Cementitious Behavior of Argon Oxygen Decarburization Stainless Steel Slag and Its Stabilization on Chromium
Abstract
:1. Introduction
2. Experiment
2.1. Raw Materials
2.2. Paste Samples
2.3. Mortar Samples
2.4. Sequential Leaching
2.4.1. Leaching Subjects
2.4.2. Leaching Procedure
3. Results and Discussion
3.1. Properties of Raw Materials
3.2. Cementitious Properties of AODS
3.2.1. Water Requirement, Soundness, and Setting Time
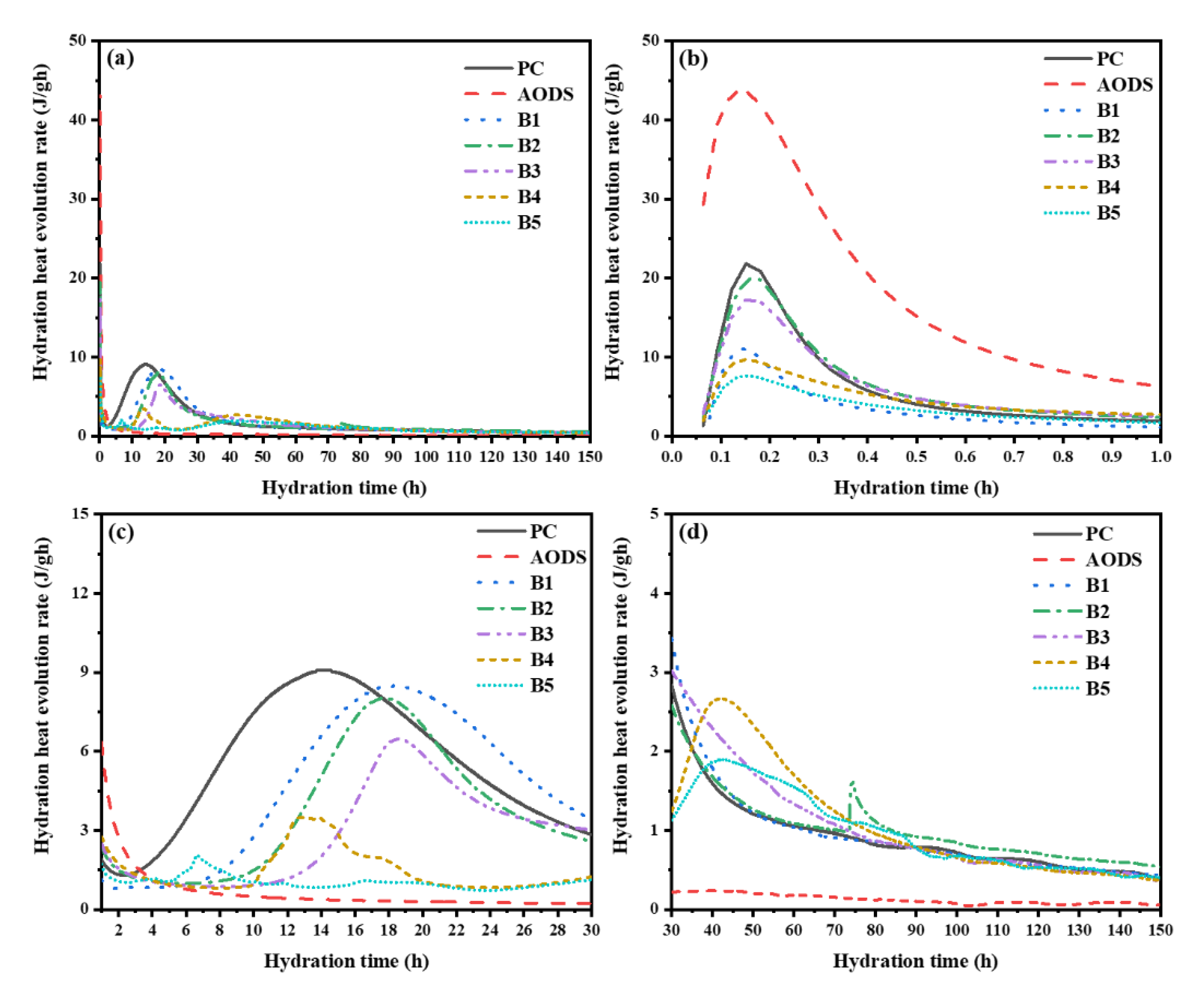
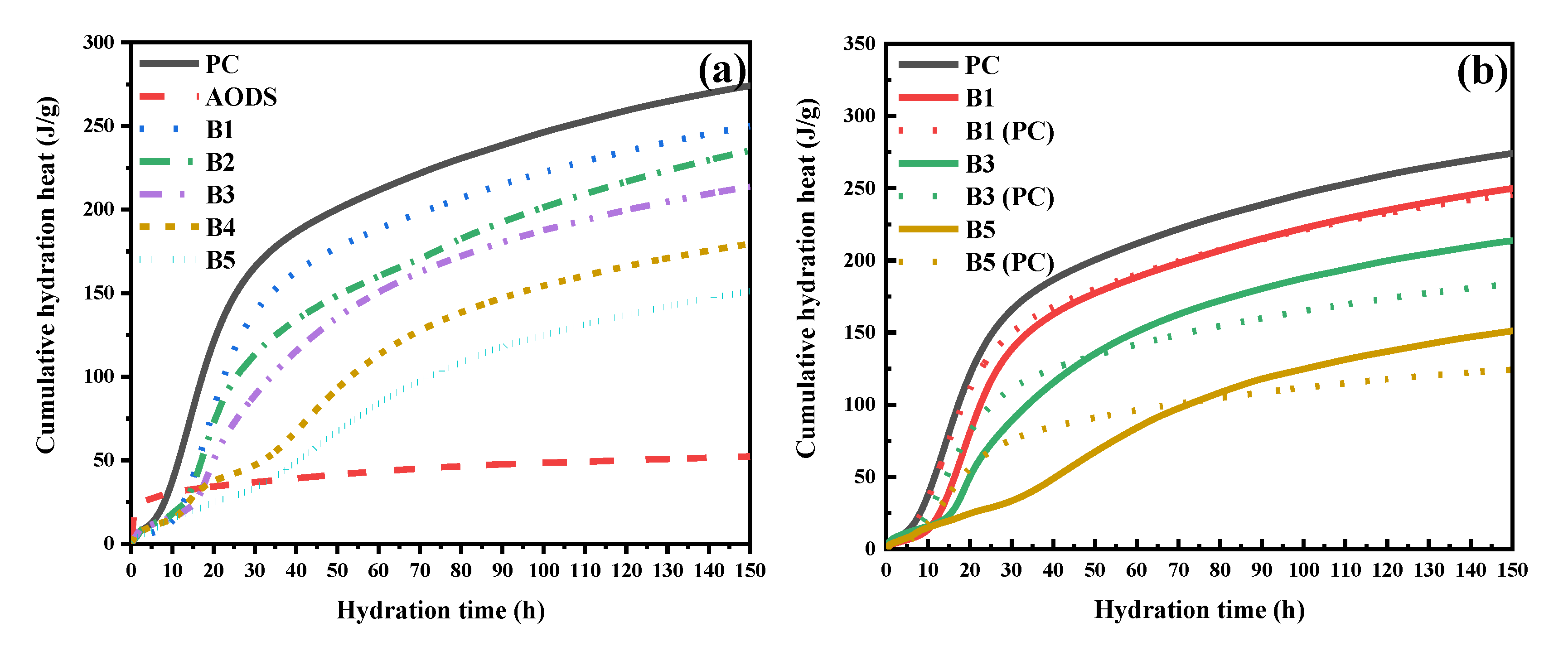
3.2.2. Hydration Exotherm
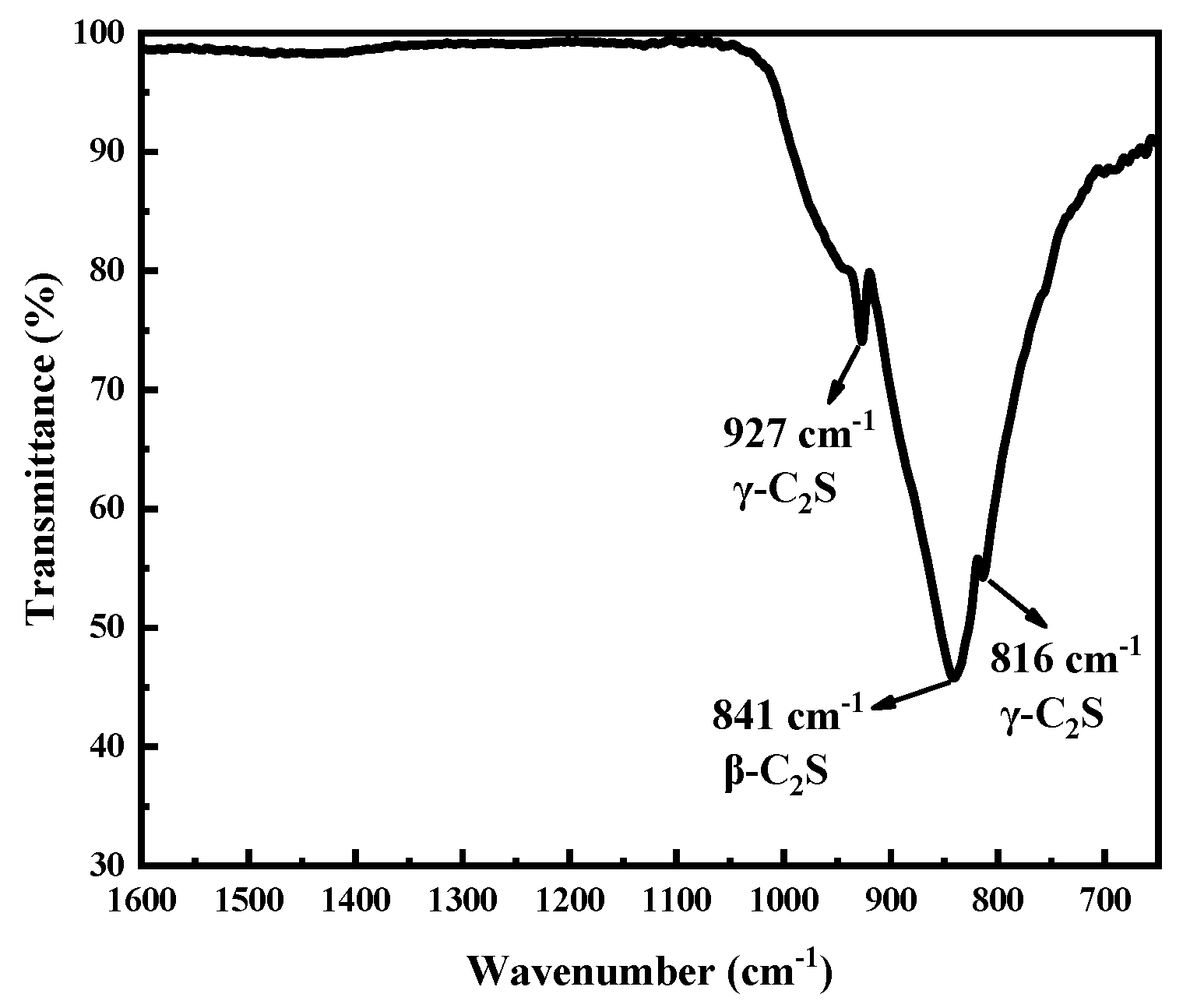
3.2.3. Hydration Products
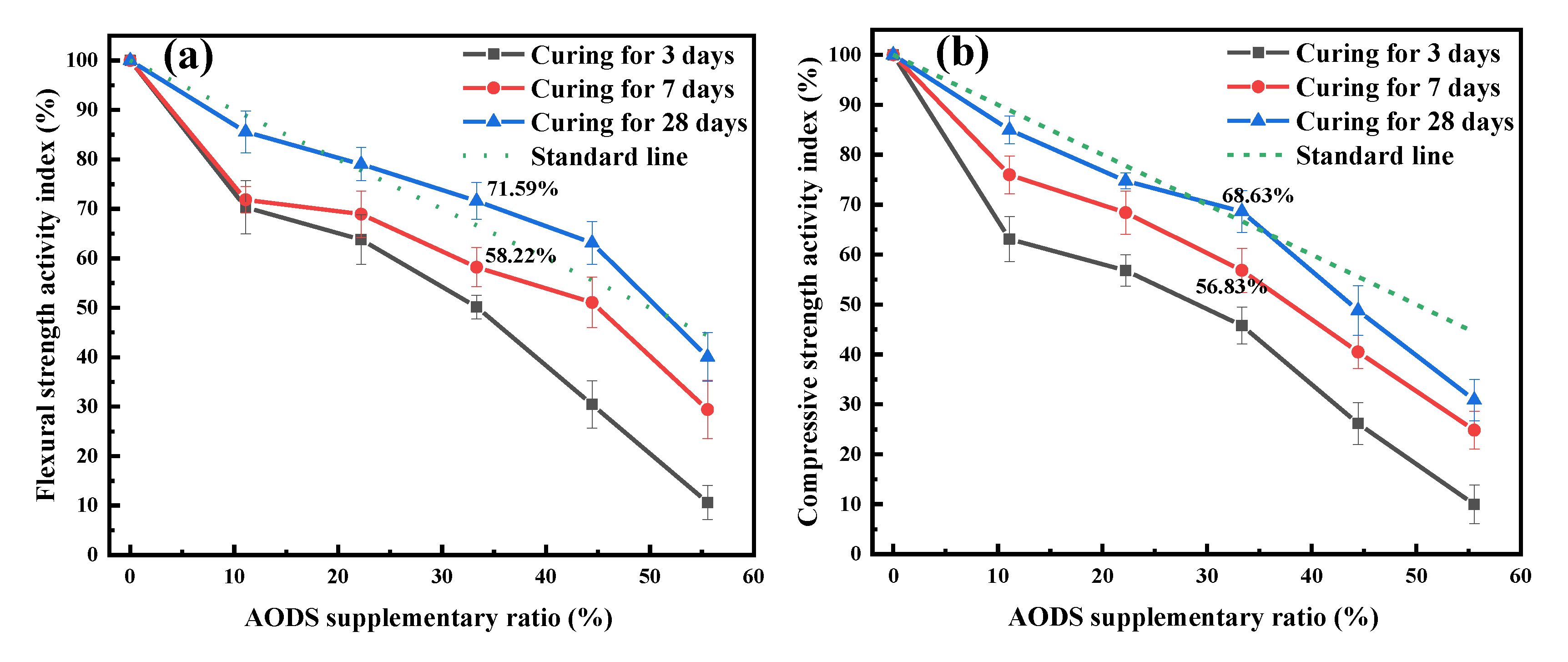
3.2.4. Mechanical Strength of Mortars
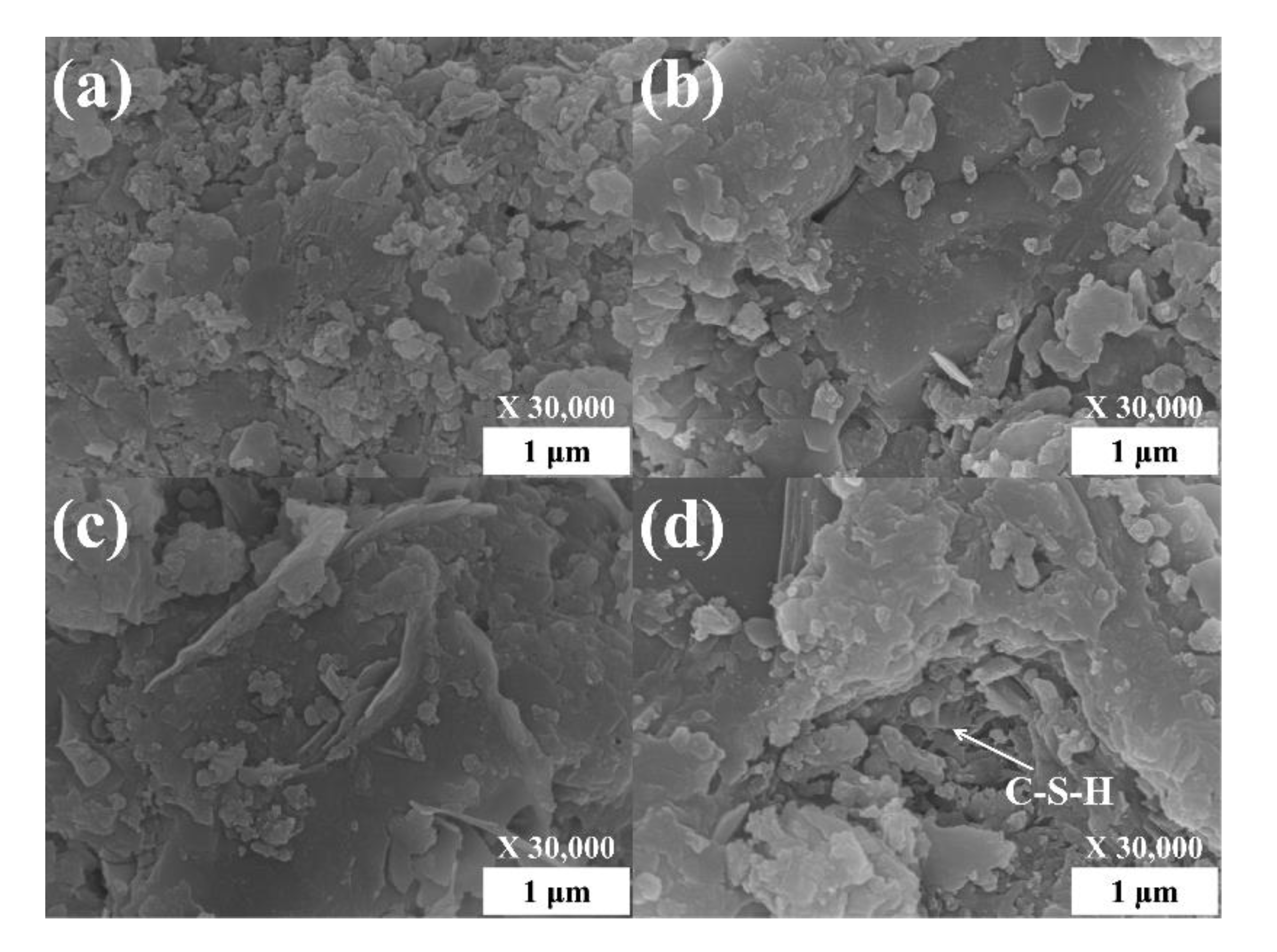
3.2.5. Morphology and Microstructure of Cured Pastes
3.3. The Chromium Leachability of Composite Pastes
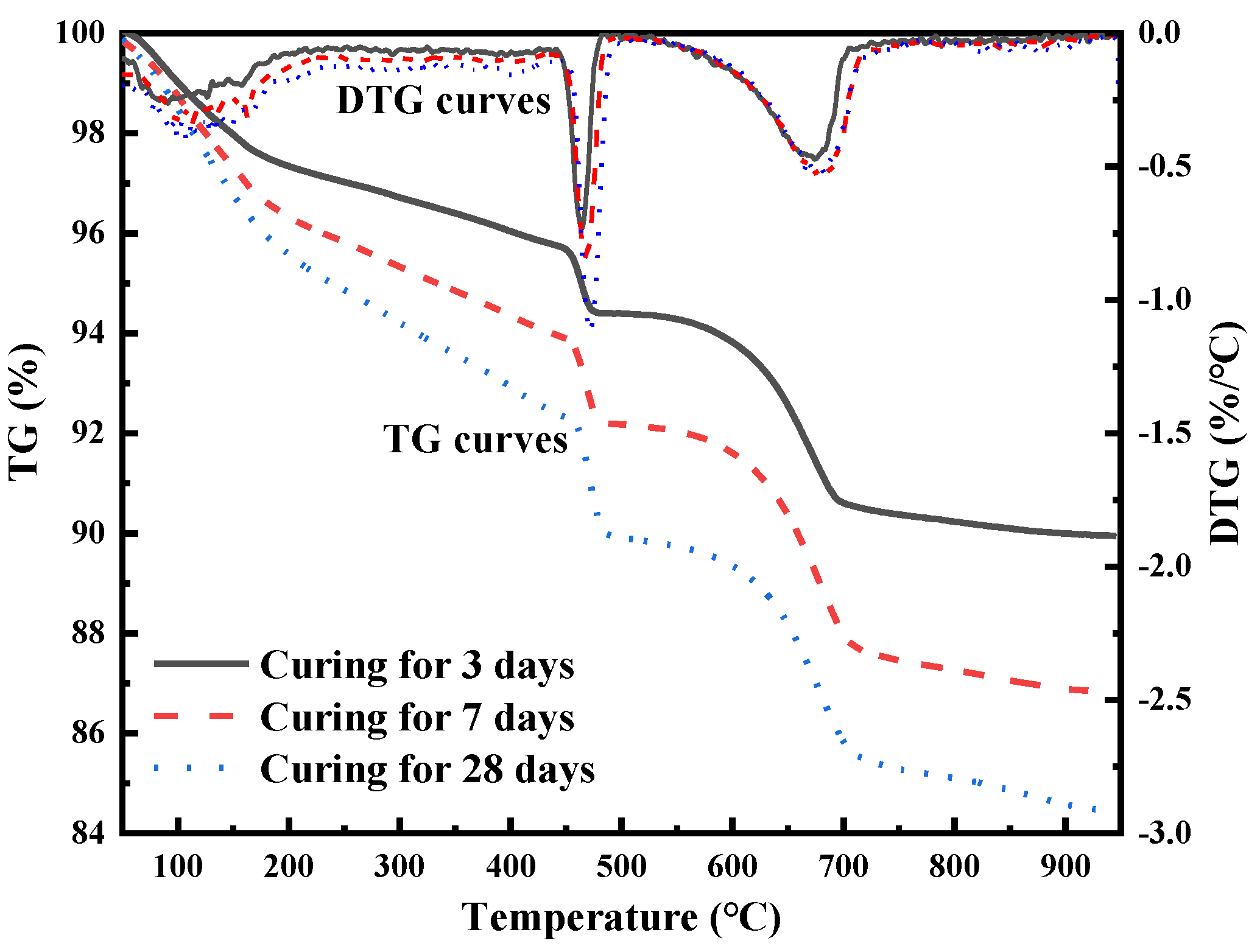
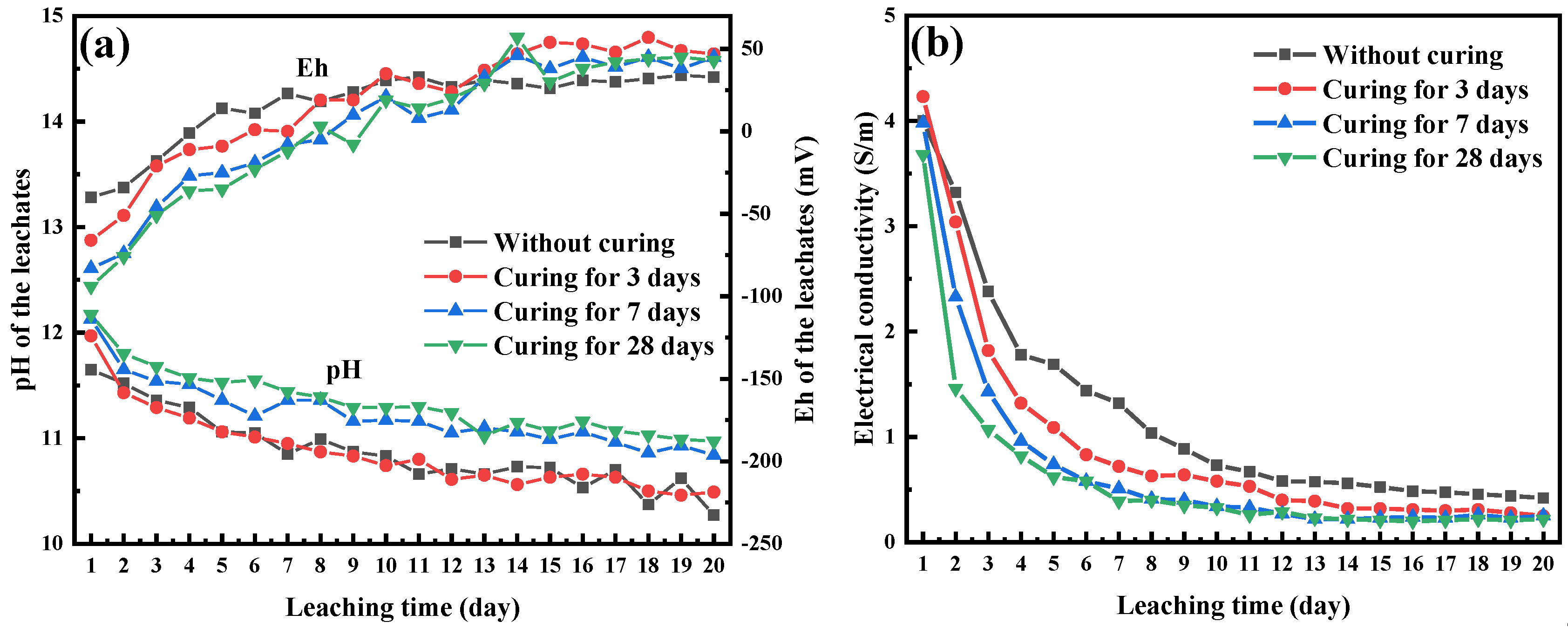
3.3.1. Effects of Curing Time on Chromium Leachability
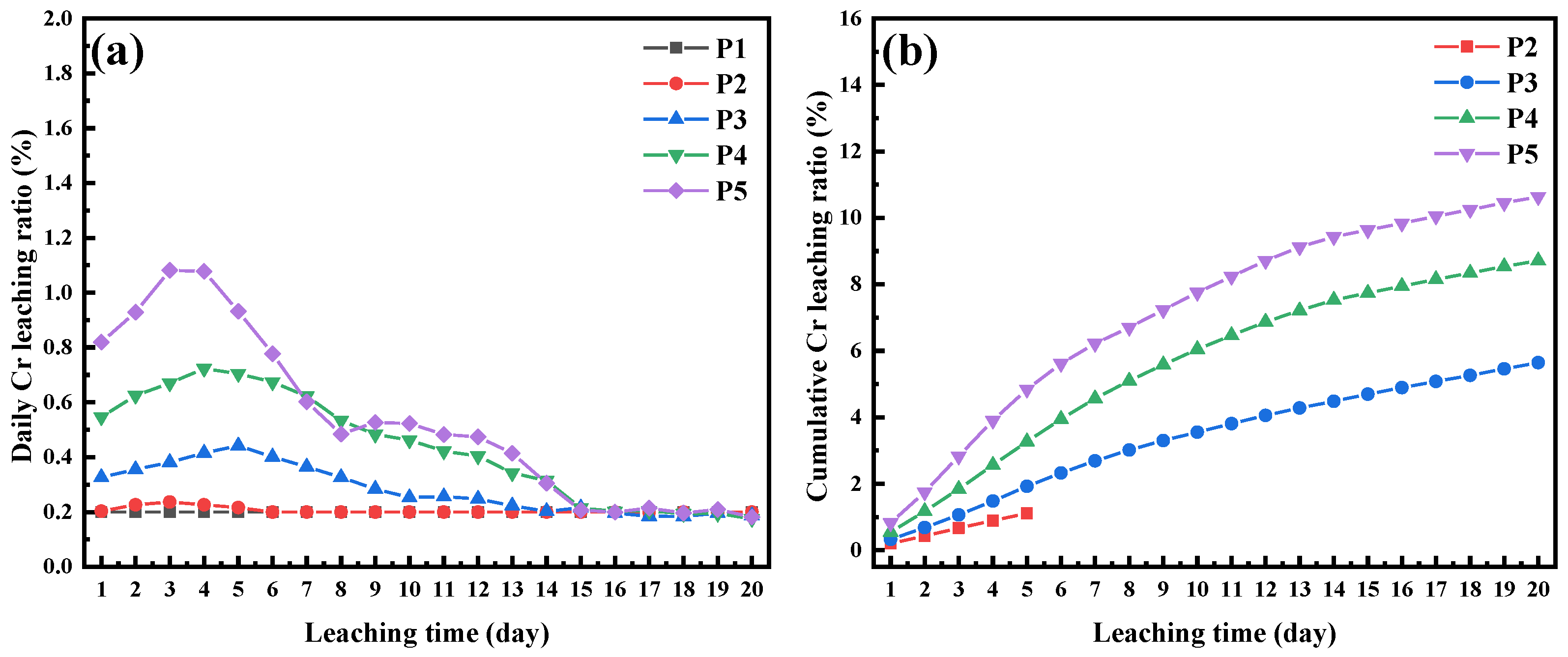
3.3.2. Effects of AODS’s Dosage on Chromium Leachability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iacobescu, R.I.; Angelopoulos, G.N.; Jones, P.T.; Blanpain, B.; Pontikes, Y. Ladle metallurgy stainless steel slag as a raw material in Ordinary Portland Cement production: A possibility for industrial symbiosis. J. Clean. Prod. 2016, 112, 872–881. [Google Scholar] [CrossRef]
- Adegoloye, G.; Beaucour, A.L.; Ortola, S.; Noumowé, A. Concretes made of EAF slag and AOD slag aggregates from stainless steel process: Mechanical properties and durability. Construct. Build. Mater. 2015, 76, 313–321. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.L.; Qu, Z.M. Physicochemical property and chromium leaching behavior in different environments of glass ceramics prepared from AOD stainless steel slag. J. Alloy. Compd. 2019, 805, 1106–1116. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Hong, S.-H. Influence of minor ions on the stability and hydration rates of β-dicalcium silicate. J. Am. Ceram. 2004, 87, 900–905. [Google Scholar] [CrossRef]
- Kriskova, L.; Eroli, M.; Iacobescu, R.I.; Onisei, S.; Vecchiocattivi, F.; Pontikes, Y. Transformation of stainless steel slag toward a reactive cementitious binder. J. Am. Ceram. Soc. 2017, 101, 1727–1736. [Google Scholar] [CrossRef]
- Li, J.G.; Liu, B.; Zeng, Y.N.; Wang, Z.M.; Gao, Z.Y. Maximum availability and mineralogical control of chromium released from AOD slag. Environ. Monit. Assess. 2017, 189, 113. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.G.; Zeng, Y.N.; Wang, Z.M. Toxicity assessment and geochemical model of chromium leaching from AOD slag. Chemosphere 2016, 114, 2052–2057. [Google Scholar] [CrossRef]
- DesMarais, T.L.; Costa, M. Mechanisms of chromium-induced toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Simonsen, A.M.T.; Solismaa, S.; Hansen, H.K.; Jensen, P.E. Evaluation of mine tailings’ potential as supplementary cementitious materials based on chemical, mineralogical and physical characteristics. Waste Manag. 2020, 102, 710–721. [Google Scholar] [CrossRef]
- Herrero, T.; Vegas, I.J.; Santamaría, A.; San-José, J.T.; Skaf, M. Effect of high-alumina ladle furnace slag as cement substitution in masonry mortars. Constr. Build. Mater. 2016, 123, 404–413. [Google Scholar] [CrossRef]
- Mladenovič, A.; Mirtič, B.; Meden, A.; Zalar Serjun, V. Calcium aluminate rich secondary stainless steel slag as a supplementary cementitious material. Constr. Build. Mater. 2016, 116, 216–225. [Google Scholar] [CrossRef]
- Shi, C. Steel slag–its production, processing, characteristics, and cementitious properties. J. Mater. Civ. Eng. 2004, 16, 230–236. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zeng, Y.-N.; Li, J.-G.; Zhang, Y.-Z.; Zhang, Y.-J.; Zhao, Q.-Z. Carbonation of argon oxygen decarburization stainless steel slag and its effect on chromium leachability. J. Clean. Prod. 2020, 256, 120377. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, M.; Yang, J. Influence of classified steel slag with particle sizes smaller than 20 μm on the properties of cement and concrete. Constr. Build. Mater. 2016, 123, 601–610. [Google Scholar]
- Park, B.; Moon, E.-J.; Choi, Y.C. Investigation of microstructure and mechanical performance of carbon-capture binder using AOD stainless steel slag. Constr. Build. Mater. 2020, 242, 118174. [Google Scholar] [CrossRef]
- Belhadj, E.; Diliberto, C.; Lecomte, A. Properties of hydraulic paste of basic oxygen furnace slag. Cement Concr. Comp. 2014, 45, 15–21. [Google Scholar] [CrossRef]
- Moon, E.-J.; Choi, Y.C. Carbon dioxide fixation via accelerated carbonation of cement-based materials: Potential for construction materials applications. Constr. Build. Mater. 2019, 199, 676–687. [Google Scholar] [CrossRef]
- Saito, T.; Sakai, E.; Morioka, M.; Otsuki, N. Carbonation of γ-Ca2SiO4 and the Mechanism of Vaterite Formation. J. Adv. Concr. Technol. 2010, 8, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Mabudo, G.M.; Lee, S.; Kang, S.; Song, M. Physical properties and carbon dioxide capture of synthetic gamma-C2S cement composites in the early days of curing. Mag. Concr. Res. 2016, 68, 1079–1084. [Google Scholar] [CrossRef]
- Gupta, T.; Sachdeva, S.N. Laboratory investigation and modeling of concrete pavements containing AOD steel slag. Cement Concr. Res. 2019, 124, 105808. [Google Scholar] [CrossRef]
- Carvalho, S.Z.; Vernilli, F.; Almeida, B.; Demarco, M.; Silva, S.N. The recycling effect of BOF slag in the portland cement properties. Resour. Conserv. Recy. 2017, 127, 216–220. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Pugazhmani, G.; Sripragadeesh, R.; Muthu, D.; Venkatasubramanian, C. Experimental study on the mechanical and durability properties of concrete with waste glass powder and ground granulated blast furnace slag as supplementary cementitious materials. Constr. Build. Mater. 2017, 156, 739–749. [Google Scholar] [CrossRef]
- Franco de Carvalho, J.M.; Fontes, W.C.; Felipe de Azevedo, C.; Brigolini, G.J.; Schmidt, W.; Fiorotti Peixoto, R.A. Enhancing the eco-efficiency of concrete using engineered recycled mineral admixtures and recycled aggregates. J. Clean. Prod. 2020, 257, 120530. [Google Scholar] [CrossRef]
- Isteri, V.; Ohenoja, K.; Hanein, T.; Kinoshita, H.; Tanskanen, P.; Illikainen, M.; Fabritius, T. Production and properties of ferrite-rich CSAB cement from metallurgical industry residues. Sci. Total Environ. 2020, 712, 136208. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Forssberg, E.; Nordström, U. Physicochemical and mineralogical properties of stainless steel slags oriented to metal recovery. Resour. Conserv. Recy. 2004, 40, 245–271. [Google Scholar] [CrossRef]
- Gupta, T.; Sachdeva, S.N. Experimental study and modeling of concrete containing AOD steel slag for pavements. Arab. J. Sci. Eng. 2020, 45, 8111–8127. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R. Influence of particle size on the carbonation of stainless steel slag for CO2 storage. Energy Procedia 2009, 1, 4859–4866. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Ling, T.-C.; Shi, C.; Pan, S.-Y. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recy. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, J.; Zhang, S. The mechanisms of heavy metal immobilization by cementitious material treatments and thermal treatments: A review. J. Environ. Manag. 2017, 193, 410–422. [Google Scholar] [CrossRef]
- Kogbara, R.B.; Al-Tabbaa, A. Mechanical and leaching behaviour of slag-cement and lime-activated slag stabilised/solidified contaminated soil. Sci. Total Environ. 2011, 409, 2325–2335. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Zhou, Y.; Rinklebe, J.; Song, H.; Kwon, E.E.; Baek, K.; Sik Ok, Y. Mechanistic insights into red mud, blast furnace slag, or metakaolin-assisted stabilization/solidification of arsenic-contaminated sediment. Environ. Int. 2020, 136, 106194. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of heavy metal in cement-based solidification/stabilisation: A review. Waste Manag. 2008, 29, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cho, D.-W.; Tsang, D.C.W.; Cao, X.; Hou, D.; Shen, Z.; Alessi, D.S.; Sik Ok, Y.; Poon, C.S. Green remediation of As and Pb contaminated soil using cement-free clay-based stabilization/solidification. Environ. Int. 2019, 126, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Kiventerä, J.; Piekkari, K.; Isteri, V.; Ohenoja, K.; Tanskanen, P.; Illikainen, M. Solidification/stabilization of gold mine tailings using calcium sulfoaluminate-belite cement. J. Clean. Prod. 2019, 239, 118008. [Google Scholar] [CrossRef]
- Su, P.; Zhang, J.; Li, Y. Solidification/stabilization of stainless steel pickling residue with aluminum potassium sulfate amended fly ash. J. Clean. Prod. 2019, 234, 400–409. [Google Scholar] [CrossRef]
- Wang, L.; Geddes, D.A.; Walkley, B.; Provis, J.L.; Mechtcherine, V.; Tsang, D.C.W. The role of zinc in metakaolin-based geopolymers. Cement Concr. Res. 2020, 136, 106194. [Google Scholar] [CrossRef]
- Meena, A.H.; Kaplan, D.I.; Powell, B.A.; Arai, Y. Chemical stabilization of chromate in blast furnace slag mixed cementitious materials. Chemosphere 2015, 138, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Panda, C.R.; Mishra, K.K.; Panda, K.C.; Nayak, B.D.; Nayak, B.B. Environmental and technical assessment of ferrochrome slag as concrete aggregate material. Constr. Build. Mater. 2013, 49, 262–271. [Google Scholar] [CrossRef]
- Serjun, V.Z.; Mladenovič, A.; Mirtič, B.; Meden, A.; Ščančar, J.; Milačič, R. Recycling of ladle slag in cement composites: Environmental impacts. Waste Manag. 2015, 43, 376–385. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, C.; Cao, L.; Zheng, X.; Jiang, M. Stability of chromium in stainless steel slag during cooling. Minerals 2018, 8, 445. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, D.; Zhuang, S. The soundness of steel slag with different free CaO and MgO contents. Constr. Build. Mater. 2017, 151, 138–146. [Google Scholar] [CrossRef]
- Kriskova, L.; Pontikes, Y.; Cizer, Ö.; Mertens, G.; Veulemans, W.; Geysen, D.; Jones, P.T.; Vandewalle, L.; Balen, K.V.; Blanpain, B. Effect of mechanical activation on the hydraulic properties of stainless steel slags. Cement Concr. Res. 2012, 42, 778–788. [Google Scholar] [CrossRef]
- Kim, E.; Spooren, J.; Broos, K.; Nielsen, P.; Horckmans, L.; Geurts, R.; Vrancken, K.C.; Quaghebeur, M. Valorization of stainless steel slag by selective chromium recovery and subsequent carbonation of the matrix material. J. Clean. Prod. 2016, 117, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef]
- Liu, S.; Li, L. Influence of fineness on the cementitious properties of steel slag. J. Therm. Anal. Calorim. 2014, 117, 629–634. [Google Scholar] [CrossRef]
- Sheen, Y.-N.; Wang, H.-Y.; Sun, T.-H. A study of engineering properties of cement mortar with stainless steel oxidizing slag and reducing slag resource materials. Constr. Build. Mater. 2013, 40, 239–245. [Google Scholar] [CrossRef]
- EI-Didamony, H.; Sharara, A.M.; Helmy, I.M.; El-Aleem, S.A. Hydration characteristics of β-C2S in the presence of some accelerators. Cement Concr. Res. 1996, 26, 1179–1187. [Google Scholar] [CrossRef]
- Zheng, L.; Xuehua, C.; Mingshu, T. Hydration and setting time of MgO-type expansive cement. Cement Concr. Res. 1992, 22, 1–5. [Google Scholar] [CrossRef]
- Péra, J.; Ambroise, J.; Chabannet, M. Properties of blast-furnace slags containing high amounts of manganese. Cement Concr. Res. 1999, 29, 171–177. [Google Scholar] [CrossRef]
- Shi, T.; Gao, Y.; Corr, D.J.; Shah, S.P. FTIR study on early-age hydration of carbon nanotubes-modified cement-based materials. Adv. Cem. Res. 2019, 31, 353–361. [Google Scholar] [CrossRef]
- Wang, W.; Shao, Y.; Hou, H.; Zhou, M. Synthesis and thermodynamic properties of arsenate and sulfate-arsenate ettringite structure phases. PLoS ONE 2017, 12, e0182160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alotaibi, J.; Saji, S.; Swain, M.V. FTIR characterization of the setting reaction of biodentine™. Dent. Mater. 2018, 34, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Sun, S.; Wu, P. Microdynamic changes of moisture-induced crystallization of amorphous calcium carbonate revealed via in situ FTIR spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 21882–21889. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lee, Y.; Cho, H.; Lee, H.; Lim, S. Improvement in carbonation resistance of portland cement mortar incorporating γ-dicalcium silicate. Adv. Mater. Sci. Eng. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Wu, L.; Liu, X.; Zhang, Y. Structural characteristics and cementitious behavior of basic oxygen furnace slag mud and electric arc furnace slag. Constr. Build. Mater. 2019, 219, 11–18. [Google Scholar] [CrossRef]
- Chang, J.; Cang, X.; Zhang, Y.Y.; Wang, D. Foaming characteristics and microstructure of aerated steel slag block prepared by accelerated carbonation. Constr. Build. Mater. 2019, 209, 222–233. [Google Scholar] [CrossRef]
- Chang, J.; Fang, Y.; Shang, X. The role of β-C2S and γ-C2S in carbon capture and strength development. Mater. Struct. 2016, 49, 4417–4424. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Zhao, Y.; Liu, X. Hydration characteristics and environmental friendly performance of a cementitious material composed of calcium silicate slag. J. Hazard Mater. 2016, 306, 67–76. [Google Scholar] [CrossRef]
- Kumar, S.; Bandopadhyay, A.; Alex, T.C.; Kumar, R. Influence of mechanical activation on the synthesis and hydraulic activity of calcium dialuminate. Ceram. Int. 2006, 32, 555–560. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Li, J.-S.; Yeung, T.L.Y.; Ding, S.M.; Poon, C.S. Green remediation of contaminated sediment by stabilization/solidification with industrial by-products and CO2 utilization. Sci. Total Environ. 2018, 631, 1321–1327. [Google Scholar] [CrossRef]
- Jeong, Y.; Yum, W.S.; Moon, J.; Oh, J.E. Utilization of precipitated CaCO3 from carbon sequestration of industrially emitted CO2 in cementless CaO-activated blast-furnace slag binder system. J. Clean. Prod. 2017, 166, 649–659. [Google Scholar] [CrossRef]
- Xu, H.C.; Xu, X.J.; Wang, K.; Huang, J.G. An analysis of factors affecting the oxidation reduction potential in drinking water. J. Univ. Sci. Technol. Suzhou Eng. Technol. 2007, 20, 63–66. [Google Scholar]
- Chen, Y.-L.; Ko, M.-S.; Lai, Y.-C.; Chang, J.-E. Hydration and leaching characteristics of cement pastes made from electroplating sludge. Waste Manag. 2011, 31, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Giergiczny, Z.; Król, A. Immobilization of heavy metals (Pb, Cu, Cr, Zn, Cd, Mn) in the mineral additions containing concrete composites. J. Hazard Mater. 2008, 160, 247–255. [Google Scholar] [CrossRef] [PubMed]





| Oxides | CaO | SiO2 | MgO | Al2O3 | Fe2O3 | SO3 | Cr2O3 | TiO2 | f-CaO |
|---|---|---|---|---|---|---|---|---|---|
| AODS | 65.78 | 24.15 | 6.55 | 1.74 | 0.26 | 0.54 | 0.50 | 0.22 | 0.33 |
| PC | 65.80 | 19.12 | 1.90 | 5.15 | 3.37 | 3.10 | 0.01 | 0.31 | 0.41 |
| Binder | AODS (g) | PC (g) | Water Requirement (%) | Initial Setting Time (min) | Final Setting Time (min) | Expansion Ratio (%) | Soundness Determination |
|---|---|---|---|---|---|---|---|
| B0 | 0 | 450 | 27.5 | 144 | 216 | 0.35 | Qualified |
| B1 | 50 | 400 | 29.11 | 163 | 231 | 0.30 | Qualified |
| B2 | 100 | 350 | 30.52 | 181 | 246 | 0.34 | Qualified |
| B3 | 150 | 300 | 31.73 | 190 | 265 | 0.38 | Qualified |
| B4 | 200 | 250 | 32.54 | 198 | 282 | 0.41 | Qualified |
| B5 | 250 | 200 | 33.06 | 203 | 301 | 0.43 | Qualified |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-J.; Zeng, Y.-N.; Li, J.-G.; Zhang, Y.-Z. Cementitious Behavior of Argon Oxygen Decarburization Stainless Steel Slag and Its Stabilization on Chromium. Crystals 2020, 10, 876. https://doi.org/10.3390/cryst10100876
Wang Y-J, Zeng Y-N, Li J-G, Zhang Y-Z. Cementitious Behavior of Argon Oxygen Decarburization Stainless Steel Slag and Its Stabilization on Chromium. Crystals. 2020; 10(10):876. https://doi.org/10.3390/cryst10100876
Chicago/Turabian StyleWang, Ya-Jun, Ya-Nan Zeng, Jun-Guo Li, and Yu-Zhu Zhang. 2020. "Cementitious Behavior of Argon Oxygen Decarburization Stainless Steel Slag and Its Stabilization on Chromium" Crystals 10, no. 10: 876. https://doi.org/10.3390/cryst10100876
APA StyleWang, Y.-J., Zeng, Y.-N., Li, J.-G., & Zhang, Y.-Z. (2020). Cementitious Behavior of Argon Oxygen Decarburization Stainless Steel Slag and Its Stabilization on Chromium. Crystals, 10(10), 876. https://doi.org/10.3390/cryst10100876






