Phase Stability of Chloroform and Dichloromethane at High Pressure
Abstract
1. Introduction
2. Materials and Methods
3. Results
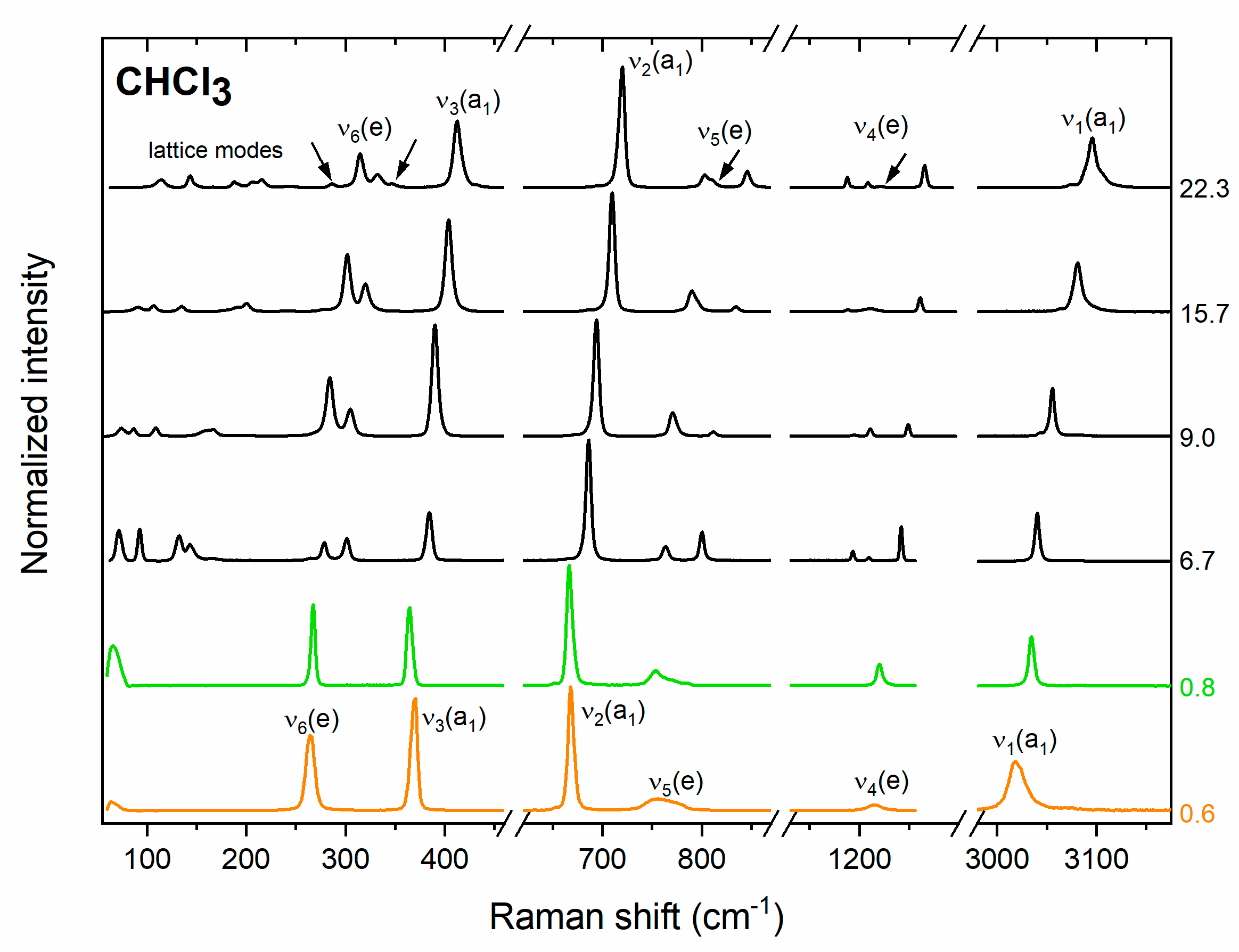
3.1. Raman Spectrum of Chloroform
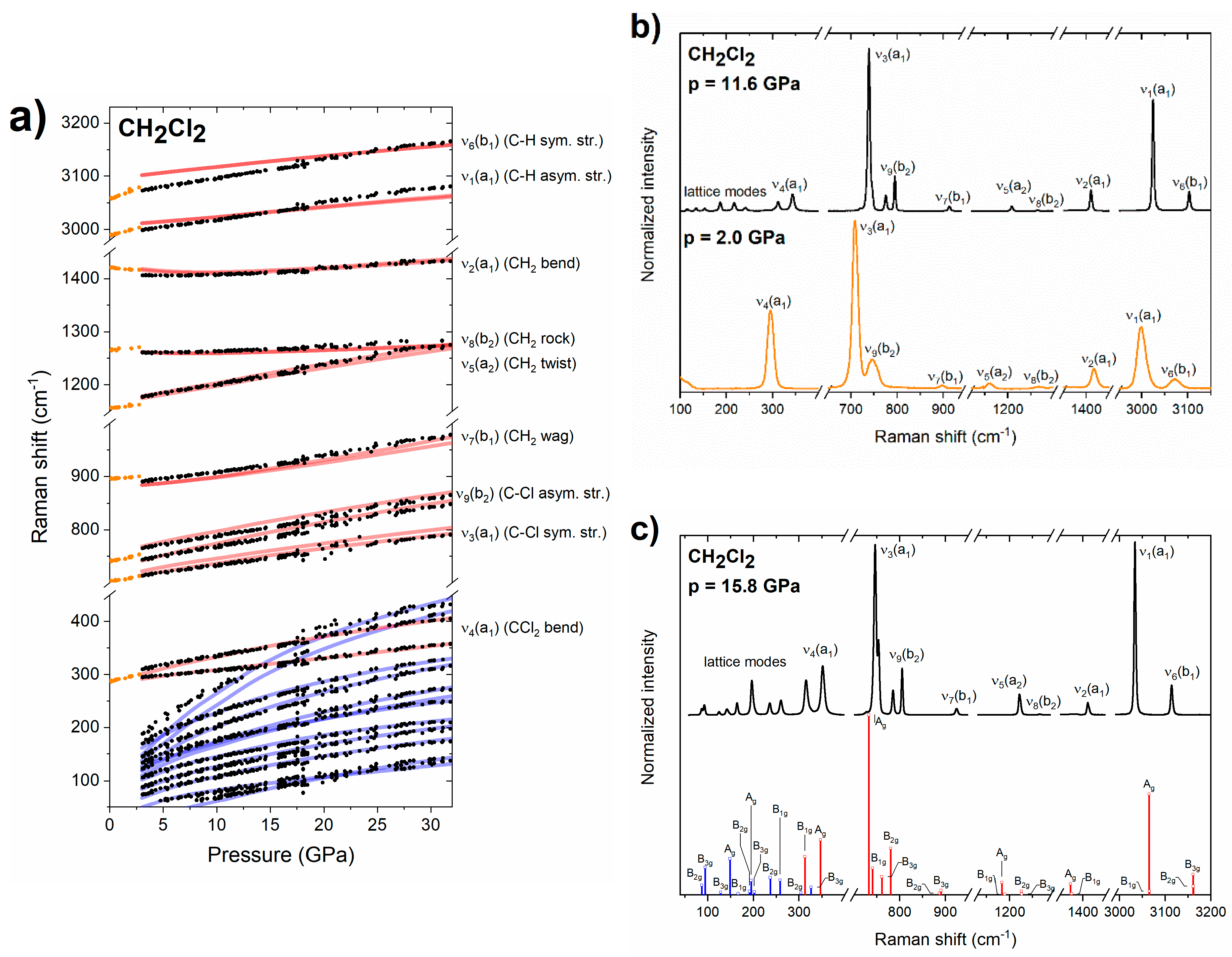
3.2. Raman Spectrum of Dichloromethane
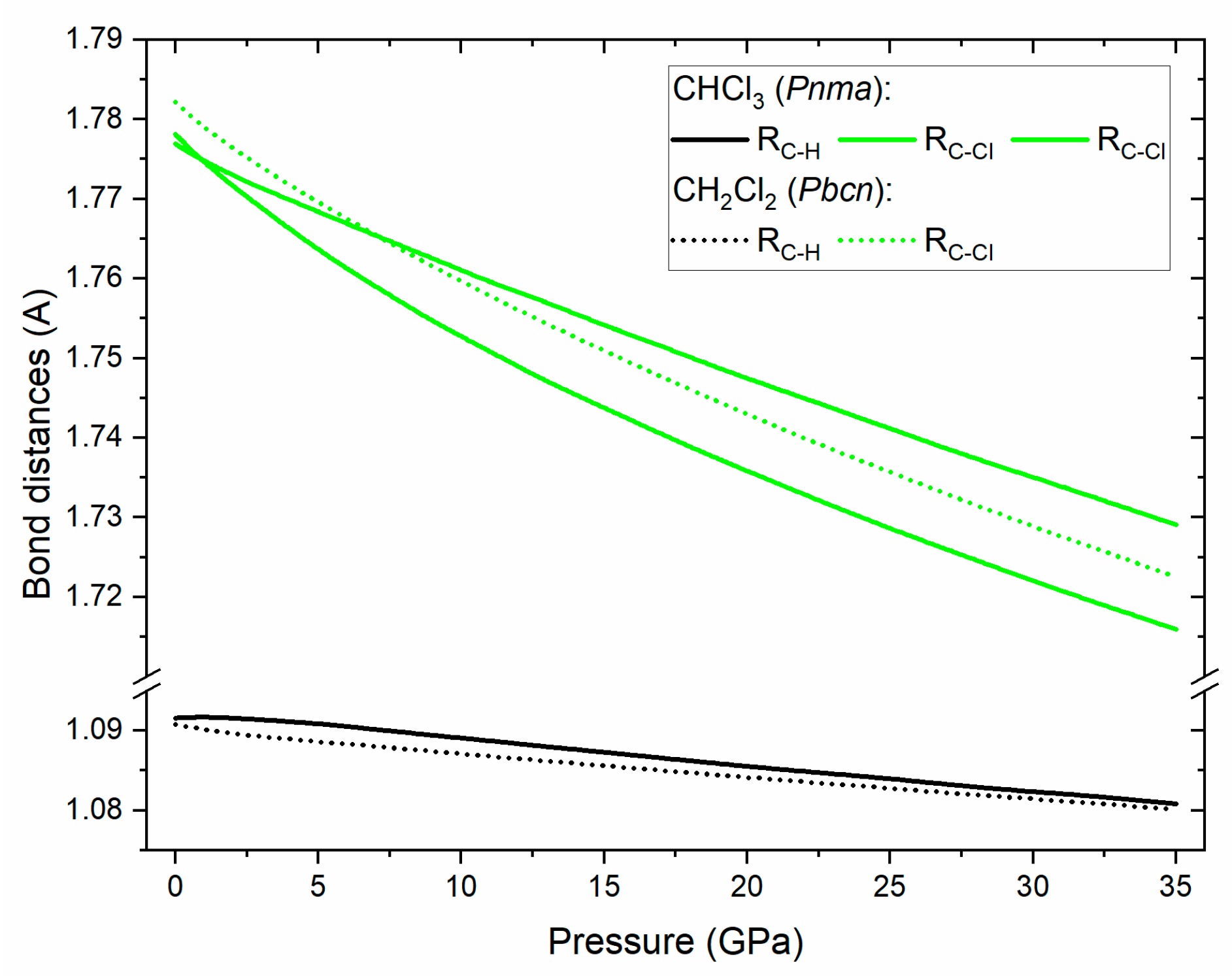
3.3. DFT Calculations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joshi, D.R.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Vidal, S. Safety First: A Recent Case of a Dichloromethane Injection Injury. ACS Central Sci. 2020, 6, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Curtin, D.Y.; Paul, I.C. Chemical consequences of the polar axis in organic solid-state chemistry. Chem. Rev. 1981, 81, 525–541. [Google Scholar] [CrossRef]
- Pidcock, E. Achiral molecules in non-centrosymmetric space groups. Chem. Commun. 2005, 3457–3459. [Google Scholar] [CrossRef]
- Shephard, J.J.; Callear, S.K.; Salzmann, C.G.; Soper, A.K.; Imberti, S.; Evans, J.S.O. Polar stacking of molecules in liquid chloroform. Chem. Commun. 2015, 51, 4770–4773. [Google Scholar] [CrossRef]
- Karnes, J.J.; Benjamin, I. On the local intermolecular ordering and dynamics of liquid chloroform. J. Mol. Liq. 2017, 248, 121–126. [Google Scholar] [CrossRef]
- Shephard, J.J.; Evans, J.S.O.; Salzmann, C. Local structure and orientational ordering in liquid bromoform. Mol. Phys. 2019, 117, 3337–3344. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Parthasarathy, R. The nature of halogen.cntdot. cntdot. cntdot.halogen interactions: Are short halogen contacts due to specific attractive forces or due to close packing of nonspherical atoms? J. Am. Chem. Soc. 1989, 111, 8725–8726. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef]
- Allen, F.H.; Wood, P.A.; Galek, P.T.A. Role of chloroform and dichloromethane solvent molecules in crystal packing: An interaction propensity study. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2013, 69, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Podsiadło, M.; Olejniczak, A.; Katrusiak, A. Halogen⋯halogen contra C–H⋯halogen interactions. CrystEngComm 2014, 16, 8279–8285. [Google Scholar] [CrossRef]
- Kaźmierczak, M.; Katrusiak, A. Quantitative estimate of cohesion forces. CrystEngComm 2015, 17, 9423–9430. [Google Scholar] [CrossRef]
- Bujak, M.; Podsiadło, M.; Katrusiak, A. Halogen and hydrogen bonds in compressed pentachloroethane. CrystEngComm 2016, 18, 5393–5397. [Google Scholar] [CrossRef]
- Boldyreva, E.V. CHAPTER 2. High Pressure Crystallography: Elucidating the Role of Intermolecular Interactions in Crystals of Organic and Coordination Compounds. In Supramolecular Amphiphiles; Royal Society of Chemistry (RSC): London, UK, 2018; pp. 32–97. [Google Scholar]
- Kurzydłowski, D.; Wang, H.B.; Troyan, I.A.; Eremets, M.I. Lone-pair interactions and photodissociation of compressed nitrogen trifluoride. J. Chem. Phys. 2014, 141, 064706. [Google Scholar] [CrossRef] [PubMed]
- Rusek, M.; Kwaśna, K.; Budzianowski, A.; Katrusiak, A. Fluorine···Fluorine Interactions in a High-Pressure Layered Phase of Perfluorobenzene. J. Phys. Chem. C 2019, 124, 99–106. [Google Scholar] [CrossRef]
- Shimizu, H.; Matsumoto, K. High-pressure Raman study of liquid and molecular crystals at room temperature. 3. Chloroform and chloroform-d. J. Phys. Chem. 1984, 88, 2934–2936. [Google Scholar] [CrossRef]
- Yongnian, Z.; Huan, L.; Xianyi, L.; Guangtian, Z. High-pressure Raman studies of liquid and molecular crystal chloroform and bromoform at room temperature. Phys. B+C 1986, 139, 526–529. [Google Scholar] [CrossRef]
- Stanila, D.; Smith, W.; Anderson, A. Infrared Spectra of Chloroform at High Pressures. Spectrosc. Lett. 2002, 35, 703–713. [Google Scholar] [CrossRef]
- Dziubek, K.F.; Katrusiak, A. Polar Symmetry in New High-Pressure Phases of Chloroform and Bromoform. J. Phys. Chem. B 2008, 112, 12001–12009. [Google Scholar] [CrossRef]
- Fourme, R. Appareillage pour études radiocristallographiques sous pression et à température variable. J. Appl. Crystallogr. 1968, 1, 23–30. [Google Scholar] [CrossRef]
- Dziubek, K.; Podsiadło, M.; Katrusiak, A. Molecular Symmetry and Isostructural Relations in Crystal Phases of Trihalomethanes CHCl3, CHBrCl2, CHBr2Cl, and CHBr3. J. Phys. Chem. B 2009, 113, 13195–13201. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Matsumoto, K. High-Pressure Raman Study of Phase Transitions in Solid Bromoform. J. Phys. Soc. Jpn. 1984, 53, 4438–4444. [Google Scholar] [CrossRef]
- Stanila, D.; Smith, W.; Anderson, A. Infrared Spectra of Bromoform at High Pressures. Spectrosc. Lett. 2001, 34, 199–210. [Google Scholar] [CrossRef]
- Stanila, D.; Smith, W.; Anderson, A. Raman and Infrared Spectra of Iodoform at High Pressures. Spectrosc. Lett. 2002, 35, 689–702. [Google Scholar] [CrossRef]
- Liu, D.; Lei, W.; Wang, K.; Bao, G.; Li, F.; Hao, J.; Liu, B.; Cui, T.; Cui, Q.; Zou, G. Compression and Probing C−H···I Hydrogen Bonds of Iodoform under High Pressure by X-ray Diffraction and Raman Scattering. J. Phys. Chem. B 2009, 113, 7430–7434. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, F.; Curetti, N.; Benna, P.; Gervasio, G. The effects of P–T changes on intermolecular interactions in crystal structure of iodoform. J. Mol. Struct. 2013, 1041, 106–112. [Google Scholar] [CrossRef]
- Shimizu, H. High-pressure Raman study of liquid and molecular crystal at room temperature. Methylene chloride. Chem. Phys. Lett. 1984, 105, 268–272. [Google Scholar] [CrossRef]
- Shimizu, H.; Xu, J.; Mao, H.; Bell, P. High-pressure FT IR measurements of crystalline methylene chloride up to 120 kbar in the diamond anvil cell. Chem. Phys. Lett. 1984, 105, 273–276. [Google Scholar] [CrossRef]
- Podsiadło, M.; Dziubek, K.; Katrusiak, A. In situ high-pressure crystallization and compression of halogen contacts in dichloromethane. Acta Crystallogr. Sect. B Struct. Sci. 2005, 61, 595–600. [Google Scholar] [CrossRef]
- Podsiadło, M.; Katrusiak, A. Isobaric and isochoric freezing of CH2BrCl and isostructural relations between CH2Cl2, CH2Br2 and CH2BrCl. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Podsiadło, M.; Katrusiak, A. Competing Patterns of Weak Directional Forces in Pressure-Frozen CH2ClI and CH2I2. J. Phys. Chem. B 2008, 112, 5355–5362. [Google Scholar] [CrossRef] [PubMed]
- Itié, J.; Chatelet, M. High-pressure Raman study of dibromomethane. Chem. Phys. Lett. 1994, 221, 393–396. [Google Scholar] [CrossRef]
- Podsiadło, M.; Dziubek, K.; Szafrański, M.; Katrusiak, A. Molecular interactions in crystalline dibromomethane and diiodomethane, and the stabilities of their high-pressure and low-temperature phases. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 1090–1098. [Google Scholar] [CrossRef]
- Podsiadło, M.; Katrusiak, A. Isostructural relations in dihalomethanes and disproportionation of bromoiodomethane. CrystEngComm 2008, 10, 1436. [Google Scholar] [CrossRef]
- Zhou, Y.; Lee, S.A.; Anderson, A. Raman Studies of Molecular Crystals at High Pressures. III. Methylene Iodide. J. Raman Spectrosc. 1996, 27, 499–502. [Google Scholar] [CrossRef]
- Bao, G.; Duan, D.; Zhou, D.; Jin, X.; Liu, B.; Cui, T. A New High-Pressure Polar Phase of Crystalline Bromoform: A First-Principles Study. J. Phys. Chem. B 2010, 114, 13933–13939. [Google Scholar] [CrossRef]
- Bao, G.; Duan, D.; Tian, F.; Wang, L.; Liu, B.; Cui, T. Structural, electronic, and optical properties of crystalline iodoform under high pressure: A first-principles study. J. Chem. Phys. 2011, 134, 34508. [Google Scholar] [CrossRef]
- Dewaele, A.; Torrent, M.; Loubeyre, P.; Mezouar, M. Compression curves of transition metals in the Mbar range: Experiments and projector augmented-wave calculations. Phys. Rev. B 2008, 78, 104102. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Für Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of Crystals with the Quasi-Newton Method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Refson, K.; Tulip, P.R.; Clark, S.J. Variational density-functional perturbation theory for dielectrics and lattice dynamics. Phys. Rev. B 2006, 73, 155114. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Bhagavantam, S.; Venkateswaran, S. The Raman spectra of some organic halogen compounds. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1930, 127, 360–373. [Google Scholar] [CrossRef]
- Fourme, R.; Renaud, M. Etude de la structure cristalline du chloroforme a 185 degree K. Comptes Rendus Des Seances L’Academie Des Sci. Ser. B 1966, 263, 69. [Google Scholar]
- Andrews, B.; Anderson, A.; Torrie, B. Raman and infrared spectra of crystalline chloroform. Chem. Phys. Lett. 1984, 104, 65–70. [Google Scholar] [CrossRef]
- Welsh, H.L.; Crawford, M.F.; Thomas, T.R.; Love, G.R. Raman Spectroscopy of Low Pressure Gases and Vapors. Can. J. Phys. 1952, 30, 577–596. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Tanaka, K.; Takeuchi, T.; Watanabé, T. The Crystal Structure of Methylene Bichloride, CH2Cl2. Bull. Chem. Soc. Jpn. 1973, 46, 62–66. [Google Scholar] [CrossRef]
- Ito, M. Raman Spectra of Polycrystalline Chloromethanes at Liquid Nitrogen Temperature. J. Chem. Phys. 1964, 40, 3128. [Google Scholar] [CrossRef]
- Brown, C.W.; Obremski, R.J.; Allkins, J.R.; Lippincott, E.R. Vibrational Spectra of Single Crystals and Polycrystalline Films of CH2Cl2 and CH2Br2. J. Chem. Phys. 1969, 51, 1376–1384. [Google Scholar] [CrossRef]
- Mukherjee, A.; Desiraju, G.R. Halogen bonds in some dihalogenated phenols: Applications to crystal engineering. IUCrJ 2013, 1, 49–60. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M. Halogen Bonding: A Halogen-Centered Noncovalent Interaction Yet to Be Understood. Inorganics 2019, 7, 40. [Google Scholar] [CrossRef]
- Rahm, M.; Hoffmann, R.; Ashcroft, N.W. Atomic and Ionic Radii of Elements 1-96. Chem. A Eur. J. 2016, 22, 14625–14632. [Google Scholar] [CrossRef]
- Rahm, M.; Ångqvist, M.; Rahm, J.M.; Erhart, P.; Cammi, R. Non-bonded Radii of the Atoms Under Compression. ChemPhysChem 2020. [Google Scholar] [CrossRef]
- The Atoms Under Pressure (AUP) database. Available online: https://www.rahmlab.com/atoms-under-pressure/ (accessed on 15 September 2020).




| Compound | Maximum Pressure | Experimental Method | Ref. |
|---|---|---|---|
| CHCl3 | 10 | Raman | [18,19] |
| 10 | IR | [20] | |
| 0.8 | X-ray diffraction | [21,22] | |
| CHBrCl2 | 2.5 | X-ray diffraction | [23] |
| CHBr2Cl | 1.2 | X-ray diffraction | [23] |
| CHBr3 | 10 | Raman | [19,24] |
| 10 | IR | [25] | |
| 0.4 | X-ray diffraction | [21] | |
| CHI3 | 22 | Raman | [26,27] |
| 5 | IR | [26] | |
| 40 | X-ray diffraction | [27,28] | |
| CH2Cl2 | 8.5 | Raman | [29] |
| 12 | IR | [30] | |
| 1.6 | X-ray diffraction | [31] | |
| CH2BrCl | 1.7 | X-ray diffraction | [32] |
| CH2ClI | 2.3 | X-ray diffraction | [33] |
| CH2Br2 | 24 | Raman | [34] |
| 0.6 | X-ray diffraction | [35] | |
| CH2BrI | 0.5 | X-ray diffraction | [36] |
| CH2I2 | <8.2 | Raman | [37] |
| 0.2 | X-ray diffraction | [35] | |
| 1.6 | X-ray diffraction | [33] |
| Mode | Description | Frequency (Liquid) [49] |
|---|---|---|
| ν1(a1) | C–H stretching | 3019 |
| ν4(e) | C–H bend | 1218 |
| ν5(e) | C–Cl asymmetric stretching | 762 |
| ν2(a1) | C–Cl symmetric stretching | 669 |
| ν3(a1) | CCl3 symmetric deformation | 367 |
| ν6(e) | CCl3 asymmetric deformation | 261 |
| Mode | Description | Frequency (Gas) [52] |
|---|---|---|
| ν6(b1) | C–H asymmetric stretching | 3019 |
| ν1(a1) | C–H symmetric stretching | 2996 |
| ν2(a1) | CH2 scissoring | 1467 |
| ν8(b2) | CH2 wagging | 1255 |
| ν5(a2) | CH2 twisting | 1153 |
| ν7(b1) | CH2 rocking | 893 |
| ν9(b2) | C–Cl asymmetric stretching | 748 |
| ν3(a1) | C–Cl symmetric stretching | 713 |
| ν4(a1) | CCl2 bend | 282 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzydłowski, D.; Chumak, T.; Rogoża, J. Phase Stability of Chloroform and Dichloromethane at High Pressure. Crystals 2020, 10, 920. https://doi.org/10.3390/cryst10100920
Kurzydłowski D, Chumak T, Rogoża J. Phase Stability of Chloroform and Dichloromethane at High Pressure. Crystals. 2020; 10(10):920. https://doi.org/10.3390/cryst10100920
Chicago/Turabian StyleKurzydłowski, Dominik, Taisiia Chumak, and Jakub Rogoża. 2020. "Phase Stability of Chloroform and Dichloromethane at High Pressure" Crystals 10, no. 10: 920. https://doi.org/10.3390/cryst10100920
APA StyleKurzydłowski, D., Chumak, T., & Rogoża, J. (2020). Phase Stability of Chloroform and Dichloromethane at High Pressure. Crystals, 10(10), 920. https://doi.org/10.3390/cryst10100920





