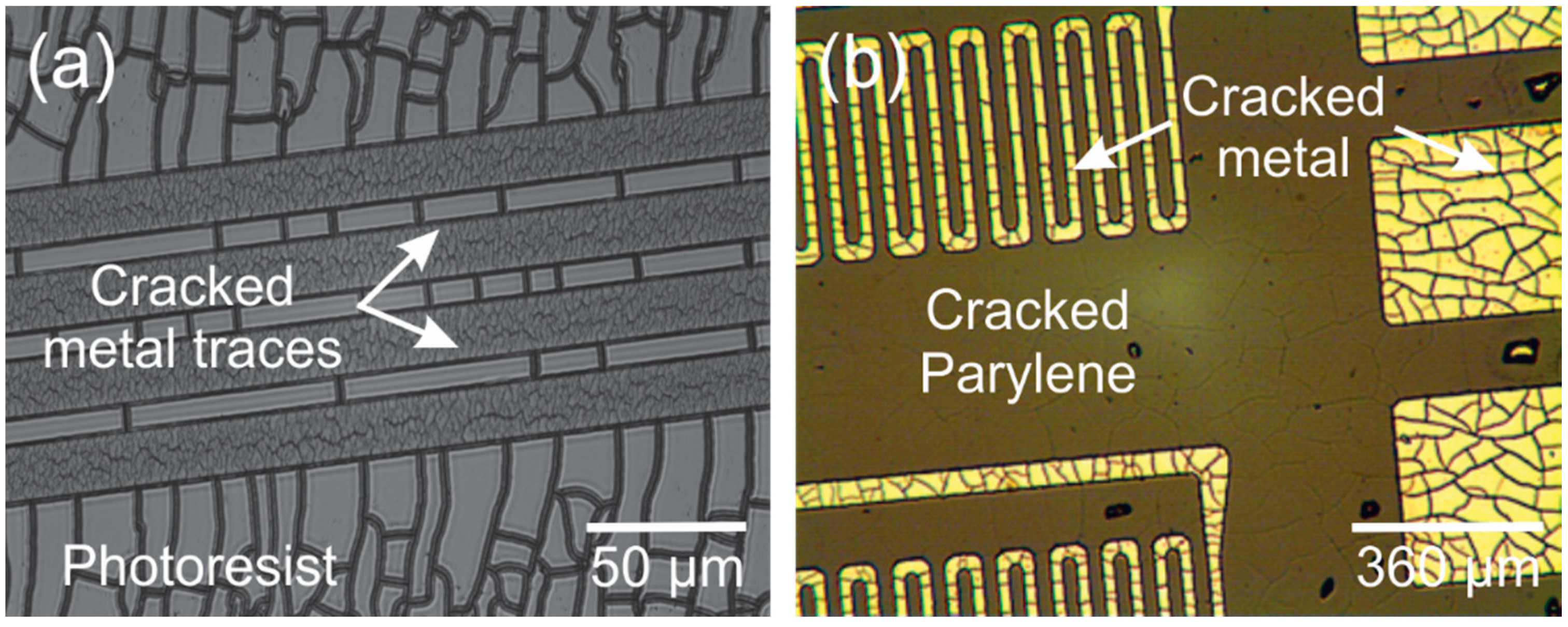
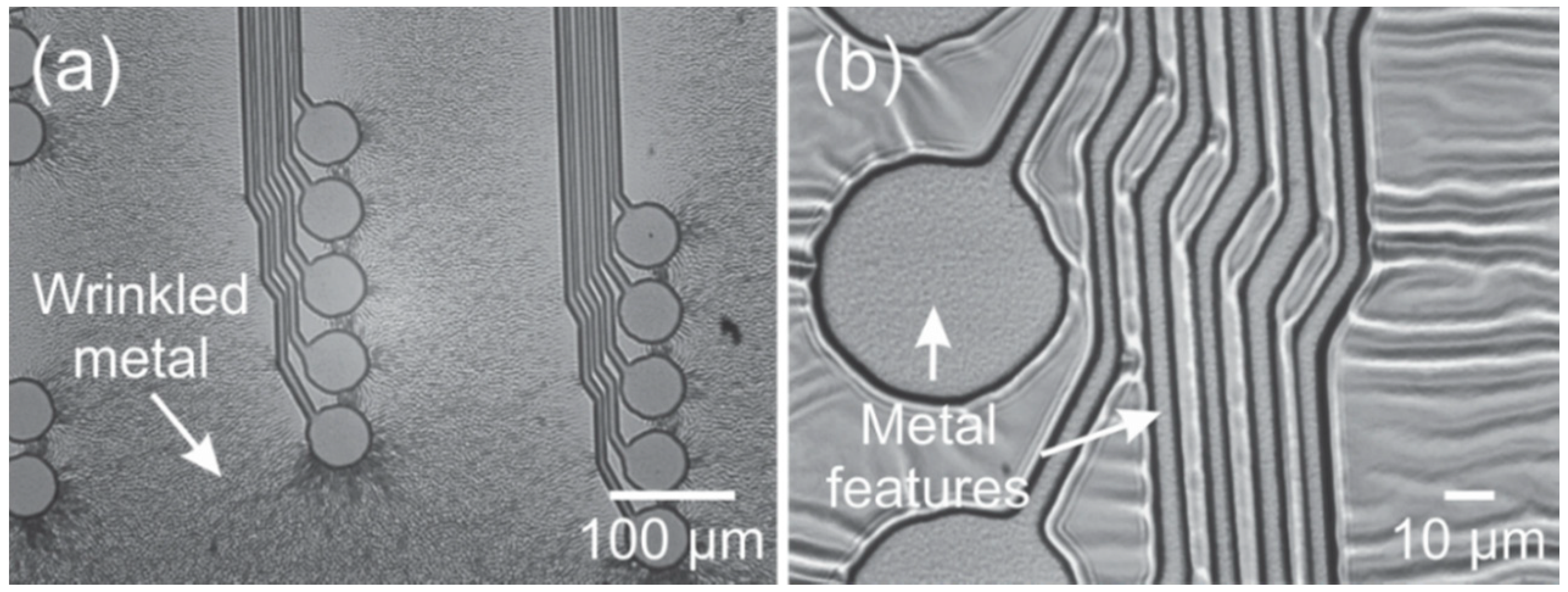
2.3. Delamination/Adhesion
2.4. Packaging and Electrical Connections
2.5. Sterilization
2.6. Handling
3. Micromachining of Parylene Films
3.1. Deposition
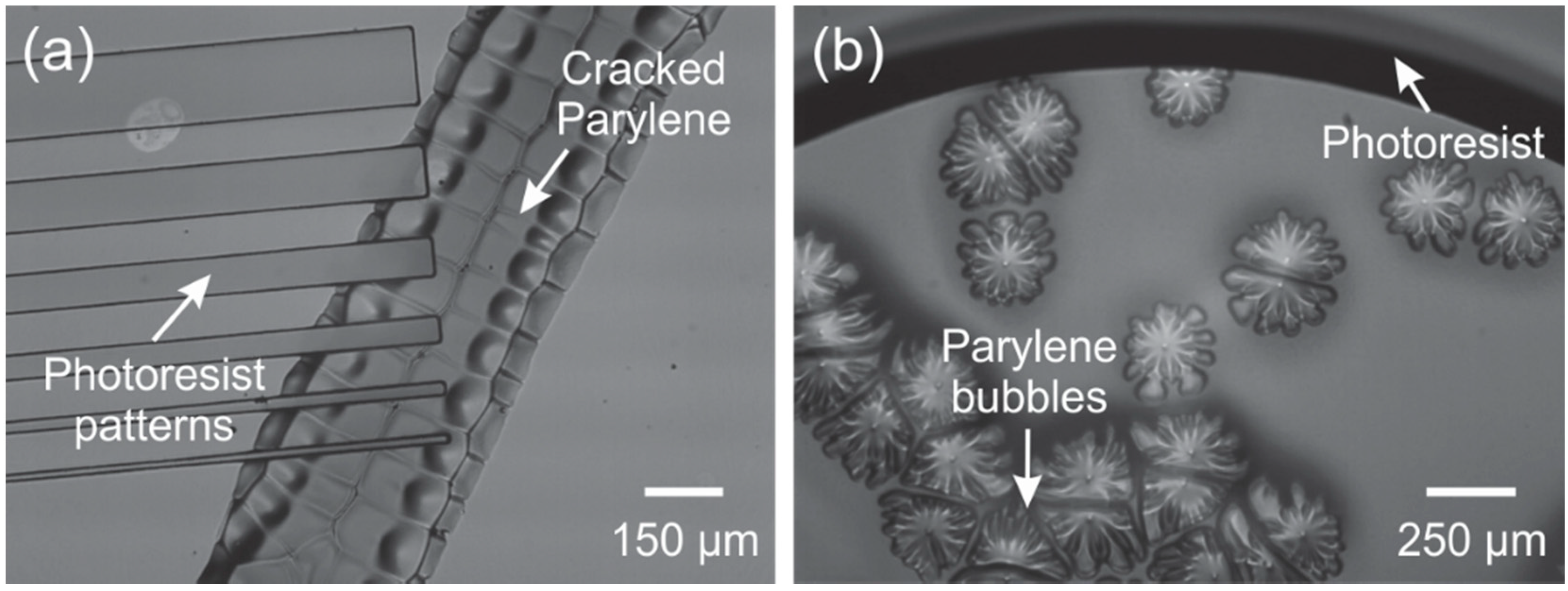
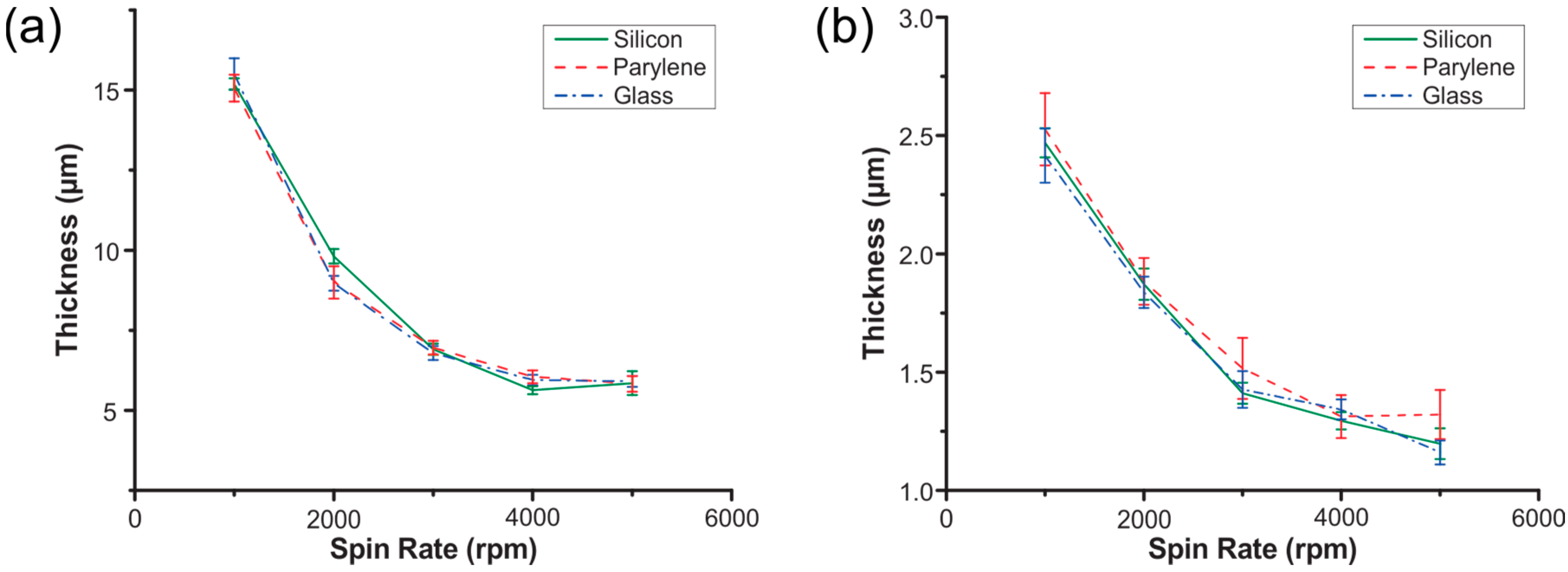
3.2. Lithographic Processes
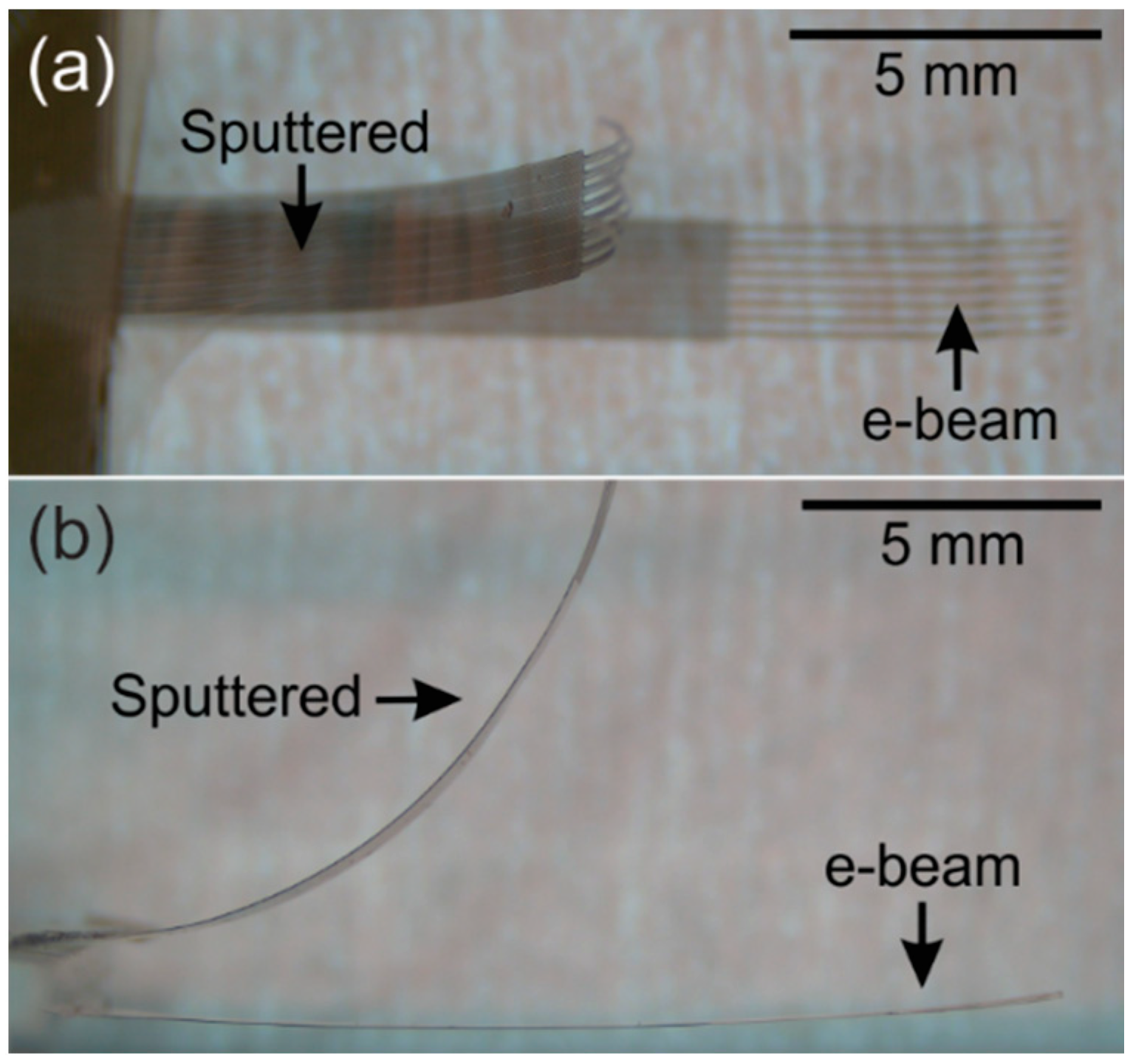
3.3. Metal Deposition
3.4. Etching
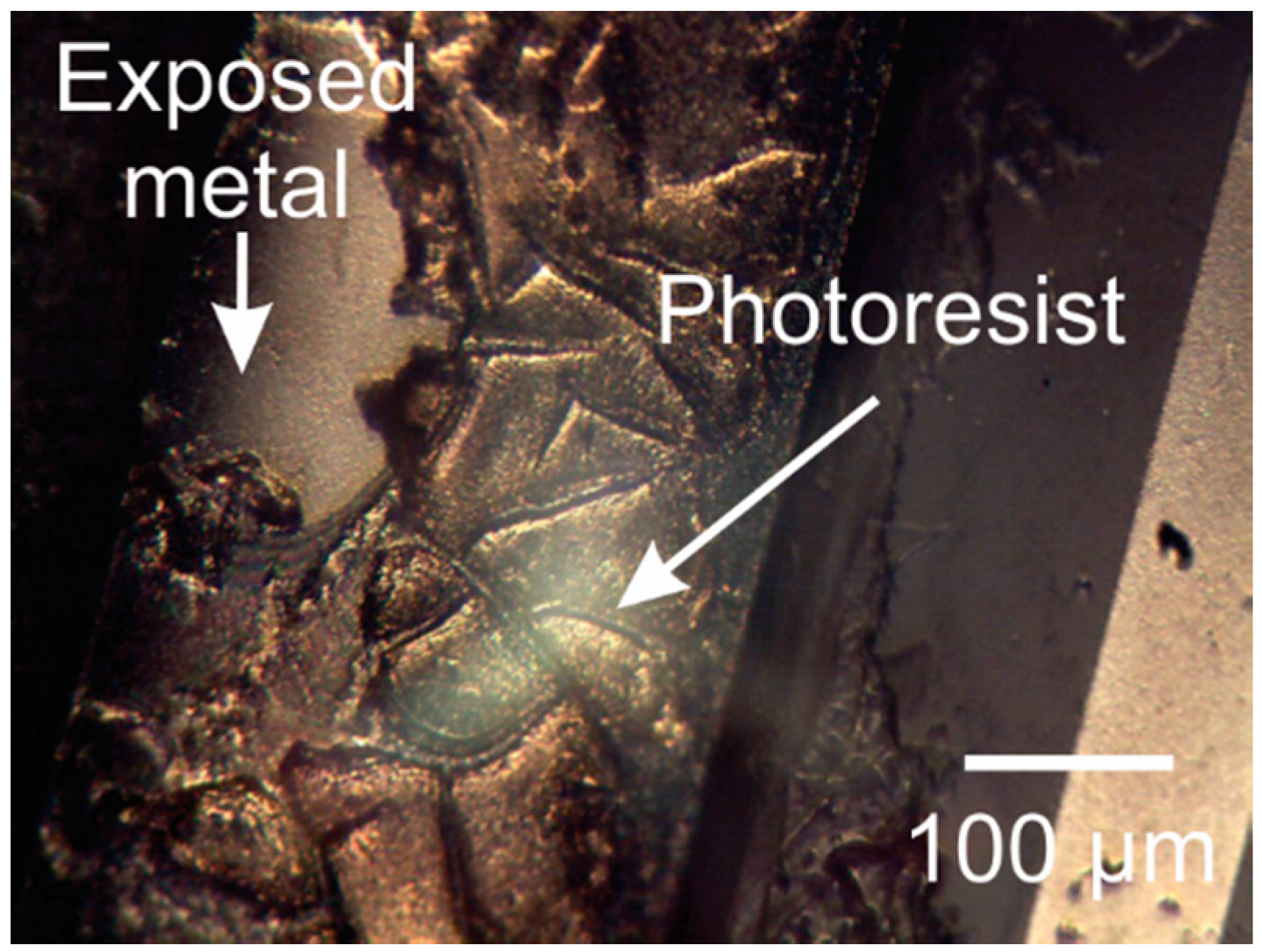
3.5. Photoresist Stripping
3.5.1. Sacrificial Layer
3.5.2. Oxygen Plasma Exposed Photoresist
3.6. Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gorham, W.F. A new, general synthetic method for the preparation of linear poly-p-xylylenes. J. Polym. Sci. Part A Polym. Chem. 1966, 4, 3027–3039. [Google Scholar] [CrossRef]
- Szwarc, M. Some remarks on the CH2 [graphic omitted] CH2 molecule. Discuss. Faraday Soc. 1947, 2, 46–49. [Google Scholar] [CrossRef]
- Kim, B.J.; Meng, E. Micromachining of Parylene C for bioMEMS. Polym. Adv. Technol. 2016, 27, 564–576. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.-M. Deposition kinetics for polymerization via the gorham route. In Chemical Vapor Deposition Polymerization; Springer: New York, NY, USA, 2004; pp. 41–55. [Google Scholar]
- Yang, G.-R.; Ganguli, S.; Karcz, J.; Gill, W.; Lu, T.-M. High deposition rate Parylene films. J. Cryst. Growth 1998, 183, 385–390. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.-M. Chemical Vapor Deposition Polymerization: The Growth and Properties of Parylene Thin Films; Springer: New York, NY, USA, 2003. [Google Scholar]
- Tai, Y.C. Parylene BioMEMS. In Proceedings of the 2005 IEEE International Conference on Robotics and Biomimetics (ROBIO), Shatin, China, 5–9 July 2005; p. xxxvii. [Google Scholar]
- Rodger, D.C.; Li, W.; Ameri, H.; Ray, A.; Weiland, J.D.; Humayun, M.S.; Tai, Y.-C. Flexible Parylene-based microelectrode technology for intraocular retinal prostheses. In Proceedings of the 1st IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Zhuhai, China, 18–21 January 2006; pp. 743–746. [Google Scholar]
- Senkevich, J.J.; Desu, S.B. Morphology of poly (chloro-p-xylylene) CVD thin films. Polymer 1999, 40, 5751–5759. [Google Scholar] [CrossRef]
- Noh, H.-S.; Huang, Y.; Hesketh, P.J. Parylene micromolding, a rapid and low-cost fabrication method for Parylene microchannel. Sens. Actuators B Chem. 2004, 102, 78–85. [Google Scholar] [CrossRef]
- Kim, B.J.; Meng, E. Review of polymer MEMS micromachining. J. Micromech. Microeng. 2015, 26, 013001. [Google Scholar] [CrossRef] [Green Version]
- Scholten, K.; Meng, E. Electron-beam lithography for polymer bioMEMS with submicron features. Microsyst. Nanoeng. 2016, 2, 16053. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Tai, Y.-C. Flexible Parylene-based 3-D coiled cable. In Proceedings of the 2010 5th IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Xiamen, China, 20–23 January 2010; pp. 317–320. [Google Scholar]
- Yu, H.; Wang, S.; Wang, W.; Li, Z. Fabrication of electroplated nickel multielectrode microprobes with flexible Parylene cable. In Proceedings of the 2012 IEEE 25th International Conference on Micro Electro Mechanical Systems (MEMS), Paris, France, 29 January–2 February 2012; pp. 239–242. [Google Scholar]
- Bhandari, R.; Negi, S.; Van Wagenen, R.; Solzbacher, F. Parylene C-based flexible interface for neuroprosthetic applications. In Proceedings of the 2010 Proceedings 60th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 1–4 June 2010; pp. 1010–1014. [Google Scholar]
- Li, W.; Rodger, D.C.; Pinto, A.; Meng, E.; Weiland, J.D.; Humayun, M.S.; Tai, Y.-C. Parylene-based integrated wireless single-channel neurostimulator. Sens. Actuators A Phys. 2011, 166, 193–200. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, Y.; Li, Z.; Yu, M.; Yuan, Q.; Li, X.; Zhang, H. Design and fabrication of flexible Parylene-based inductors with electroplated nife magnetic core for wireless power transmission system. In Proceedings of the 2012 7th IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Kyoto, Japan, 5–8 March 2012; pp. 238–242. [Google Scholar]
- Baldwin, A.; Yu, L.; Pratt, M.; Scholten, K.; Meng, E. Passive, wireless transduction of electrochemical impedance across thin-film microfabricated coils using reflected impedance. Biomed. Microdevices 2017, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Crum, B.; Li, W. Parylene-based fold-and-bond wireless pressure sensor. In Proceedings of the 2013 8th IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Suzhou, China, 7–10 April 2013; pp. 1155–1158. [Google Scholar]
- Yu, L.; Gutierrez, C.A.; Meng, E. An electrochemical microbubble-based MEMS pressure sensor. J. Microelectromech. Syst. 2016, 25, 144–152. [Google Scholar] [CrossRef]
- Meng, E.; Chen, P.-J.; Rodger, D.; Tai, Y.-C.; Humayun, M. Implantable Parylene MEMS for glaucoma therapy. In Proceedings of the 3rd IEEE/EMBS Special Topic Conference on Microtechnology in Medicine and Biology, Kahuku, HI, USA, 12–15 May 2005; pp. 116–119. [Google Scholar]
- Meng, E.; Tai, Y.-C. A Parylene MEMS flow sensing array. In Proceedings of the 12th International Conference on TRANSDUCERS, Solid-State Sensors, Actuators and Microsystems, Boston, MA, USA, 8–12 June 2003; pp. 686–689. [Google Scholar]
- Baldwin, A.; Yu, L.; Meng, E. An electrochemical impedance-based thermal flow sensor for physiological fluids. J. Microelectromech. Syst. 2016, 25, 1015–1024. [Google Scholar] [CrossRef]
- Li, C.; Wu, P.-M.; Hartings, J.A.; Wu, Z.; Ahn, C.H.; Narayan, R.K. Cerebral blood flow sensor with in situ temperature and thermal conductivity compensation. In Proceedings of the 2012 IEEE 25th International Conference on the Micro Electro Mechanical Systems (MEMS), Paris, France, 29 January–2 February 2012; pp. 1021–1024. [Google Scholar]
- Yu, H.; Ai, L.; Rouhanizadeh, M.; Patel, D.; Kim, E.S.; Hsiai, T.K. Flexible polymer sensors for in vivo intravascular shear stress analysis. J. Microelectromech. Syst. 2008, 17, 1178–1186. [Google Scholar]
- Park, J.; Pine, J.; Tai, Y.-C. Flexible neurocage array for live neural network study. In Proceedings of the 2013 IEEE 26th International Conference on Micro Electro Mechanical Systems (MEMS), Taipei, Taiwan, 20–24 January 2013; pp. 295–298. [Google Scholar]
- He, Q.; Meng, E.; Tai, Y.-C.; Rutherglen, C.M.; Erickson, J.; Pine, J. Parylene neuro-cages for live neural networks study. In Proceedings of the 12th International Conference on TRANSDUCERS, Solid-State Sensors, Actuators and Microsystems, Boston, MA, USA, 8–12 June 2003; pp. 995–998. [Google Scholar]
- Tooker, A.; Meng, E.; Erickson, J.; Tai, Y.-C.; Pine, J. Development of biocompatible Parylene neurocages. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–4 September 2004; pp. 2542–2545. [Google Scholar]
- Sheybani, R.; Gensler, H.; Meng, E. A MEMS electrochemical bellows actuator for fluid metering applications. Biomed. Microdevices 2013, 15, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-Y.; Sheybani, R.; Gutierrez, C.A.; Kuo, J.T.; Meng, E. A Parylene bellows electrochemical actuator. J. Microelectromech. Syst. 2010, 19, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Gensler, H.; Sheybani, R.; Meng, E. Rapid non-lithography based fabrication process and characterization of Parylene C bellows for applications in MEMS electrochemical actuators. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference (TRANSDUCERS), Beijing, China, 5–9 June 2011; pp. 2347–2350. [Google Scholar]
- Kim, B.J.; Jin, W.; Baldwin, A.; Yu, L.; Christian, E.; Krieger, M.D.; McComb, J.G.; Meng, E. Parylene MEMS patency sensor for assessment of hydrocephalus shunt obstruction. Biomed. Microdevices 2016, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-Y.; Givrad, T.K.; Holschneider, D.P.; Maarek, J.-M.I.; Meng, E. A Parylene MEMS electrothermal valve. J. Microelectromech. Syst. 2009, 18, 1184–1197. [Google Scholar] [PubMed]
- Kuo, J.T.; Kim, B.J.; Hara, S.A.; Lee, C.D.; Gutierrez, C.A.; Hoang, T.Q.; Meng, E. Novel flexible Parylene neural probe with 3d sheath structure for enhancing tissue integration. Lab Chip 2013, 13, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kuo, J.T.; Hara, S.A.; Lee, C.D.; Yu, L.; Gutierrez, C.; Hoang, T.; Pikov, V.; Meng, E. 3d Parylene sheath neural probe for chronic recordings. J. Neural Eng. 2013, 10, 045002. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.A.; Kim, B.J.; Kuo, J.T.; Lee, C.D.; Meng, E.; Pikov, V. Long-term stability of intracortical recordings using perforated and arrayed Parylene sheath electrodes. J. Neural Eng. 2016, 13, 066020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschberg, A.W.; Xu, H.; Scholten, K.; Berger, T.W.; Song, D.; Meng, E. Development of an anatomically conformal Parylene neural probe array for multi-region hippocampal recordings. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 129–132. [Google Scholar]
- Seymour, J.P.; Langhals, N.B.; Anderson, D.J.; Kipke, D.R. Novel multi-sided, microelectrode arrays for implantable neural applications. Biomed. Microdevices 2011, 13, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, Y.; Nishino, M.; Saito, I.; Suzuki, T.; Mabuchi, K. Flexible intracortical neural probe with biodegradable polymer for delivering bioactive components. In Proceedings of the 2006 International Conference on Microtechnologies in Medicine and Biology, Okinawa, Japan, 9–12 May 2006; pp. 143–146. [Google Scholar]
- Ledochowitsch, P.; Olivero, E.; Blanche, T.; Maharbiz, M.M. A transparent μECoG array for simultaneous recording and optogenetic stimulation. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC, Boston, MA, USA, 30 August–3 September 2011; pp. 2937–2940. [Google Scholar]
- Ledochowitsch, P.; Félus, R.; Gibboni, R.; Miyakawa, A.; Bao, S.; Maharbiz, M. Fabrication and testing of a large area, high density, Parylene MEMS µECoG array. In Proceedings of the 2011 IEEE 24th International Conference on Micro Electro Mechanical Systems (MEMS), Cancun, Mexico, 23–27 January 2011; pp. 1031–1034. [Google Scholar]
- Chou, L.-C.; Tsai, S.-W.; Chang, W.-L.; Chiou, J.-C.; Chiu, T.-W. A Parylene-C based 16 channels flexible bio-electrode for ECoG recording. In Proceedings of the 2014 IEEE SENSORS, Valencia, Spain, 2–5 November 2014; pp. 877–880. [Google Scholar]
- Takeuchi, S.; Ziegler, D.; Yoshida, Y.; Mabuchi, K.; Suzuki, T. Parylene flexible neural probes integrated with microfluidic channels. Lab Chip 2005, 5, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kotake, N.; Mabuchi, K.; Takeuchi, S. Flexible regeneration-type nerve electrode with integrated microfluidic channels. In Proceedings of the 2006 International Conference on Microtechnologies in Medicine and Biology, Okinawa, Japan, 9–12 May 2006; pp. 303–305. [Google Scholar]
- Ziegler, D.; Suzuki, T.; Takeuchi, S. Fabrication of flexible neural probes with built-in microfluidic channels by thermal bonding of Parylene. J. Microelectromech. Syst. 2006, 15, 1477–1482. [Google Scholar] [CrossRef]
- Yu, H.; Xiong, W.; Zhang, H.; Wang, W.; Li, Z. A Parylene self-locking cuff electrode for peripheral nerve stimulation and recording. J. Microelectromech. Syst. 2014, 23, 1025–1035. [Google Scholar] [CrossRef]
- Kang, X.; Liu, J.-Q.; Tian, H.; Yang, B.; Nuli, Y.; Yang, C. Self-closed Parylene cuff electrode for peripheral nerve recording. J. Microelectromech. Syst. 2015, 24, 319–332. [Google Scholar] [CrossRef]
- Cobo, A.M.; Boyajian, B.; Larson, C.; Scholten, K.; Pikov, V.; Meng, E. A Parylene cuff electrode for peripheral nerve recording and drug delivery. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 506–509. [Google Scholar]
- Rodger, D.C.; Fong, A.J.; Li, W.; Ameri, H.; Ahuja, A.K.; Gutierrez, C.; Lavrov, I.; Zhong, H.; Menon, P.R.; Meng, E. Flexible Parylene-based multielectrode array technology for high-density neural stimulation and recording. Sens. Actuators B Chem. 2008, 132, 449–460. [Google Scholar] [CrossRef]
- Nandra, M.S.; Lavrov, I.A.; Edgerton, V.R.; Tai, Y.-C. A Parylene-based microelectrode array implant for spinal cord stimulation in rats. In Proceedings of the 2011 IEEE 24th International Conference on Micro Electro Mechanical Systems (MEMS), Cancun, Mexico, 23–27 January 2011; pp. 1007–1010. [Google Scholar]
- Wang, R.; Huang, X.; Liu, G.; Wang, W.; Dong, F.; Li, Z. Fabrication and characterization of a Parylene-based three-dimensional microelectrode array for use in retinal prosthesis. J. Microelectromech. Syst. 2010, 19, 367–374. [Google Scholar] [CrossRef]
- Liu, Y.; Park, J.; Lang, R.J.; Emami-Neyestanak, A.; Pellegrino, S.; Humayun, M.S.; Tai, Y.-C. Parylene origami structure for intraocular implantation. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, Spain, 16–20 June 2013; pp. 1549–1552. [Google Scholar]
- Johnson, A.C.; Wise, K.D. A self-curling monolithically-backed active high-density cochlear electrode array. In Proceedings of the 2012 IEEE 25th International Conference on Micro Electro Mechanical Systems (MEMS), Paris, France, 29 January–2 February 2012; pp. 914–917. [Google Scholar]
- Reiser, A.; Huang, J.; He, X.; Yeh, T.; Jha, S.; Shih, H.; Kim, M.; Han, Y.; Yan, K. The molecular mechanism of novolak–diazonaphthoquinone resists. Eur. Polym. J. 2002, 38, 619–629. [Google Scholar] [CrossRef]
- Kim, B.J.; Washabaugh, E.P.; Meng, E. Annealing effects on flexible multi-layered Parylene-based sensors. In Proceedings of the 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–28 January 2014; pp. 825–828. [Google Scholar]
- Ortigoza-Diaz, J.; Scholten, K.; Meng, E. Characterization and modification of adhesion in dry and wet environments in thin-film Parylene systems. J. Microelectromech. Syst. 2018. [Google Scholar] [CrossRef]
- Hassler, C.; von Metzen, R.P.; Ruther, P.; Stieglitz, T. Characterization of Parylene C as an encapsulation material for implanted neural prostheses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-M.; Rieth, L.; Normann, R.A.; Tathireddy, P.; Solzbacher, F. Encapsulation of an integrated neural interface device with Parylene C. IEEE Trans. Biomed. Eng. 2009, 56, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Spivack, M.; Ferrante, G. Determination of the water vapor permeability and continuity of ultrathin Parylene membranes. J. Electrochem. Soc. 1969, 116, 1592–1594. [Google Scholar] [CrossRef]
- Von Metzen, R.P.; Stieglitz, T. The effects of annealing on mechanical, chemical, and physical properties and structural stability of Parylene C. Biomed. Microdevices 2013, 15, 727–735. [Google Scholar] [CrossRef] [PubMed]
- SCS. SCS Parylene Properties. Available online: https://scscoatings.com/wp-content/uploads/2017/09/02-SCS-Parylene-Properties-1016.pdf (accessed on 17 August 2018).
- Coating, P.T. Parylene Properties. Available online: https://www.parylene.com/Portals/0/CWPS-Parylene-Properties-Chart.pdf (accessed on 20 August 2018).
- Menon, P.; Li, W.; Tooker, A.; Tai, Y. Characterization of water vapor permeation through thin film Parylene C. In Proceedings of the International Solid-State Sensors, Actuators and Microsystems Conference, TRANSDUCERS, Denver, CO, USA, 21–25 June 2009; pp. 1892–1895. [Google Scholar]
- ASTM International. Standard Test Methods for Water Vapor Transmission of Organic Coating Films; ASTM D1653-03; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Mordelt, G.; Heim, P. Parylene: High-tech-beschichtung der zukunft. Metalloberfläche 1998, 52, 368–371. [Google Scholar]
- Seymour, J.P.; Elkasabi, Y.M.; Chen, H.-Y.; Lahann, J.; Kipke, D.R. The insulation performance of reactive Parylene films in implantable electronic devices. Biomaterials 2009, 30, 6158–6167. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Rieth, L.; Tathireddy, P.; Solzbacher, F. Long-term in-vivo investigation of Parylene-C as encapsulation material for neural interfaces. Procedia Eng. 2011, 25, 483–486. [Google Scholar] [CrossRef]
- Li, W.; Rodger, D.; Menon, P.; Tai, Y.-C. Corrosion behavior of Parylene-metal-Parylene thin films in saline. ECS Trans. 2008, 11, 1–6. [Google Scholar]
- Li, W.; Rodger, D.C.; Meng, E.; Weiland, J.D.; Humayun, M.S.; Tai, Y.-C. Flexible Parylene packaged intraocular coil for retinal prostheses. In Proceedings of the 2006 International Conference on Microtechnologies in Medicine and Biology, Okinawa, Japan, 4–8 June 2006; pp. 105–108. [Google Scholar]
- Huang, R.; Tai, Y. Parylene to silicon adhesion enhancement. In Proceedings of the International Solid-State Sensors, Actuators and Microsystems Conference, TRANSDUCERS, Denver, CO, USA, 21–25 June 2009; pp. 1027–1030. [Google Scholar]
- Charmet, J.; Bitterli, J.; Sereda, O.; Liley, M.; Renaud, P.; Keppner, H. Optimizing Parylene C adhesion for MEMS processes: Potassium hydroxide wet etching. J. Microelectromech. Syst. 2013, 22, 855–864. [Google Scholar] [CrossRef]
- Yamagishi, F.G. Investigations of plasma-polymerized films as primers for Parylene-C coatings on neural prosthesis materials. Thin Solid Films 1991, 202, 39–50. [Google Scholar] [CrossRef]
- Hwang, K.S.; Park, J.H.; Lee, J.H.; Yoon, D.S.; Kim, T.S.; Han, I.; Noh, J.H. Effect of atmospheric-plasma treatments for enhancing adhesion of au on Parylene-C-coated protein chips. J. Korean Phys. Soc. 2004, 44, 1168–1172. [Google Scholar]
- Schmidt, E.; McIntosh, J.; Bak, M. Long-term implants of Parylene-C coated microelectrodes. Med. Biol. Eng. Comput. 1988, 26, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.M.; Benetatos, N.M.; Regnault, W.F.; Winey, K.I.; Elabd, Y.A. The influence of thermal history on structure and water transport in Parylene C coatings. Polymer 2011, 52, 5378–5386. [Google Scholar] [CrossRef]
- Lee, C.D.; Meng, E. Mechanical properties of thin-film Parylene–metal–Parylene devices. Front. Mech. Eng. 2015, 1, 10. [Google Scholar] [CrossRef]
- Radun, V.; von Metzen, R.; Stieglitz, T.; Bucher, V.; Stett, A. Evaluation of adhesion promoters for Parylene C on gold metallization. Curr. Dir. Biomed. Eng. 2015, 1, 493–497. [Google Scholar] [CrossRef]
- Xie, Y.; Pei, W.; Guo, D.; Zhang, L.; Zhang, H.; Guo, X.; Xing, X.; Yang, X.; Wang, F.; Gui, Q. Improving adhesion strength between layers of an implantable Parylene-C electrode. Sens. Actuators A Phys. 2017, 260, 117–123. [Google Scholar] [CrossRef]
- Bienkiewicz, J. Plasma-enhanced Parylene coating. Med. Device Technol. 2006, 17, 10. [Google Scholar] [PubMed]
- Randles, J.E.B. Kinetics of rapid electrode reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Weltman, A.; Yoo, J.; Meng, E. Flexible, penetrating brain probes enabled by advances in polymer microfabrication. Micromachines 2016, 7, 180. [Google Scholar] [CrossRef]
- Lu, D.; Wong, C. Materials for Advanced Packaging; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Gutierrez, C.A.; Lee, C.; Kim, B.; Meng, E. Epoxy-less packaging methods for electrical contact to Parylene-based flat flexible cables. In Proceedings of the 2011 16th International, Solid-State Sensors, Actuators and Microsystems Conference (TRANSDUCERS), Beijing, China, 5–9 June 2011; pp. 2299–2302. [Google Scholar]
- Lecomte, A.; Castagnola, V.; Descamps, E.; Dahan, L.; Blatché, M.; Dinis, T.; Leclerc, E.; Egles, C.; Bergaud, C. Silk and peg as means to stiffen a Parylene probe for insertion in the brain: Toward a double time-scale tool for local drug delivery. J. Micromech. Microeng. 2015, 25, 125003. [Google Scholar] [CrossRef]
- Wu, F.; Tien, L.; Chen, F.; Kaplan, D.; Berke, J.; Yoon, E. A multi-shank silk-backed Parylene neural probe for reliable chronic recording. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, Spain, 16–20 June 2013; pp. 888–891. [Google Scholar]
- Rios, G.; Lubenov, E.V.; Chi, D.; Roukes, M.L.; Siapas, A.G. Nanofabricated neural probes for dense 3-D recordings of brain activity. Nano Lett. 2016, 16, 6857–6862. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.; Le, H.-P.; Li, W.; Ledochowitsch, P.; Gambini, S.; Bjorninen, T.; Koralek, A.; Carmena, J.M.; Maharbiz, M.M.; Alon, E. A minimally invasive 64-channel wireless μECoG implant. IEEE J. Solid State Circ. 2015, 50, 344–359. [Google Scholar] [CrossRef]
- Lecomte, A.; Degache, A.; Descamps, E.; Dahan, L.; Bergaud, C. In vitro and in vivo biostability assessment of chronically-implanted Parylene C neural sensors. Sens. Actuators B Chem. 2017, 251, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Rodger, D.C.; Weiland, J.D.; Humayun, M.S.; Tai, Y.-C. Scalable high lead-count Parylene package for retinal prostheses. Sens. Actuators B Chem. 2006, 117, 107–114. [Google Scholar] [CrossRef]
- Chang, J.H.-C.; Huang, R.; Tai, Y.-C. High density 256-channel chip integration with flexible Parylene pocket. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference (TRANSDUCERS), Beijing, China, 5–9 June 2011; pp. 378–381. [Google Scholar]
- von Metzen, R.; Stieglitz, T. Impact of sterilization procedures on the stability of Parylene based flexible multilayer structures. Biomed. Eng. Biomed. Tech. 2013. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.; Betts, R. Drug Reservoir Stent. U.S. Patent US20070173923A1, 26 July 2007. [Google Scholar]
- Beshchasna, N.; Adolphi, B.; Granovsky, S.; Braunschweig, M.; Schneider, W.; Uhlemann, J.; Wolter, K.-J. Influence of artificial body fluids and medical sterilization procedures on chemical stability of Parylene C. In Proceedings of the 2010 Proceedings 60th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 1–4 June 2010; pp. 1846–1852. [Google Scholar]
- Kokko, K.; Harjunpää, H.; Heino, P.; Kellomäki, M. Influence of medical sterilization on ACA flip chip joints using conformal coating. Microelectron. Reliab. 2009, 49, 92–98. [Google Scholar] [CrossRef]
- Scarantino, C.W.; Ruslander, D.M.; Rini, C.J.; Mann, G.G.; Nagle, H.T.; Black, R.D. An implantable radiation dosimeter for use in external beam radiation therapy. Med. Phys. 2004, 31, 2658–2671. [Google Scholar] [CrossRef] [PubMed]
- Rodger, D.; Fong, A.; Li, W.; Ameri, H.; Lavrov, I.; Zhong, H.; Saati, S.; Menon, P.; Meng, E.; Burdick, J. High-density flexible Parylene-based multielectrode arrays for retinal and spinal cord stimulation. In Proceedings of the International Solid-State Sensors, Actuators and Microsystems Conference, TRANSDUCERS, Lyon, France, 10–14 June 2007; pp. 1385–1388. [Google Scholar]
- Stieglitz, T. Manufacturing, assembling and packaging of miniaturized neural implants. Microsyst. Technol. 2010, 16, 723–734. [Google Scholar] [CrossRef]
- Henle, C.; Hassler, C.; Kohler, F.; Schuettler, M.; Stieglitz, T. Mechanical characterization of neural electrodes based on PDMS-Parylene C-PDMS sandwiched system. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC, Boston, MA, USA, 30 August–3 September 2011; pp. 640–643. [Google Scholar]
- Hsu, J.-M.; Kammer, S.; Jung, E.; Rieth, L.; Normann, R.; Solzbacher, F. Characterization of Parylene-C film as an encapsulation material for neural interface devices. In 4m 2007: Proceedings of the 3rd International Conference on Multi-Material Micro Manufacture; Whittles Publishing: Dunbeath, UK, 2007. [Google Scholar]
- Bonner, J.; Tudryn, C.D.; Choi, S.J.; Eulogio, S.E.; Roberts, T.J. The Use of Liquid Isopropyl Alcohol and Hydrogen Peroxide Gas Plasma to Biologically Decontaminate Spacecraft Electronics; Jet Propulsion Laboratory, National Aeronautics and Space Administration: Pasadena, CA, USA, 2006.
- Perry, D.; Grayden, D.; Shepherd, R.; Fallon, J. A fully implantable rodent neural stimulator. J. Neural Eng. 2012, 9, 014001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grinberg, O.; Natan, M.; Lipovsky, A.; Varvak, A.; Keppner, H.; Gedanken, A.; Banin, E. Antibiotic nanoparticles embedded into the Parylene C layer as a new method to prevent medical device-associated infections. J. Mater. Chem. B 2015, 3, 59–64. [Google Scholar] [CrossRef]
- Loeb, G.E.; Bak, M.; Salcman, M.; Schmidt, E. Parylene as a chronically stable, reproducible microelectrode insulator. IEEE Trans. Biomed. Eng. 1977, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Loeb, G.; Peck, R.; Martyniuk, J. Toward the ultimate metal microelectrode. J. Neurosci. Methods 1995, 63, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Bera, M.; Rivaton, A.; Gandon, C.; Gardette, J. Comparison of the photodegradation of Parylene C and Parylene N. Eur. Polym. J. 2000, 36, 1765–1777. [Google Scholar] [CrossRef]
- Fortin, J.; Lu, T.-M. Ultraviolet radiation induced degradation of poly-para-xylylene (Parylene) thin films. Thin Solid Films 2001, 397, 223–228. [Google Scholar] [CrossRef]
- Solvent Resistance of the Parylenes. Available online: https://scscoatings.com/wp-content/uploads/2015/09/solvent_resistance.pdf (accessed on 20 August 2018).
- Meng, E.; Tai, Y.-C. Parylene etching techniques for microfluidics and bioMEMS. In Proceedings of the 18th IEEE International Conference on Micro Electro Mechanical Systems, Miami Beach, FL, USA, 30 January 2005; pp. 568–571. [Google Scholar]
- Nowlin, T.; Smith, D.F. Surface characterization of plasma-treated poly-p-xylylene films. J. Appl. Polym. Sci. 1980, 25, 1619–1632. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Desai, A.; Tai, Y.-C.; Licklider, L.; Lee, T.D. Polymer-based electrospray chips for mass spectrometry. In Proceedings of the Twelfth IEEE International Conference on Micro Electro Mechanical Systems, Orlando, FL, USA, 17–21 January 1999; pp. 523–528. [Google Scholar]
- Levy, B.P.; Campbell, S.L.; Rose, T.L. Definition of the geometric area of a microelectrode tip by plasma etching of Parylene. IEEE Trans. Biomed. Eng. 1986, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Ratier, B.; Jeong, Y.S.; Moliton, A.; Audebert, P. Vapor deposition polymerization and reactive ion beam etching of poly (p-xylylene) films for waveguide applications. Opt. Mater. 1999, 12, 229–233. [Google Scholar] [CrossRef]
- Meng, E.; Li, P.-Y.; Tai, Y.-C. Plasma removal of Parylene C. J. Micromech. Microeng. 2008, 18, 045004. [Google Scholar] [CrossRef] [Green Version]
- Majid, N.; Dabral, S.; McDonald, J. The Parylene-aluminum multilayer interconnection system for wafer scale integration and wafer scale hybrid packaging. J. Electron. Mater. 1989, 18, 301–311. [Google Scholar] [CrossRef]
- Yeh, J.; Grebe, K. Patterning of poly-para-xylylenes by reactive ion etching. J. Vacuum Sci. Technol. A Vacuum Surf. Films 1983, 1, 604–608. [Google Scholar] [CrossRef]
- Lecomte, A.; Lecestre, A.; Bourrier, D.; Blatché, M.-C.; Jalabert, L.; Descamps, E.; Bergaud, C. Deep plasma etching of Parylene C patterns for biomedical applications. Microelectron. Eng. 2017, 177, 70–73. [Google Scholar] [CrossRef]
- Selvarasah, S.; Chao, S.; Chen, C.-L.; Sridhar, S.; Busnaina, A.; Khademhosseini, A.; Dokmeci, M. A reusable high aspect ratio Parylene-C shadow mask technology for diverse micropatterning applications. Sens. Actuators A Phys. 2008, 145, 306–315. [Google Scholar] [CrossRef]
- Mercanzini, A.; Cheung, K.; Buhl, D.L.; Boers, M.; Maillard, A.; Colin, P.; Bensadoun, J.-C.; Bertsch, A.; Renaud, P. Demonstration of cortical recording using novel flexible polymer neural probes. Sens. Actuators A Phys. 2008, 143, 90–96. [Google Scholar] [CrossRef]




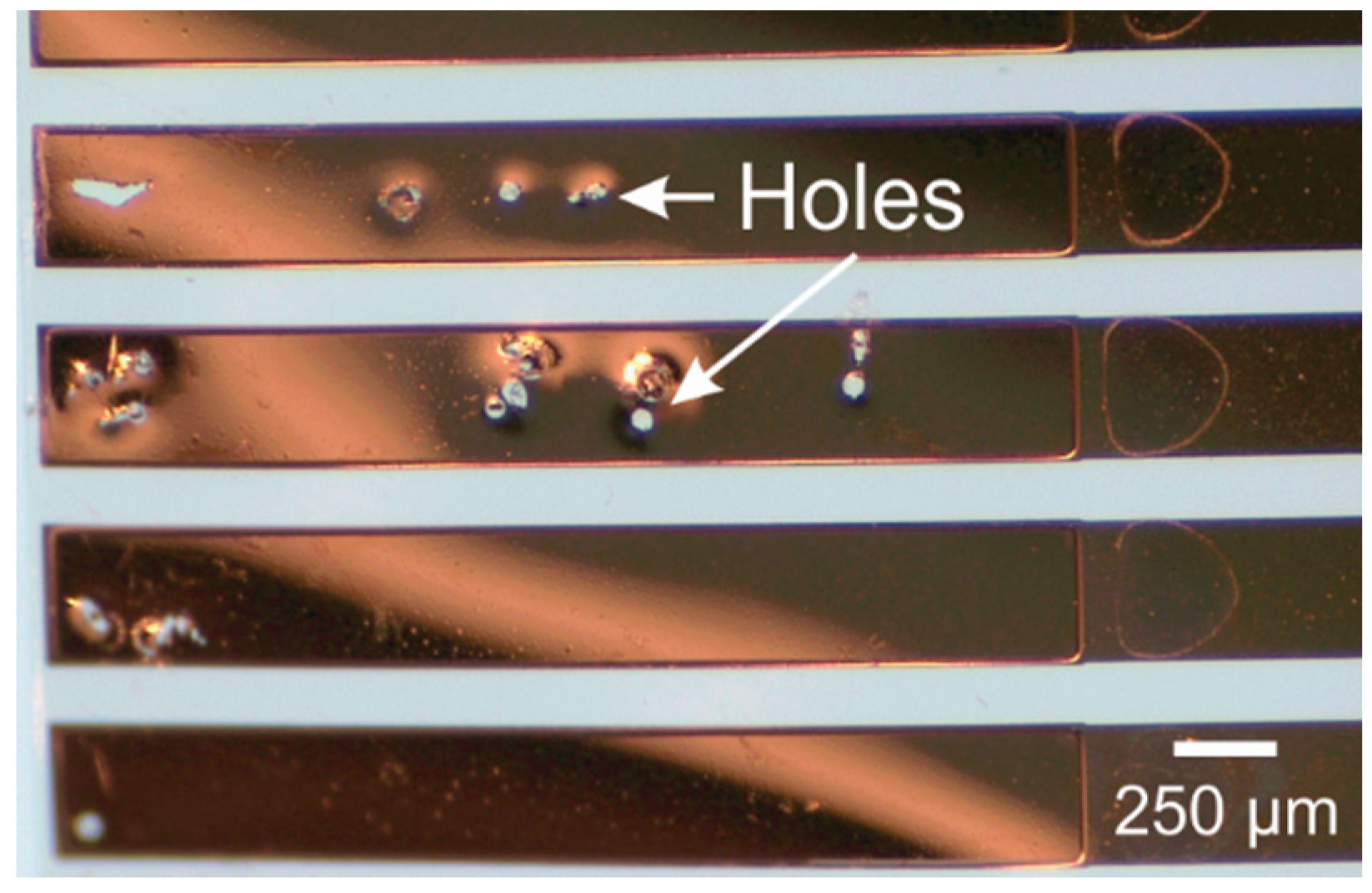
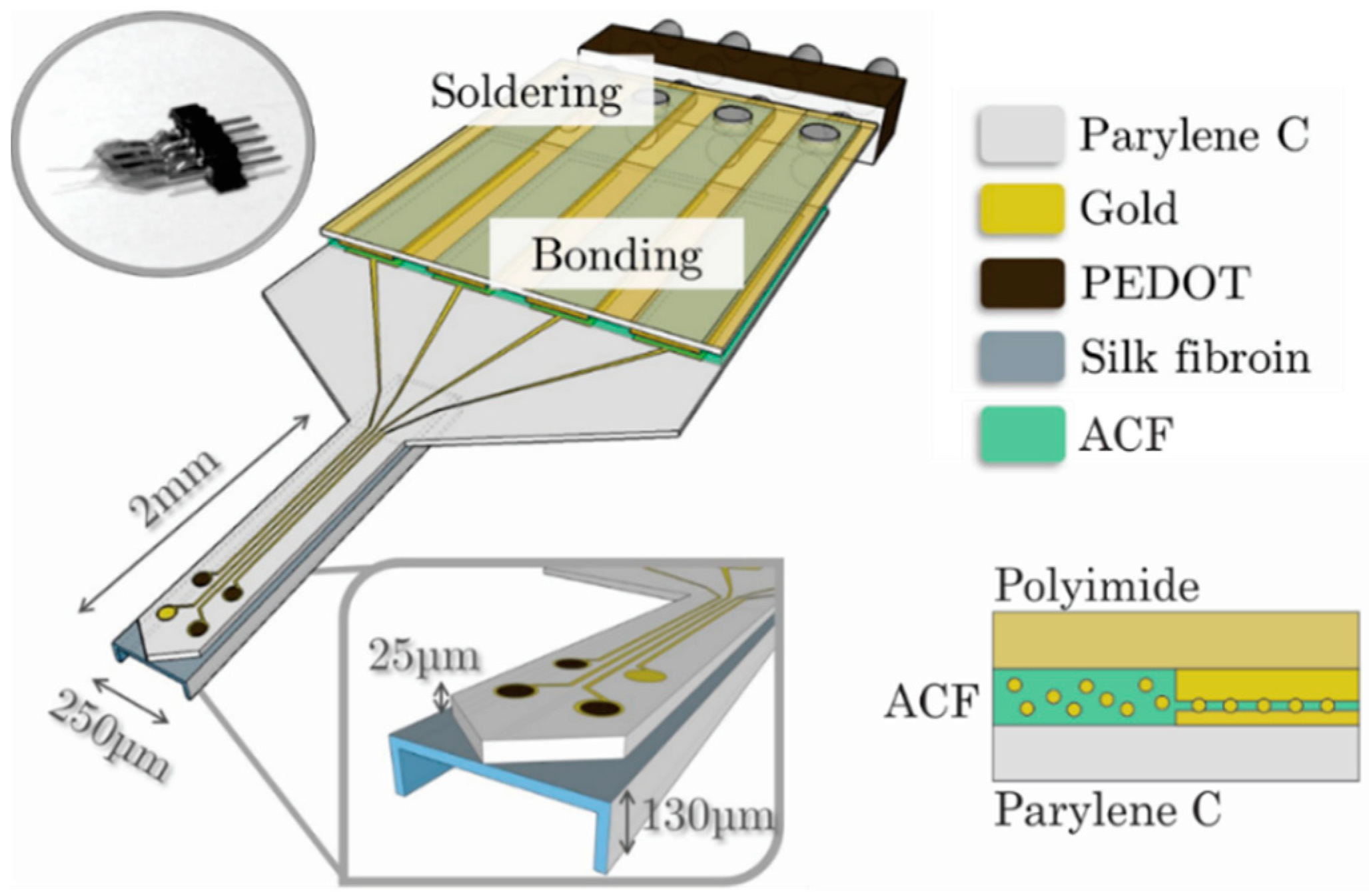
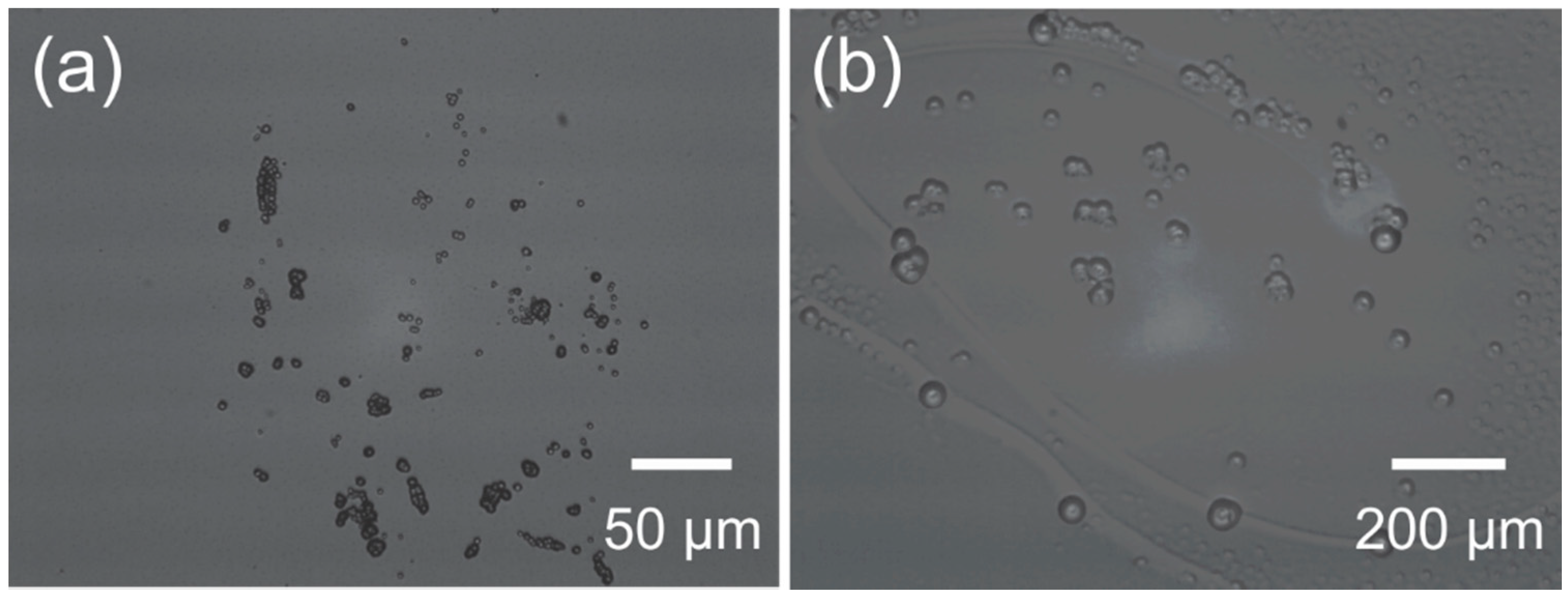
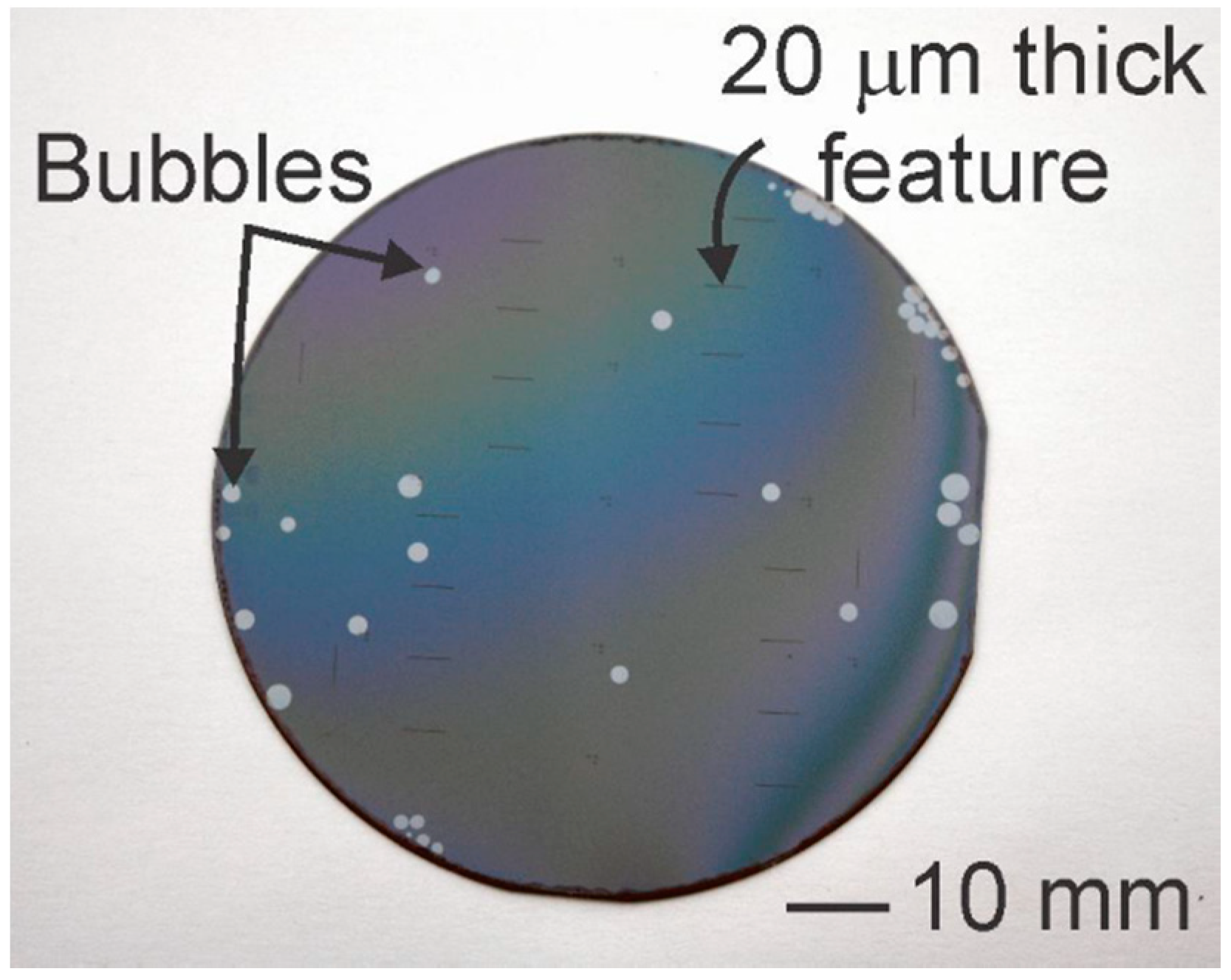


| Reference | Thickness (µm) | Mean WVTR (g·mm/m2·day) | Standard Error | Temperature and Relative Humidity (Mean ± SE) | |
|---|---|---|---|---|---|
| This work | Un-annealed | 5 | 0.0194 | 0.0012 | 20.6 ± 0.14 °C, 34 ± 3% |
| 10 | 0.0176 | 0.0010 | 20.7 ± 0.13 °C, 32 ± 3% | ||
| 15 | 0.0185 | 0.0025 | 20.8 ± 0.12 °C, 33 ± 3% | ||
| Annealed | 5 | 0.0185 | 0.0014 | 20.6 ± 0.14 °C, 33 ± 3% | |
| 10 | 0.0128 | 0.0010 | 20.7 ± 0.14 °C, 34 ± 3% | ||
| 15 | 0.0139 | 0.0024 | 20.7 ± 0.13 °C, 35 ± 3% | ||
| Specialty Coating Systems | - | 0.0830 | - | 37 °C, 90% ASTM F1249 | |
| Para Tech | - | 0.0550 | - | 37 °C, 90% ASTM F1249 | |
| Menon et al., 2009 | Un-annealed | 9 | 0.0547 | - | 20 °C, 30% ASTM D1653 |
| Annealed | 9 | 0.0276 | - | ||
| Sterilization Method | Effect on Bulk Parylene | Effect on Parylene-Parylene Adhesion | Effect on Parylene to Metal Adhesion | Effect on Parylene Adhesion to Other Materials | Reference |
|---|---|---|---|---|---|
| Electron beam ISO 11137 | Chemical structure changed: partial breakage of C-Cl bonds, ionization of polymer, crystallinity decrease | No adverse effects recorded | No adverse effects recorded | n/a | [92,93] |
| Gamma radiation ISO 11137 | Recombination, cross-linking (increases strength and decreases elongation), loss in bond strength | n/a | Decreased adhesion, causing loss of electrical insulation capabilities | To silicon wafer: Crystallinity increased, no change in Young’s modulus | [91,94] |
| Ethylene oxide ISO 11135 | Formation of inorganic chlorides, reduction of chlorine amount. EtO is toxic, carcinogenic, flammable, explosive | For sterilization after thermal annealing, no adverse effects recorded | Decreased electrical insulation capabilities (but not as damaging as gamma sterilization | To glass: No adverse effects recorded | [93,95,96] |
| Autoclave (steam) ISO 17665 | Parylene became brittle and hard, decreased adhesion, changed chemical stability, did not contaminate | Decreased adhesion | Decreased adhesion | To silicon: Significant decrease in adhesion, Parylene crystallinity increased, electrical stability decreased | [57,91,97,98,99] |
| H2O2 Plasma ISO/NP 22441 * | No change in adhesion. Recommended “best suitability for Parylene” (based on XRD testing), successfully killed bacteria without degradation of Parylene coating | n/a | n/a | To Silastic: Parylene withstood implantation and was unaffected. However, coating was easily peeled off after implantation, suggesting poor Parylene-Silastic adhesion | [93,100,101] |
| Antibiotic coating No standard | 0.5–0.75 mg/mL concentration of tetracycline nanoparticles completely eradicated E. coli but not aureus bacteria | n/a | n/a | n/a | [102] |
| AZ P4620 | AZ 5214E-IR | |
|---|---|---|
| Surfaces | Silicon, Parylene-coated silicon, glass (100 mm wafers) | |
| Pre-spin | 500 rpm for 5 s | 500 rpm for 8 s |
| Spin acceleration | ~1000 rpm/sec | |
| Main spin | 1000, 2000, 3000, 4000, 5000 rpm for 45 s | |
| Bake | 5 min at 90 °C | 70 s at 90 °C |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortigoza-Diaz, J.; Scholten, K.; Larson, C.; Cobo, A.; Hudson, T.; Yoo, J.; Baldwin, A.; Weltman Hirschberg, A.; Meng, E. Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems. Micromachines 2018, 9, 422. https://doi.org/10.3390/mi9090422
Ortigoza-Diaz J, Scholten K, Larson C, Cobo A, Hudson T, Yoo J, Baldwin A, Weltman Hirschberg A, Meng E. Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems. Micromachines. 2018; 9(9):422. https://doi.org/10.3390/mi9090422
Chicago/Turabian StyleOrtigoza-Diaz, Jessica, Kee Scholten, Christopher Larson, Angelica Cobo, Trevor Hudson, James Yoo, Alex Baldwin, Ahuva Weltman Hirschberg, and Ellis Meng. 2018. "Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems" Micromachines 9, no. 9: 422. https://doi.org/10.3390/mi9090422
APA StyleOrtigoza-Diaz, J., Scholten, K., Larson, C., Cobo, A., Hudson, T., Yoo, J., Baldwin, A., Weltman Hirschberg, A., & Meng, E. (2018). Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems. Micromachines, 9(9), 422. https://doi.org/10.3390/mi9090422





