Rapid Construction of Liquid-like Surfaces via Single-Cycle Polymer Brush Grafting for Enhanced Antifouling in Microfluidic Systems
Abstract
:1. Introduction
2. Materials and Methods
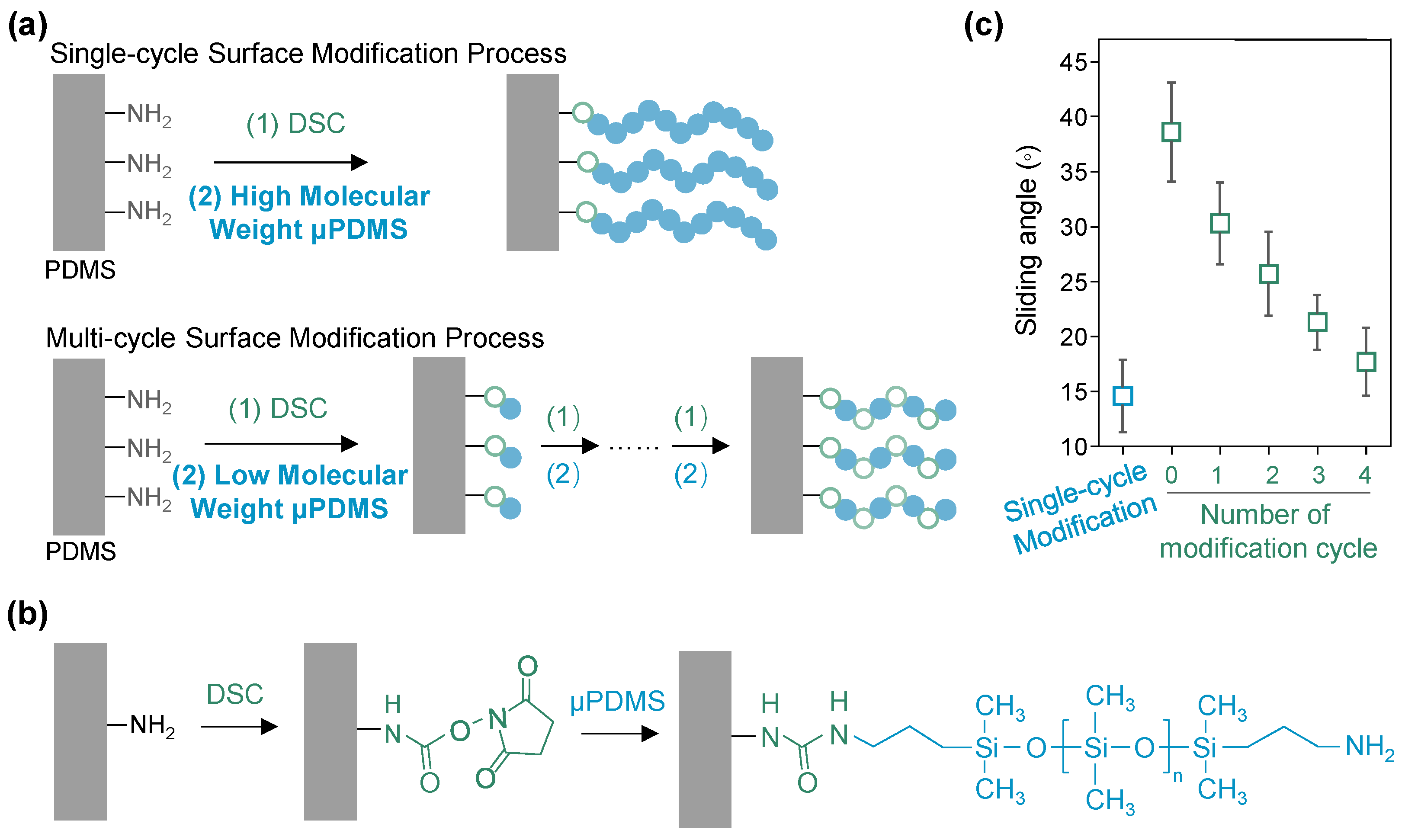
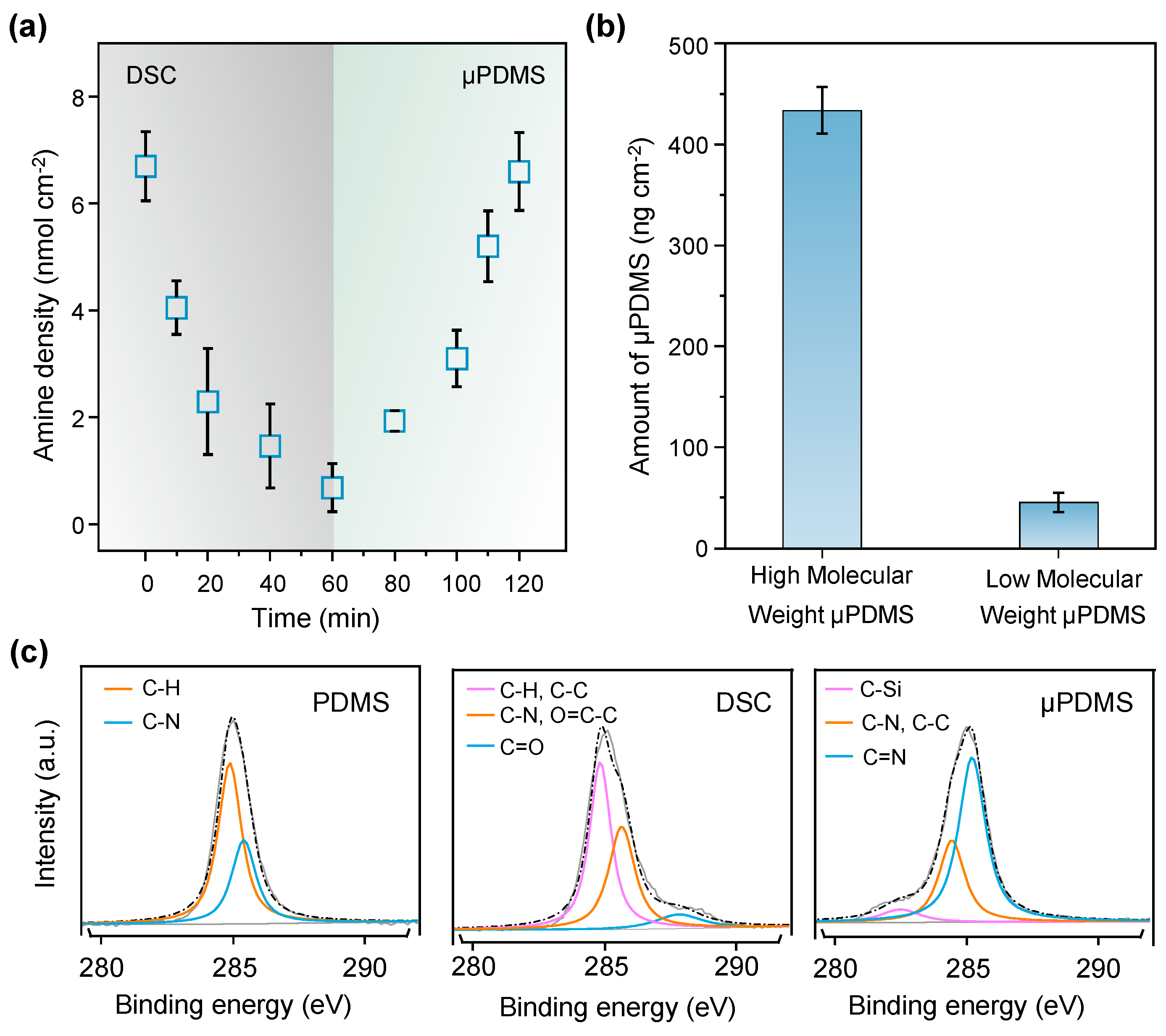
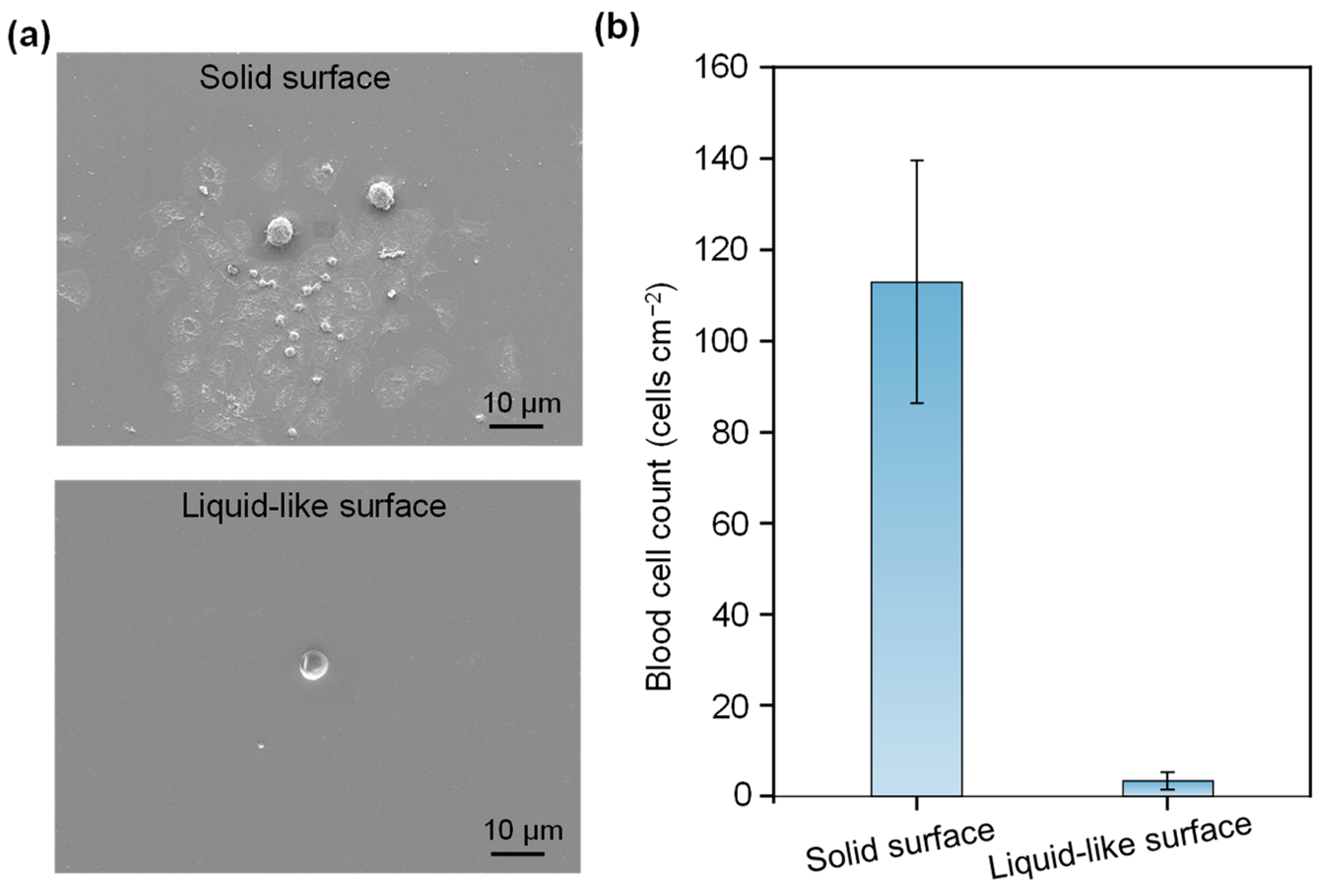
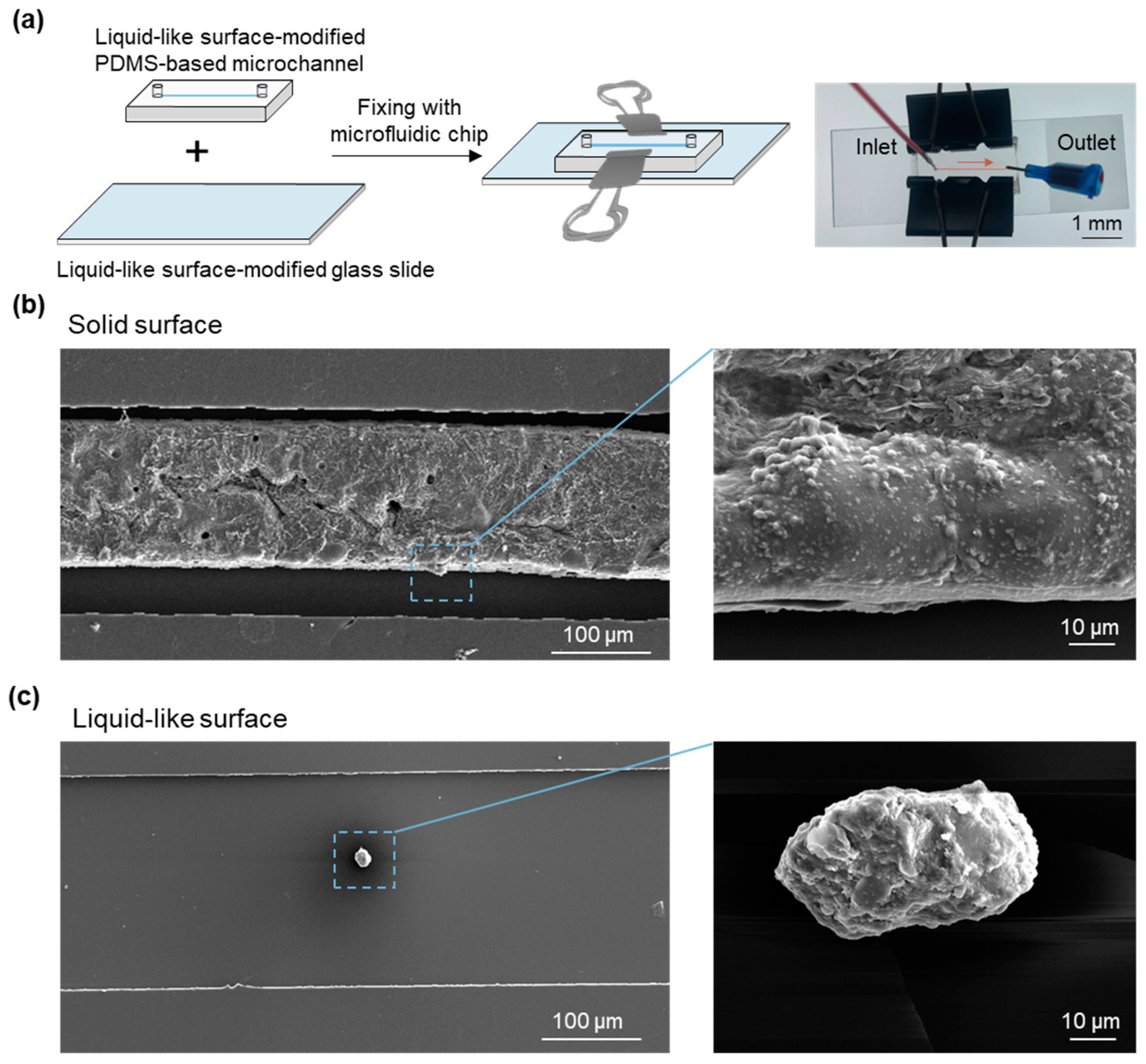
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Chen, X.; Wang, S.; Zhou, R.; Wang, C.; Yu, L.; Zheng, J.; Yang, C.; Hou, X. Green synthesized liquid-like dynamic polymer chains with decreased nonspecific adhesivity for high-purity capture of circulating tumor cells. CCS Chem. 2024, 6, 507–517. [Google Scholar] [CrossRef]
- Li, K.; Lu, X.; Liao, J.; Chen, H.; Lin, W.; Zhao, Y.; Tang, D.; Li, C.; Tian, Z.; Zhu, Z. DNA-DISK: Automated end-to-end data storage via enzymatic single-nucleotide DNA synthesis and sequencing on digital microfluidics. Proc. Natl. Acad. Sci. USA 2024, 121, e2410164121. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, H.; Zhang, D.; Zhao, K.; Gao, J.; Lin, B.; Huang, C.; Song, Y.; Zhao, G.; Ma, Y. Reversible immunoaffinity interface enables dynamic manipulation of trapping force for accumulated capture and efficient release of circulating rare cells. Adv. Sci. 2021, 8, 2102070. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Kong, X.; Liu, Y.; Wang, S.; Chen, Z.; Hou, X. Microfluidic-based isolation of circulating tumor cells with high-efficiency and high-purity. Chin. Chem. Lett. 2024, 35, 109754. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Yang, Y.; Zhang, K.; Liang, W.; Xu, Z.; Wu, Y.; Luo, J.; Zhuang, C.; Cai, X. Recent progress and perspectives on neural chip platforms integrating PDMS-based microfluidic devices and microelectrode arrays. Micromachines 2023, 14, 709. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Z.; Zhang, Z.; Weitz, D.; Zhang, H.; Zhang, Y.; Cui, W. Advanced microfluidic devices for fabricating multi-structural hydrogel microsphere. Exploration 2021, 1, 20210036. [Google Scholar] [CrossRef]
- Wang, K.; Huang, K.; Wang, L.; Lin, X.; Tan, M.; Su, W. Microfluidic strategies for encapsulation, protection, and controlled delivery of probiotics. J. Agric. Food Chem. 2024, 72, 15092–15105. [Google Scholar] [CrossRef]
- Lovegrove, J.T.; Kent, B.; Förster, S.; Garvey, C.J.; Stenzel, M.H. The flow of anisotropic nanoparticles in solution and in blood. Exploration 2023, 3, 20220075. [Google Scholar] [CrossRef]
- Saxena, S.; Lu, Y.; Zhang, Z.; Li, Y.; Soleymani, L.; Hoare, T. Zwitter-repel: An anti-fouling coating promoting electrochemical biosensing in biological fluids. Chem. Eng. J. 2024, 495, 153522. [Google Scholar] [CrossRef]
- Hou, X.; Li, J.; Tesler, A.B.; Yao, Y.; Wang, M.; Min, L.; Sheng, Z.; Aizenberg, J. Dynamic air/liquid pockets for guiding microscale flow. Nat. Commun. 2018, 9, 733. [Google Scholar] [CrossRef]
- Cirillo, A.I.; Tomaiuolo, G.; Guido, S. Membrane fouling phenomena in microfluidic systems: From technical challenges to scientific opportunities. Micromachines 2021, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Yan, H.; Tian, S.; Luo, R.; Zhao, Y.; Wang, J. Anti-fouling coatings for blood-contacting devices. Smart Mater. Med. 2024, 5, 166–180. [Google Scholar] [CrossRef]
- Mercader, A.; Ye, S.-H.; Kim, S.; Orizondo, R.A.; Cho, S.K.; Wagner, W.R. PDMS-zwitterionic hybrid for facile, antifouling microfluidic device fabrication. Langmuir 2022, 38, 3775–3784. [Google Scholar] [CrossRef]
- Leslie, D.C.; Waterhouse, A.; Berthet, J.B.; Valentin, T.M.; Watters, A.L.; Jain, A.; Kim, P.; Hatton, B.D.; Nedder, A.; Donovan, K. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 2014, 32, 1134–1140. [Google Scholar] [CrossRef]
- Tong, P.; Chen, L.; Sun, X.; Li, H.; Feng, Y.; Li, J.; Guan, S. Surface modification of biodegradable magnesium alloy with poly (L-lactic acid) and sulfonated hyaluronic acid nanoparticles for cardiovascular application. Int. J. Biol. Macromol. 2023, 237, 124191. [Google Scholar] [CrossRef] [PubMed]
- Sahin, F.; Celik, N.; Ceylan, A.; Pekdemir, S.; Ruzi, M.; Onses, M.S. Antifouling superhydrophobic surfaces with bactericidal and SERS activity. Chem. Eng. J. 2022, 431, 133445. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Y.; Li, J.; Zhang, K.; Chong, F. Bioinspired strategies for functionalization of mg-based stents. Crystals 2022, 12, 1761. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Y.; Xu, J.; Pan, C. Bioinspired surface design for magnesium alloys with corrosion resistance. Metals 2022, 12, 1404. [Google Scholar] [CrossRef]
- Rasitha, T.; Vanithakumari, S.; Krishna, D.N.G.; George, R.; Srinivasan, R.; Philip, J. Facile fabrication of robust superhydrophobic aluminum surfaces with enhanced corrosion protection and antifouling properties. Prog. Org. Coat. 2022, 162, 106560. [Google Scholar] [CrossRef]
- Shi, R.; Shang, L.; Zhou, C.; Zhao, Y.; Zhang, T. Interfacial wettability and mass transfer characterizations for gas–liquid–solid triple-phase catalysis. Exploration 2022, 2, 20210046. [Google Scholar] [CrossRef]
- Wu, F.; Xu, J.; Wang, Z.; Jiang, J.; Liu, Y.; Zhang, N.; Zhang, K.; Chong, F.; Li, J. Functional liquid-infused PDMS sponge-based catheter with antithrombosis, antibacteria, and anti-inflammatory properties. Colloids Surf. B Biointerfaces 2023, 224, 113208. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Tian, X.; Wang, Z.; Zhang, L.; Zhang, F.; Yang, Y.; Liu, L. Versatile dynamic bioactive lubricant-infused surface for effective isolation of circulating tumor cells. Anal. Chem. 2023, 95, 5307–5315. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wen, G.; Guo, Z. What are the design principles, from the choice of lubricants and structures to the preparation method, for a stable slippery lubricant-infused porous surface? Mater. Horiz. 2020, 7, 1697–1726. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, R.; Wang, S.; Meng, J. Liquid-like surfaces with enhanced de-wettability and durability: From structural designs to potential applications. Adv. Mater. 2024, 36, 2407315. [Google Scholar] [CrossRef]
- Yang, C.; Wu, Q.; Zhong, L.; Lyu, C.; He, G.; Yang, C.; Li, X.; Huang, X.; Hu, N.; Chen, M. Liquid-like polymer-based self-cleaning coating for effective prevention of liquid foods contaminations. J. Colloid Interface Sci. 2021, 589, 327–335. [Google Scholar] [CrossRef]
- Huang, S.; Li, J.; Liu, L.; Zhou, L.; Tian, X. Lossless fast drop self-transport on anisotropic omniphobic surfaces: Origin and elimination of microscopic liquid residue. Adv. Mater. 2019, 31, 1901417. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, X.; Chen, L.; Liu, S.; Xu, X.; Zhao, S.; Huang, S.; Tian, X. Dynamic poly(dimethylsiloxane) brush coating shows even better antiscaling capability than the low-surface-energy fluorocarbon counterpart. Environ. Sci. Technol. 2021, 55, 8839–8847. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Z.; Sarma, J.; Dai, X. Passive removal of highly wetting liquids and ice on quasi-liquid surfaces. ACS Appl. Mater. Interfaces 2020, 12, 20084–20095. [Google Scholar] [CrossRef]
- Kou, F.; Liu, C.; Wang, L.; Yasin, A.; Li, J.; Guan, S. Fabrication of citric acid/RGD multilayers on Mg-Zn-Y-Nd alloy via layer-by-layer self-assembly for promoting surface biocompatibility. Adv. Mater. Interfaces 2021, 8, 2002241. [Google Scholar] [CrossRef]
- Hu, Q.; Du, Y.; Bai, Y.; Xing, D.; Wu, C.; Li, K.; Lang, S.; Liu, X.; Liu, G. Smart zwitterionic coatings with precise pH-responsive antibacterial functions for bone implants to combat bacterial infections. Biomater. Sci. 2024, 12, 4471–4482. [Google Scholar] [CrossRef]
- Torma, V.; Gyenes, T.; Szakács, Z.; Noszál, B.; Némethy, Á.; Zrínyi, M. Novel amino acid-based polymers for pharmaceutical applications. Polym. Bull. 2007, 59, 311–318. [Google Scholar] [CrossRef]
- Qin, X.-H.; Senturk, B.; Valentin, J.; Malheiro, V.; Fortunato, G.; Ren, Q.; Rottmar, M.; Maniura-Weber, K. Cell-membrane-inspired silicone interfaces that mitigate proinflammatory macrophage activation and bacterial adhesion. Langmuir 2018, 35, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Tan, P.; Nie, G.; Zhu, M. Biomimetic and bioinspired nano-platforms for cancer vaccine development. Exploration 2023, 3, 20210263. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castro, K.; Acuña-Umaña, K.; Lesser-Rojas, L.; Reyes, D.R. Microfluidic Blood Separation: Key Technologies and Critical Figures of Merit. Micromachines 2023, 14, 2117. [Google Scholar] [CrossRef]
- Corral-Nájera, K.; Chauhan, G.; Serna-Saldívar, S.O.; Martínez-Chapa, S.O.; Aeinehvand, M.M. Polymeric and biological membranes for organ-on-a-chip devices. Microsyst. Nanoeng. 2023, 9, 107. [Google Scholar] [CrossRef]
- Shome, A.; Martinez, I.; Pinon, V.D.; Moses, J.C.; Garren, M.; Sapkota, A.; Crutchfield, N.; Francis, D.J.; Brisbois, E.J.; Handa, H. “Reactive” chemical strategy to attain substrate independent “liquid-like” omniphobic solid anti-biofouling coatings. Adv. Funct. Mater. 2024, 34, 2401387. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Xu, J.; Liu, Y.; Sun, H.; Zhang, L.; Liu, Y.; Wang, W.; Chong, F.; Zou, D.; Wang, S. Rapid Construction of Liquid-like Surfaces via Single-Cycle Polymer Brush Grafting for Enhanced Antifouling in Microfluidic Systems. Micromachines 2024, 15, 1241. https://doi.org/10.3390/mi15101241
Wu F, Xu J, Liu Y, Sun H, Zhang L, Liu Y, Wang W, Chong F, Zou D, Wang S. Rapid Construction of Liquid-like Surfaces via Single-Cycle Polymer Brush Grafting for Enhanced Antifouling in Microfluidic Systems. Micromachines. 2024; 15(10):1241. https://doi.org/10.3390/mi15101241
Chicago/Turabian StyleWu, Feng, Jing Xu, Yuanyuan Liu, Hua Sun, Lishang Zhang, Yixuan Liu, Weiwei Wang, Fali Chong, Dan Zou, and Shuli Wang. 2024. "Rapid Construction of Liquid-like Surfaces via Single-Cycle Polymer Brush Grafting for Enhanced Antifouling in Microfluidic Systems" Micromachines 15, no. 10: 1241. https://doi.org/10.3390/mi15101241
APA StyleWu, F., Xu, J., Liu, Y., Sun, H., Zhang, L., Liu, Y., Wang, W., Chong, F., Zou, D., & Wang, S. (2024). Rapid Construction of Liquid-like Surfaces via Single-Cycle Polymer Brush Grafting for Enhanced Antifouling in Microfluidic Systems. Micromachines, 15(10), 1241. https://doi.org/10.3390/mi15101241







