Investigation of the Structural and Luminescent Properties and the Chromium Ion Valence of Li2CaGeO4 Crystals Doped with Cr4+ Ions
Abstract
1. Introduction
2. Experimental Procedure
2.1. Sample Preparation
2.2. Characterizations
3. Results and Discussion
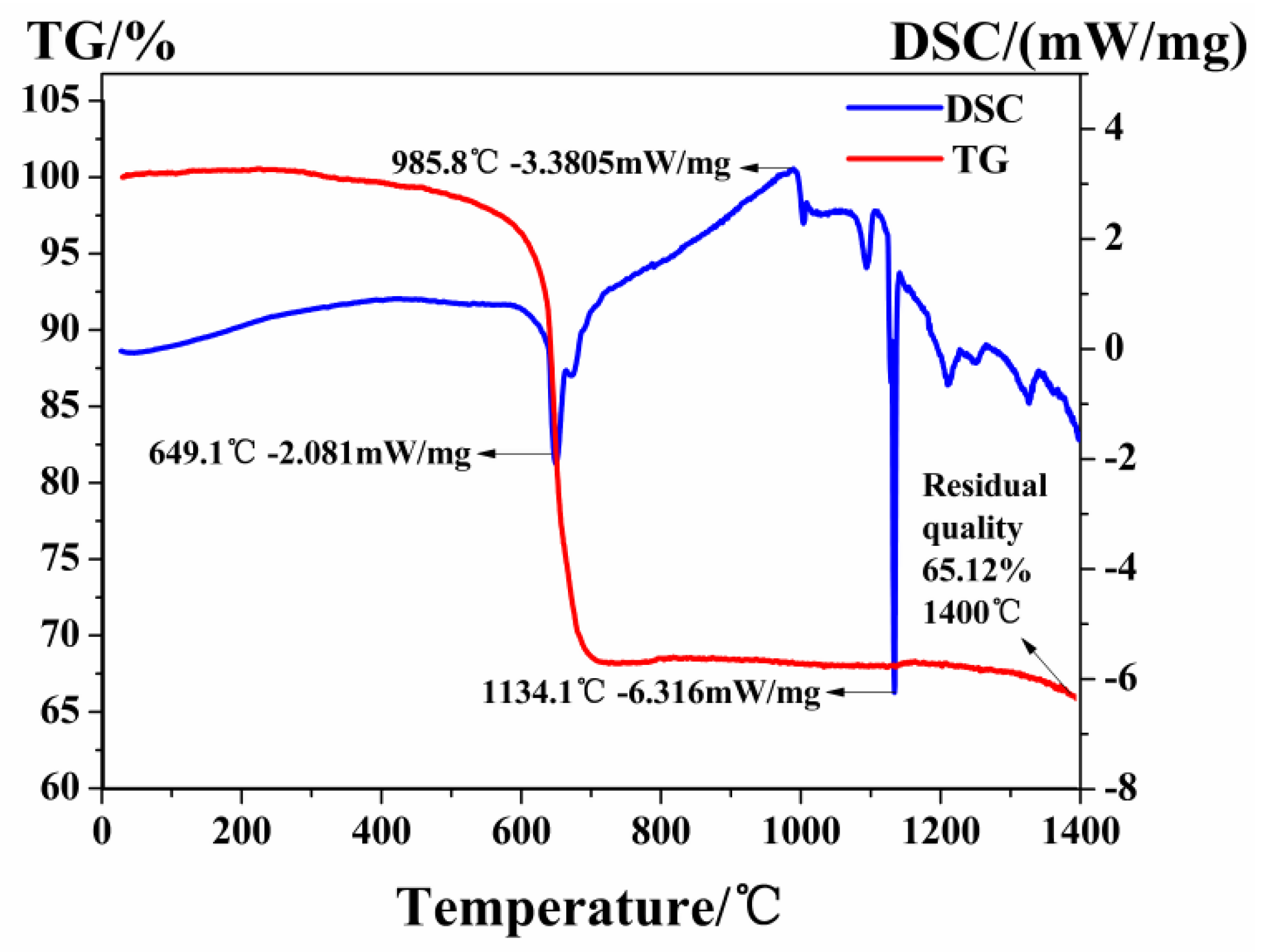
3.1. The Phase Formation Temperature
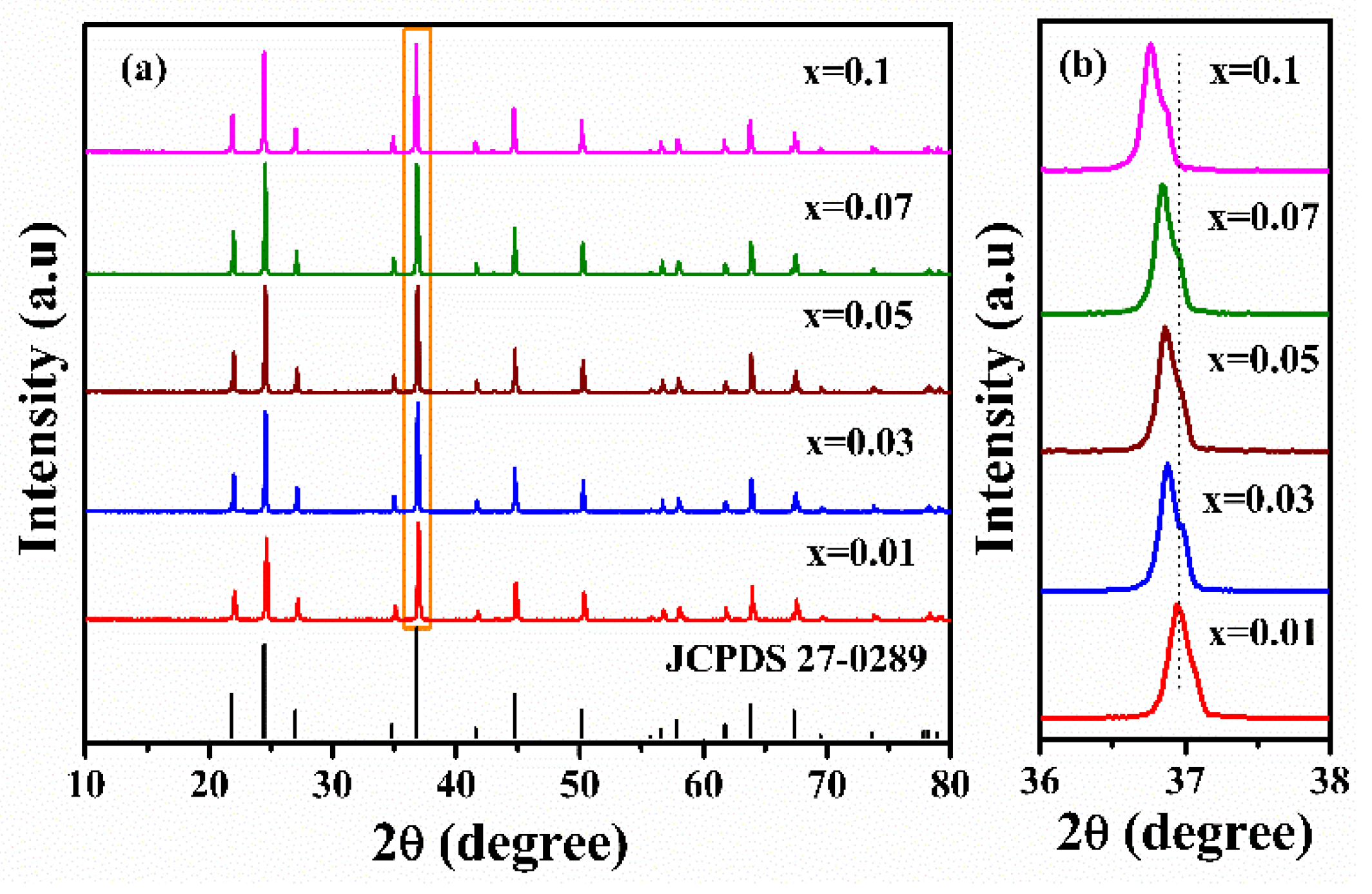
3.2. Crystal Structure Analysis
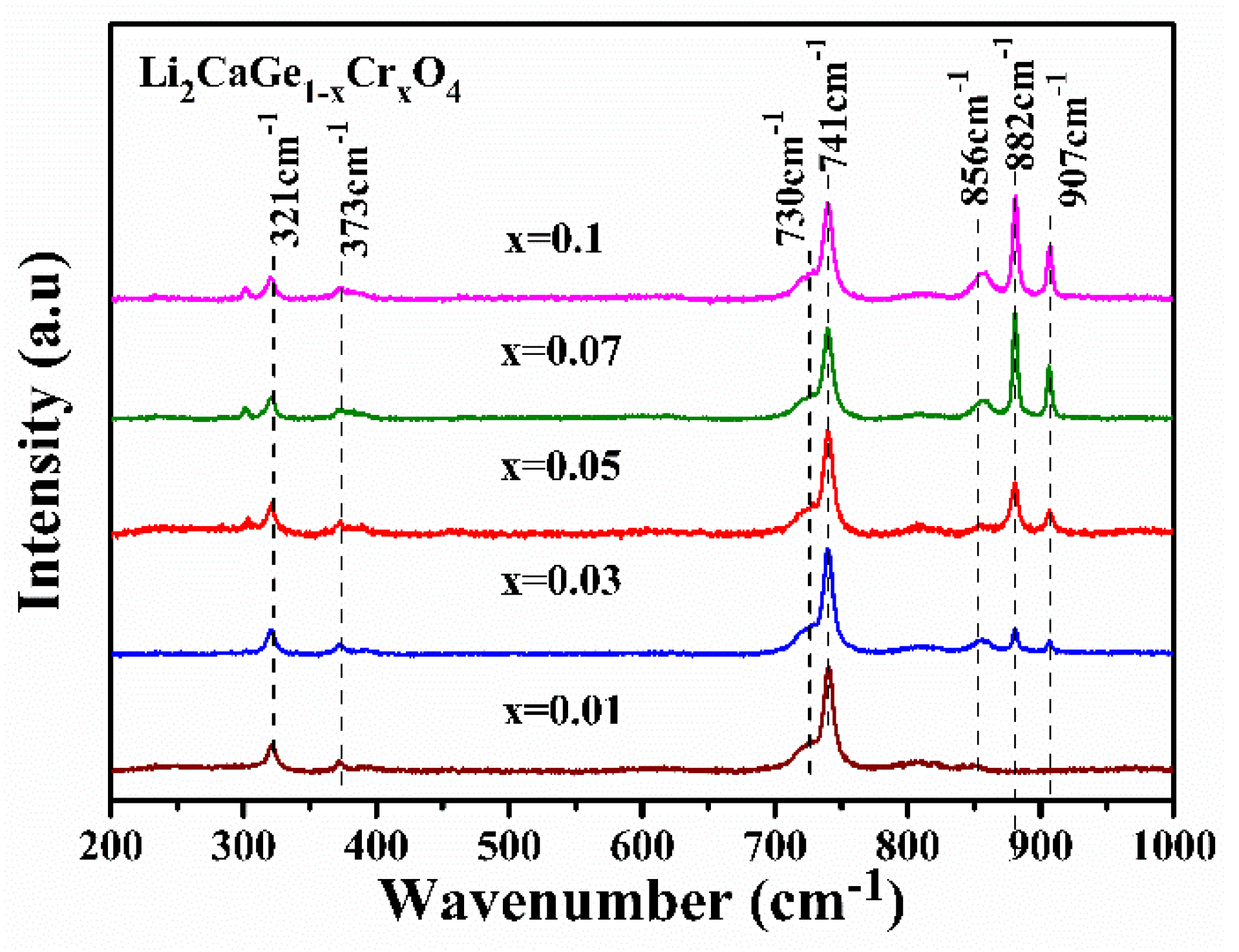
3.3. Fourier Infrared Spectrum Spectroscopy Analysis
3.4. Raman Spectrum Analysis
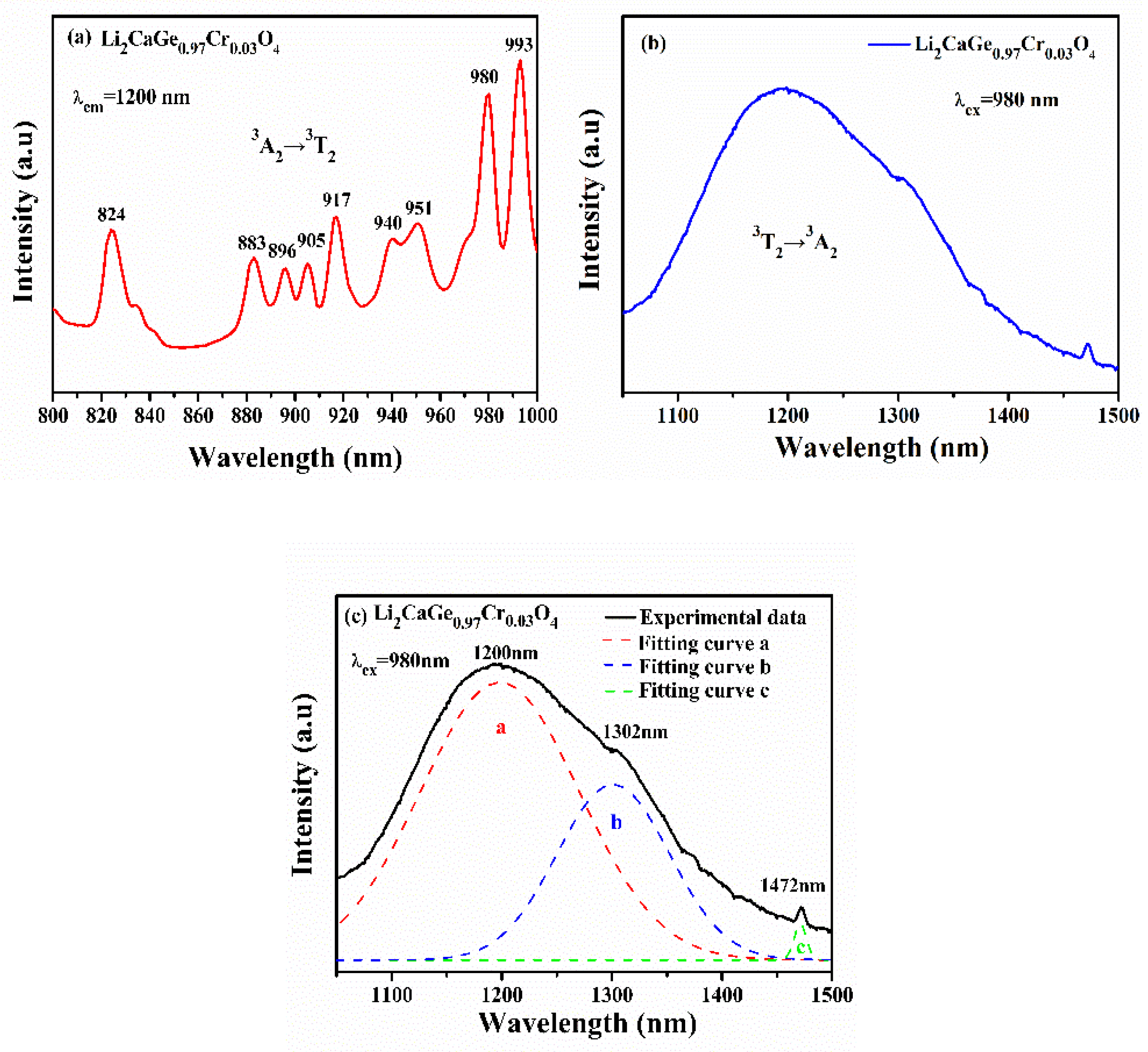
3.5. Luminescence Properties and Analysis
3.6. Fluorescence Lifetime Analysis
3.7. XPS Analysis of the Li2CaGe0.95Cr0.05O4 Crystals
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Angert, N.B.; Borodin, N.I.; Garmash, V.M.; Zhitnyuk, V.A.; Okhrimchuk, A.G.; Siyuchenko, O.G.; Shestakov, A.V. Lasing due to impurity color centers in yttrium aluminum garnet crystals at wavelengths in the range 1.35–1.45 μm. Sov. J. Quantum Electron. 1988, 18, 73–74. [Google Scholar] [CrossRef]
- Petričević, V.; Gayen, S.K.; Alfano, R.; Yamagishi, K.; Anzai, H.; Yamaguchi, Y. Laser action in chromium-doped forsterite. Appl. Phys. Lett. 1988, 52, 1040–1042. [Google Scholar] [CrossRef]
- Verdun, H.R.; Thomas, L.M.; Andrauskas, D.M.; Mccollum, T.; Pinto, A. Chromium-doped forsterite laser pumped with 1.06 μm radiation. Appl. Phys. Lett. 1988, 53, 2593–2595. [Google Scholar] [CrossRef]
- Spälter, S.; Böhm, M.; Burk, M.; Mikulla, B.; Fluck, R.; Jung, I.; Zhang, G.; Keller, U.; Sizmann, A.; Leuchs, G. Self-starting soliton-modelocked femtosecond Cr4+:YAG laser using an antiresonant Fabry-Pérot saturable absorber. Appl. Phys. A 1997, 65, 335–338. [Google Scholar] [CrossRef]
- Sennaroglu, A.; Carrig, T.J.; Pollock, C.R. Femtosecond pulse generation by using an additive-pulse mode-locked chromium-doped forsterite laser operated at 77 K. Opt. Lett. 1992, 17, 1216–1218. [Google Scholar] [CrossRef] [PubMed]
- Kück, S. Laser-related spectroscopy of ion-doped crystals for tunable solid-state lasers. Appl. Phys. A 2001, 72, 515–562. [Google Scholar] [CrossRef]
- Moncorge, R.; Manaa, H.; Boulon, G. Cr4+ and Mn5+ active centers for new solid state laser materials. Opt. Mater. 1994, 4, 139–151. [Google Scholar] [CrossRef]
- Kuo, Y.-K.; Huang, M.-F.; Birnbaum, M. Tunable Cr/sup 4+/:YSO Q-switched Cr:LiCAF laser. IEEE J. Quantum Electron. 1995, 31, 657–663. [Google Scholar] [CrossRef]
- Sennaroglu, A. Broadly tunable Cr4+-doped solid-state lasers in the near infrared and visible. Prog. Quantum Electron. 2002, 26, 287–352. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.K.; Alsous, M.B.; Ranganathan, K.; Sharma, S.K.; Gupta, P.K.; George, J.; Nathan, T.P.S. Simultaneous Q-switching and mode-locking in an intracavity frequency doubled diode-pumped Nd:YVO4/KTP green laser with Cr4+:YAG. Opt. Commun. 2003, 222, 399–404. [Google Scholar] [CrossRef]
- Gilmore, D.A.; Cvijin, P.V.; Atkinson, G.H. Intracavity laser spectroscopy in the 1.38–1.55 gm spectral region using a multimode Cr4+:YAG laser. Opt. Commun. 1993, 103, 370–374. [Google Scholar] [CrossRef]
- Chai, Y.; Leburn, C.G.; Lagatsky, A.A.; Brown, C.T.A.; Penty, R.; White, I.; Sibbett, W. 1.36-Tb/s spectral slicing source based on a Cr/sup 4+/-YAG femtosecond laser. J. Light. Technol. 2005, 23, 1319–1324. [Google Scholar] [CrossRef]
- Soubbotin, K.; Smirnov, V.; Kovaliov, S.; Scheel, H.; Zharikov, E. Growth and spectroscopic investigation of new promising laser crystal chromium (IV) doped germanoeucryptite Cr4+:LiAlGeO4. Opt. Mater. 2000, 13, 405–410. [Google Scholar] [CrossRef]
- Deka, C.; Chai, B.H.T.; Shimony, Y.; Zhang, X.X.; Munin, E. Bass, Laser performance of Cr4+:Y2SiO5. Appl. Phys. Lett. 1992, 61, 2141–2143. [Google Scholar] [CrossRef]
- Merkie, L.D.; Allik, T.H.; Chai, H.T. Crystal growth and spectroscopic properties of Cr4+ in Ca2A12SiO7 and Ca2Ga2SiO7. Opt. Mater. 1992, 1, 91–100. [Google Scholar] [CrossRef]
- Heyns, A.M.; Harden, P.M. Evidence for the existence of Cr(IV) in chromium-doped malayaite Cr4+:CaSnOSiO4: A resonance Raman Study. J. Phys. Chem. Solids 1999, 60, 277–284. [Google Scholar] [CrossRef]
- Kück, S.; Hartung, S. Comparative study of the spectroscopic properties of Cr4+-doped LiAlO2 and LiGaO2. Chem. Phys. 1999, 240, 387–401. [Google Scholar] [CrossRef]
- Chen, W.; Spariosu, K.; Stultz, R.; Kuo, Y.K.; Birnbaum, M.; Shestakov, A. Cr4+: GSGG saturable absorber Q-switch for the ruby laser. Opt. Commun. 1993, 104, 71–74. [Google Scholar] [CrossRef]
- Petermann, K.; Pohlmann, U.; Huber, G.; Kück, S.; Schönhoff, U. Tunable room-temperature laser action of Cr4+-doped Y3ScxAl5−xO12. Appl. Phys. A 1994, 58, 153–156. [Google Scholar] [CrossRef]
- Hazenkamp, M.; Oetliker, U.; Gudel, H.; Kesper, U.; Reinen, D. Absorption and luminescence spectroscopy of Cr4+-doped Ca2GeO4. A potential near infrared laser material. Chem. Phys. Lett. 1995, 233, 466–470. [Google Scholar] [CrossRef]
- Moncorge, R.; Manaa, H.; Deghoul, F.; Guyot, Y.; Kalisky, Y.; Pollack, S.; Zharikov, E.; Kokta, M. Saturable and excited state absorption measurements in Cr4+:LuAG single crystals. Opt. Commun. 1996, 132, 279–284. [Google Scholar] [CrossRef]
- Pollock, C.; Barber, D.; Mass, J.; Markgraf, S. Cr/sup 4+/ lasers: Present performance and prospects for new host lattices. IEEE J. Sel. Top. Quantum Electron. 1995, 1, 62–66. [Google Scholar] [CrossRef]
- Okhrimchuk, A.; Shestakov, A. Performance of YAG: Cr4+ laser crystal. Opt. Mater. 1994, 3, 1–13. [Google Scholar] [CrossRef]
- Sharonov, M.Y.; Bykov, A.B.; Owen, S.; Petricevic, V.; Alfano, R.R. Crystal growth and optical properties of Cr4+:Li2TiGeO5. J. Appl. Phys. 2003, 93, 1044–1047. [Google Scholar] [CrossRef]
- Gard, J.; West, A.R. Preparation and crystal structure of Li2CaSiO4 and isostructural Li2CaGeO4. J. Solid State Chem. 1973, 7, 422–427. [Google Scholar] [CrossRef]
- Marychev, M.; Koseva, I.; Gencheva, G.; Stoyanova, R.; Kukeva, R.; Nikolov, V. Cr doped Ca2GeO4, Ca5Ge3O11 and Li2CaGeO4 single crystals grown by the flux method. J. Cryst. Growth 2017, 461, 46–52. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. J. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Q.; Wang, X.; Liu, J.; Xiao, Y.; Li, C.; Lin, H.; Zeng, F.; Su, Z.-M. A novel scheme to acquire enhanced up-conversion emissions of Ho3+ and Yb3+ co-doped Sc2O3. Curr. Appl. Phys. 2020, 20, 82–88. [Google Scholar] [CrossRef]
- Mayerhöfer, T.; Dunken, H. Single-crystal IR spectroscopic investigation on fresnoite, Sr-fresnoite and Ge-fresnoite. Vib. Spectrosc. 2001, 25, 185–195. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Hu, L. Statistical structure analysis of GeO2 modified Yb3+: Phosphate glasses based on Raman and FTIR study. J. Alloys Compd. 2017, 698, 103–113. [Google Scholar] [CrossRef]
- Mguedla, R.; Kharrat, A.B.J.; Saadi, M.; Khirouni, K.; Chniba-Boudjada, N.; Boujelben, W.; Khirouni, K. Structural, electrical, dielectric and optical properties of PrCrO3 ortho-chromite. J. Alloys Compd. 2020, 812, 152130. [Google Scholar] [CrossRef]
- Zhang, X. Raman Spectrum Analysis on the Solid-Liquid Boundary Layer of BGO Crystal Growth. J. Chin. Phys. Lett. 2007, 24, 1898–1900. [Google Scholar]
- Sharonov, M.; Bykov, A.; Myint, T.; Petricevic, V.; Alfano, R. Spectroscopic study of chromium-doped transparent calcium germanate glass-ceramics. Opt. Commun. 2007, 275, 123–128. [Google Scholar] [CrossRef]
- Weinstock, N.; Schulze, H. Assignment of n2(E) and n4(F2) of tetrahedral species by the calculation of the relative Raman intensities: The vibrational spectra of , , , , , , , RuO4 and OsO4. J. Chem. Phys. 1973, 59, 5063–5067. [Google Scholar] [CrossRef]
- Michel, G.; Machiroux, R. Raman Spectroscopic Investigations of the CrO42-/Cr2O72- Equilibrium in Aqueous Solution. J. Raman Spectrosc. 1983, 14, 22–27. [Google Scholar] [CrossRef]
- Wu, X.; Huang, S.; Hömmerich, U.; Yen, W.; Aitken, B.; Newhouse, M. Spectroscopy of Cr4+ in MgCaBa aluminate glass. The coupling of 3T2 and 1E states. Chem. Phys. Lett. 1995, 233, 28–32. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.; Liu, W.; Zheng, D.; Zhang, S.; Zhang, Y.; Lin, H.; Liu, L.; Liu, J.; Zeng, F. Synthesis and characterization of Cr4+-doped Ca2GeO4 tunable crystal. J. Alloys Compd. 2015, 636, 211–215. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer between inequivalent Eu2+ ions. J. Solid State Chem. 1986, 62, 207–211. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, Y. Tunable blue–green color emission and energy transfer properties of Li2CaGeO4:Ce3+, Tb3+ phosphors for near-UV white-light LEDs. J. Alloys Compd. 2014, 610, 695–700. [Google Scholar] [CrossRef]
- Dexter, D.L.; Schulman, J.H. Theory of Concentration Quenching in Inorganic Phosphors. J. Chem. Phys. 1954, 22, 1063–1070. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, Y.; Wu, H.; Duan, H.; Chen, L.; Fu, Y.; Ju, G.; Mu, Z.; He, M. A deep red phosphor Li2MgTiO4:Mn4+ exhibiting abnormal emission: Potential application as color converter for warm w-LEDs. Chem. Eng. J. 2016, 288, 596–607. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Y.; Qu, J.; Ding, J.; Lin, H.; Liu, L.; Zhou, Y.; Zeng, F.; Li, C.; Su, Z.-M. Up-conversion photoluminescence properties and energy transfer process of Ho3+, Yb3+ Co-doped BaY2F8 fine fibers. J. Lumin. 2019, 212, 154–159. [Google Scholar] [CrossRef]
- Annadurai, G.; Kennedy, S.M.M.; Sivakumar, V. Photoluminescence properties of a novel orange-red emitting Ba2CaZn2Si6O17:Sm3+ phosphor. J. Rare Earths 2016, 34, 576–582. [Google Scholar] [CrossRef]
- Aquino, J.C.R.; Aragón, F.F.; Coaquira, J.; Gratens, X.; Chitta, V.A.; Gonzales, I.; Macedo, W.A.A.; Morais, P.C. Evidence of Cr3+ and Cr4+ Coexistence in Chromium-Doped SnO2 Nanoparticles: A Structural and Magnetic Study. J. Phys. Chem. C 2017, 121, 21670–21677. [Google Scholar] [CrossRef]
- Ikemoto, I.; Ishii, K.; Kinoshita, S.; Kuroda, H.; Franco, M.A.; Thomas, J. X-ray photoelectron spectroscopic studies of CrO2 and some related chromium compounds. J. Solid State Chem. 1976, 17, 425–430. [Google Scholar] [CrossRef]
- Fukaya, S.; Adachi, K.; Obara, M.; Kumagai, H. The growth of Cr4+: YAG and Cr4+:GGG thin films by pulsed laser deposition. Opt. Commun. 2001, 187, 373–377. [Google Scholar] [CrossRef]
- Li, J.-T.; Maurice, V.; Światowska, J.; Seyeux, A.; Zanna, S.; Klein, L.; Sun, S.-G.; Marcus, P. XPS, time-of-flight-SIMS and polarization modulation IRRAS study of Cr2O3 thin film materials as anode for lithium ion battery. Electrochim. Acta 2009, 54, 3700–3707. [Google Scholar] [CrossRef]
- Asuvathraman, R.; Gnanasekar, K.; Clinsha, P.; Ravindran, T.; Kutty, K.G. Investigations on the charge compensation on Ca and U substitution in CePO4 by using XPS, XRD and Raman spectroscopy. Ceram. Int. 2015, 41, 3731–3739. [Google Scholar] [CrossRef]
- Ramesh, B.; Dillip, G.; Reddy, G.R.; Raju, B.D.P.; Joo, S.; Sushma, N.J.; Rambabu, B. Luminescence properties of CaZn2(PO4)2:Sm3+ phosphor for lighting application. Optik 2018, 156, 906–913. [Google Scholar] [CrossRef]
- Lisboa-Filho, P.; Mastelaro, V.R.; Schreiner, W.; Messaddeq, S.; Li, M.S.; Messaddeq, Y.; Hammer, P.; Ribeiro, S.J.L.; Parent, P.; Laffon, C. Photo-induced effects in Ge25Ga10S65 glasses studied by XPS and XAS. Solid State Ion. 2005, 176, 1403–1409. [Google Scholar] [CrossRef]


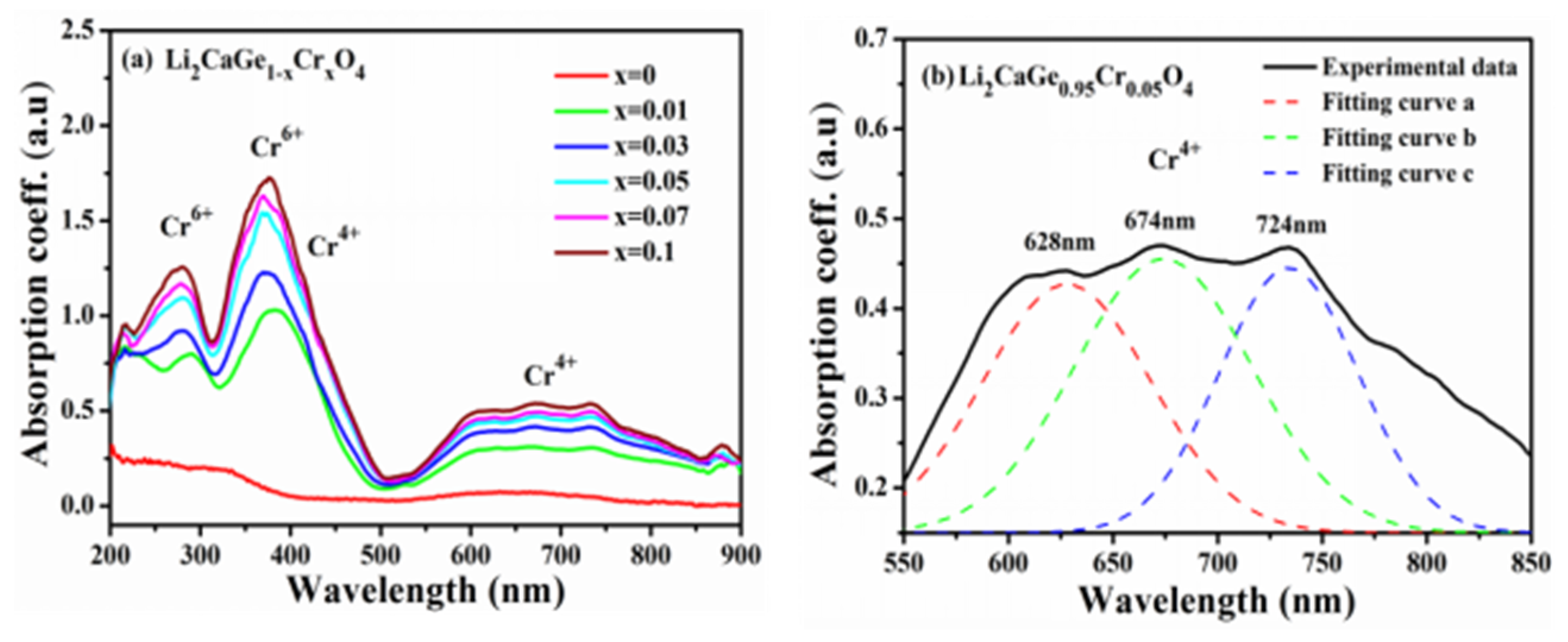
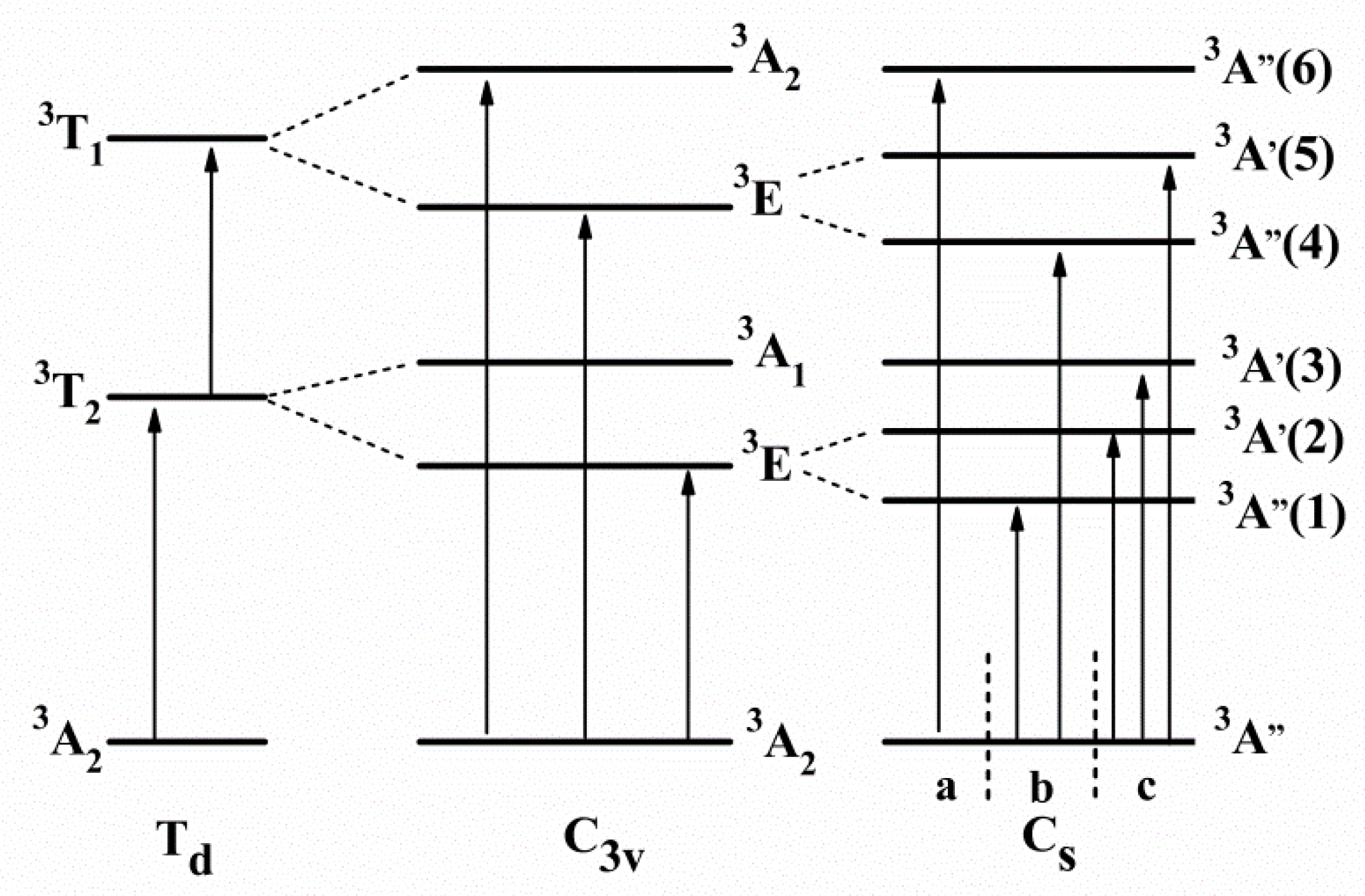

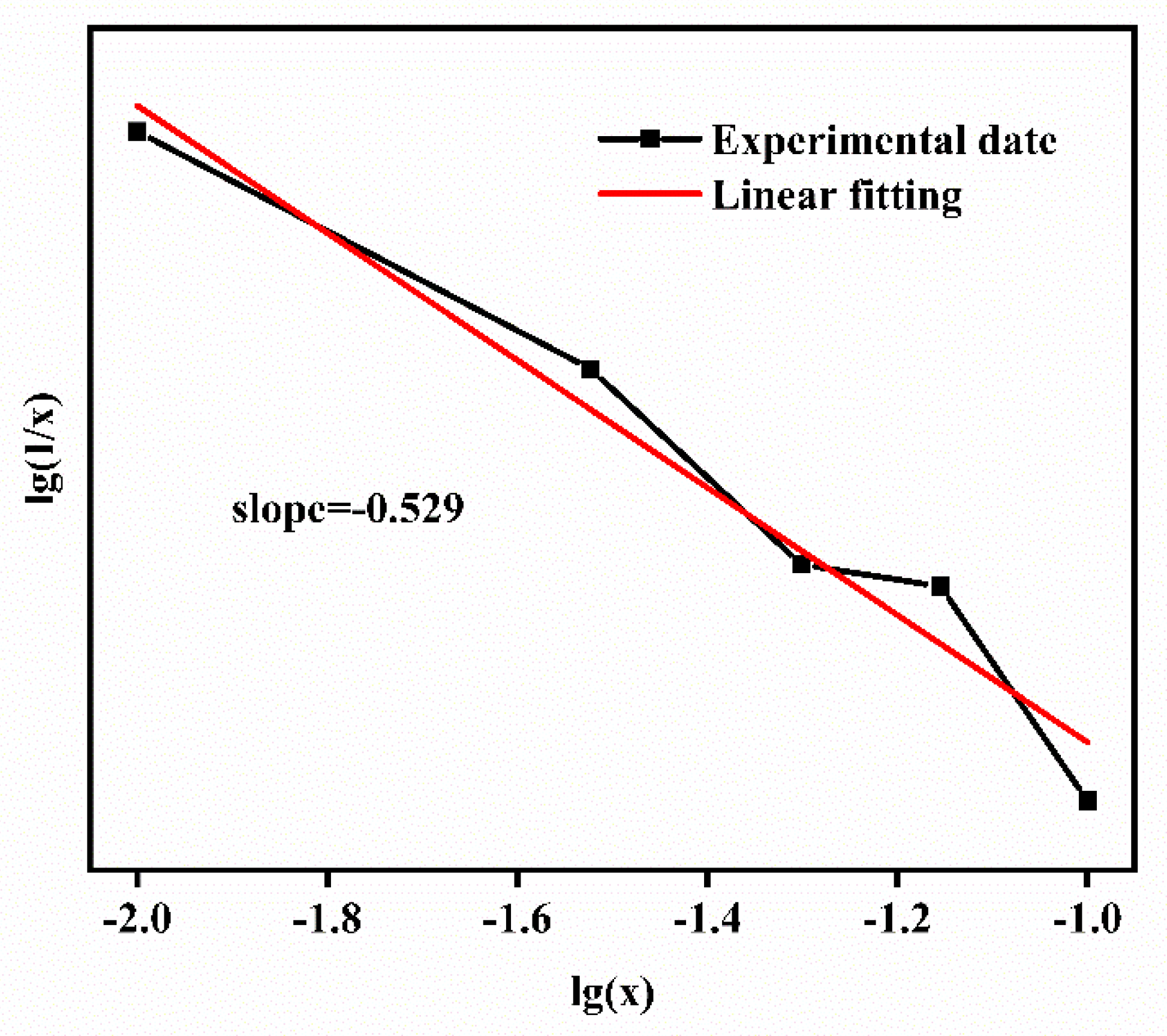


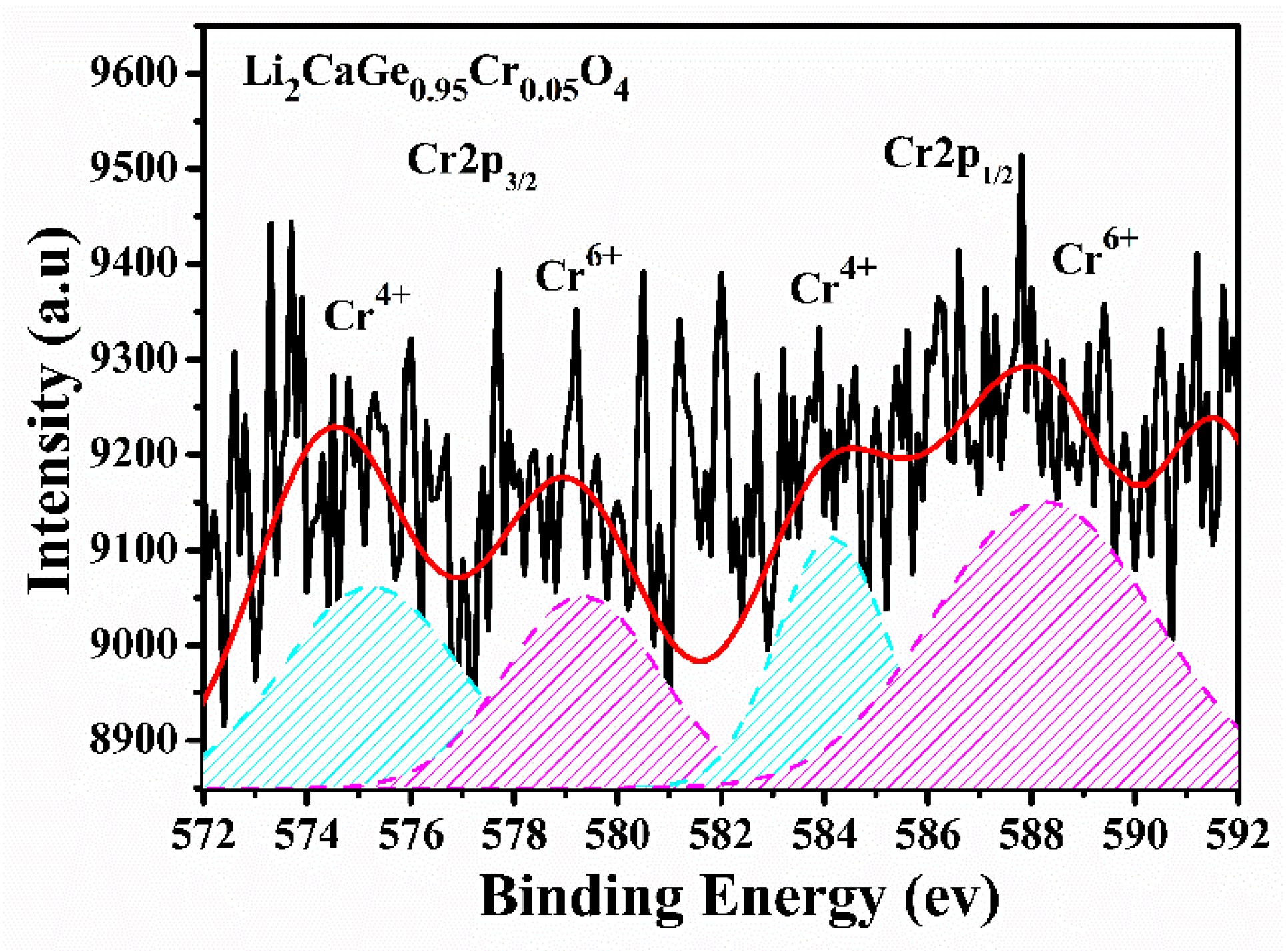

| Atom | Wyckoff Position | x/a | y/b | z/c | Chemical Valence |
|---|---|---|---|---|---|
| Li | 4d | 0 | 1/2 | 1/4 | 1 |
| Ca | 2b | 0 | 0 | 1/2 | 2 |
| Ge | 2a | 0 | 0 | 0 | 4 |
| O | 8i | 0.199 | 0.199 | 0.149 | −2 |
| Element | Peak BE |
|---|---|
| C1s | 284 |
| Cr2p | 579 |
| Ca2p | 346 |
| Ge3d | 31 |
| Li1s | 54 |
| O1s | 531 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhang, X.; Wang, X.; Leng, Z.; Yang, Q.; Ji, W.; Lin, H.; Zeng, F.; Li, C.; Su, Z. Investigation of the Structural and Luminescent Properties and the Chromium Ion Valence of Li2CaGeO4 Crystals Doped with Cr4+ Ions. Crystals 2020, 10, 1019. https://doi.org/10.3390/cryst10111019
Wang D, Zhang X, Wang X, Leng Z, Yang Q, Ji W, Lin H, Zeng F, Li C, Su Z. Investigation of the Structural and Luminescent Properties and the Chromium Ion Valence of Li2CaGeO4 Crystals Doped with Cr4+ Ions. Crystals. 2020; 10(11):1019. https://doi.org/10.3390/cryst10111019
Chicago/Turabian StyleWang, Dongmei, Xiaowei Zhang, Xinyu Wang, Zhuang Leng, Qianqian Yang, Wen Ji, Hai Lin, Fanming Zeng, Chun Li, and Zhongmin Su. 2020. "Investigation of the Structural and Luminescent Properties and the Chromium Ion Valence of Li2CaGeO4 Crystals Doped with Cr4+ Ions" Crystals 10, no. 11: 1019. https://doi.org/10.3390/cryst10111019
APA StyleWang, D., Zhang, X., Wang, X., Leng, Z., Yang, Q., Ji, W., Lin, H., Zeng, F., Li, C., & Su, Z. (2020). Investigation of the Structural and Luminescent Properties and the Chromium Ion Valence of Li2CaGeO4 Crystals Doped with Cr4+ Ions. Crystals, 10(11), 1019. https://doi.org/10.3390/cryst10111019




