Service Life Modeling of Concrete with SCMs Using Effective Diffusion Coefficient and a New Binding Model
Abstract
1. Introduction
2. Materials and Experimental Investigation
3. Methodology
3.1. Effective Diffusion Model
3.2. New Binding Model
3.3. Solution of the Transport Model
- Multiplying the residual (the differential equation) with a weighting function usually taken as the shape function, and integrating over the volume;
- Rearranging differential terms; and
- Applying divergence theorem.
4. Results and Discussion
4.1. Transport Model Using Experimental Binding Data
4.2. Transport Model Using the New Binding Model
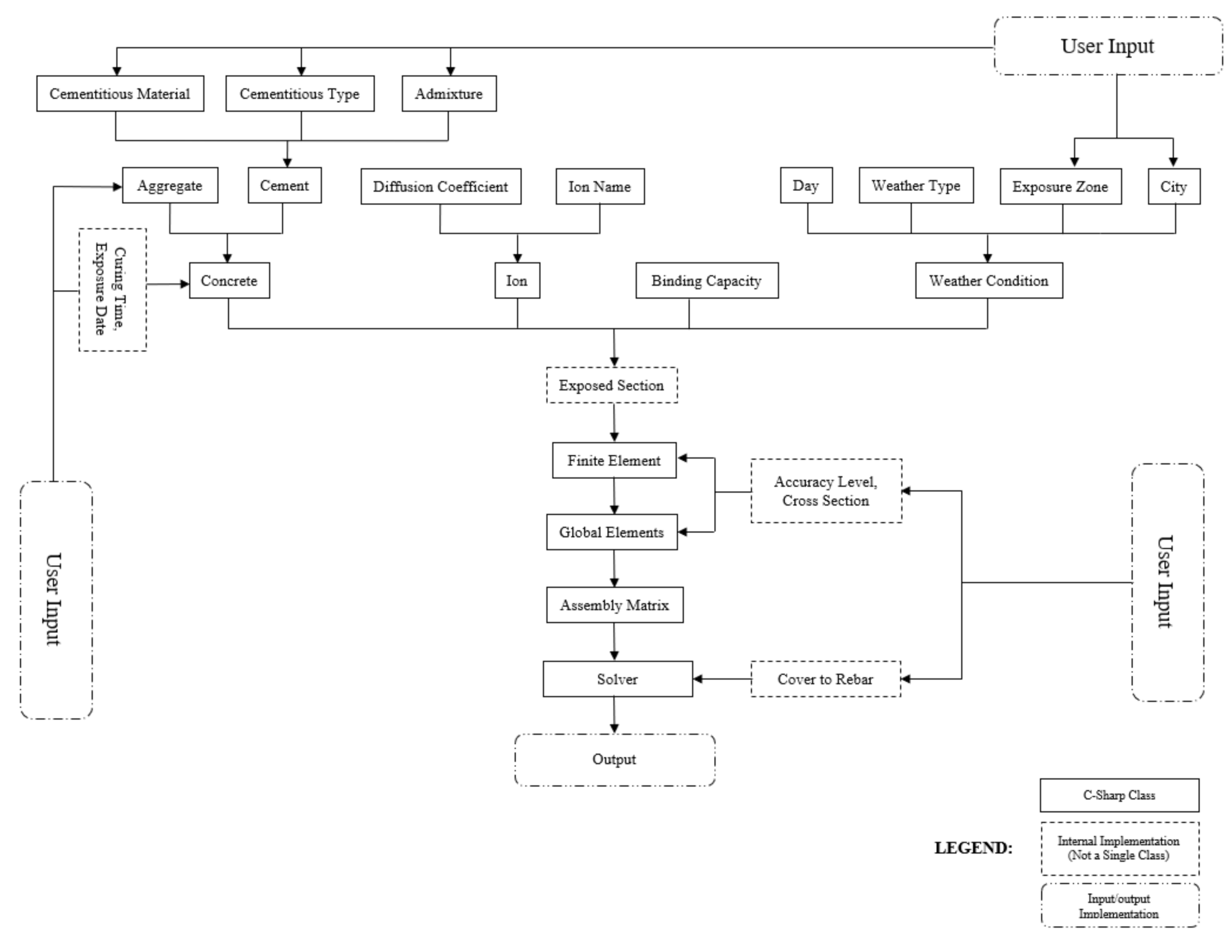
5. Software Implementation
5.1. Development of GUI
5.1.1. Weather Condition Details
5.1.2. Reinforced Concrete Properties and Member Geometric Details
5.2. Modeling Outputs
6. Conclusions
- ▪
- A reliable value of the effective diffusion coefficient is developed from the apparent diffusion coefficient by considering the binding capacity of concrete and other environmental impacts. The effective diffusivity decouples the impact of different environmental impact and embeds their effects in its calibration at each step of solution.
- ▪
- A reliable FEM transport model is developed to predict chlorides concentrations for concrete in tidal and harsh environments. The model uses solely calibrated diffusion coefficient and binding data in Fick’s law, and produces accurate results. Hence, the other terms in the transport laws, such as chemical activities and electrical flux, might not be valuable to the solution because the expected refinement against time of processing is marginal.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luping, T.; Nilsson, L.-O.; Basheer, P.A.M. Resistance of Concrete to Chloride Ingress, 1st ed.; Spon Press: London, UK, 2012. [Google Scholar]
- Bernal, J.; Fenaux, M.; Moragues, A.; Reyes, E.; Gálvez, J.C. Study of chloride penetration in concretes exposed to high-mountain weather conditions with presence of deicing salts. Constr. Build. Mater. 2016, 127, 971–983. [Google Scholar] [CrossRef]
- Kirkpatrick, T.J.; Weyers, R.E.; Anderson-Cook, C.M.; Sprinkel, M.M. Probabilistic model for the chloride-induced corrosion service life of bridge decks. Cem. Concr. Res. 2002, 32, 1943–1960. [Google Scholar] [CrossRef]
- Guzmán, S.; Gálvez, J.C.; Sancho, J.M. Modelling of chloride ingress into concrete through a single-ion approach: Application to an idealized surface crack pattern. Int. J. Numer. Anal. Methods Geomech. 2014, 38, 1683–1706. [Google Scholar] [CrossRef]
- Ababneh, A.; Xi, Y. An experimental study on the effect of chloride penetration on moisture diffusion in concrete. Mater. Struct. 2002, 35, 659–663. [Google Scholar] [CrossRef]
- Ababneh, A.; Benboudjema, F.; Xi, Y. Chloride penetration in Nonsaturated concrete. J. Mater. Civ. Eng. 2003, 15, 183–191. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Huang, Z.; Zhu, J.; Liu, W.; Zhang, W. Effect of Fly Ash as Cement Replacement on Chloride Diffusion, Chloride Binding Capacity, and Micro-Properties of Concrete in a Water Soaking Environment. Appl. Sci. 2020, 10, 6271. [Google Scholar] [CrossRef]
- Lee, T.; Lee, J. Evaluation of chloride resistance of early-strength concrete using blended binder and polycarboxylate-based chemical admixture. Appl. Sci. Switz. 2002, 10, 2972–2974. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.Z.; Zheng, J.J.; Ye, H.L.; Yang, J. Experimental investigation and analytical modeling of chloride diffusivity of fly ash concrete. Materials 2020, 13, 862. [Google Scholar] [CrossRef]
- Xi, Y.; Bažant, Z.P. Modeling Chloride Penetration in Saturated Concrete. J. Mater. Civ. Eng. 1999, 11, 58–65. [Google Scholar] [CrossRef]
- Samson, E.; Marchand, J.; Snyder, K.A.; Beaudoin, J.J. Modeling ion and fluid transport in unsaturated cement systems in isothermal conditions. Cem. Concr. Res. 2005, 35, 141–153. [Google Scholar] [CrossRef]
- Samson, E.; Marchand, J. Modeling the transport of ions in unsaturated cement-based materials. Comput. Struct. 2007, 85, 1740–1756. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Petković, J.; Dangla, P.; Baroghel-Bouny, V. Modelling of coupled ion and moisture transport in porous building materials. Constr. Build. Mater. 2008, 22, 2185–2195. [Google Scholar] [CrossRef]
- Saetta, A.V.; Scotta, R.V.; Vitaliani, R.V. Analysis of chloride diffusion into partially saturated concrete. ACI Mater. J. 1993, 90, 441–451. [Google Scholar]
- Samson, E.; Marchand, J. Numerical Solution of the Extended Nernst-Planck Model. J. Colloid Interface Sci. 1999, 215, 1–8. [Google Scholar] [CrossRef]
- Fenaux, M.; Reyes, E.; Gálvez, J.C.; Moragues, A. Modelling the transport of chloride and other ions in cement-based materials. Cem. Concr. Compos. 2019, 97, 33–42. [Google Scholar] [CrossRef]
- Luping, T.; Nilsson, L.O. Chloride Binding Capacity, Penetration and Pore Structures of Blended Cement Pastes with Slag and Fly Ash. In Proceedings of the Blended Cement in Construction, International Conference, University of Sheffield, Sheffield, UK, 9–12 September 1991. [Google Scholar]
- Tuutti, K. Corrosion of Steel in Concrete; Swedish Cement and Concrete Research Institute: Stockholm, Sweden, 1982. [Google Scholar]
- Blunk, G.; Gunkel, P.; Smolczyk, H.G. On the distribution of chloride between the hardening cement paste and its por solution. In Proceedings of the 8th International Congress on the Chemistry of Cement, Rio de Janeiro, Brazil, 22–27 September 1986; pp. 85–89. [Google Scholar]
- Tritthart, J. Chloride binding in cement I. Investigations to determine the composition of porewater in hardened cement. Cem. Concr. Res. 1989, 19, 586–594. [Google Scholar] [CrossRef]
- Tritthart, J. Chloride binding in cement II. The influence of the hydroxide concentration in the pore solution of hardened cement paste on chloride binding. Cem. Concr. Res. 1989, 19, 683–691. [Google Scholar] [CrossRef]
- Luping, T.; Nilsson, L.-O. Chloride binding capacity and binding isotherms of OPC pastes and mortars. Cem. Concr. Res. 1993, 23, 247–253. [Google Scholar] [CrossRef]
- Samson, E.; Lemaire, G.; Marchand, J.; Beaudoin, J.J. Modeling chemical activity effects in strong ionic solutions. Comput. Mater. Sci. 1999, 15, 285–294. [Google Scholar] [CrossRef]
- Pradelle, S.; Thiéry, M.; Baroghel-Bouny, V. Comparison of existing chloride ingress models within concretes exposed to seawater. Mater. Struct. Mater. Constr. 2016, 49, 4497–4516. [Google Scholar] [CrossRef]
- Riding, K.A.; Thomas, M.D.; Folliard, K.J. Apparent diffusivity model for concrete containing supplementary cementitious materials. ACI Mater. J. 2013, 110, 705–714. [Google Scholar]
- Abd El Fattah, A.; Al-Duais, I.; Riding, K.; Thomas, M.; Al-Dulaijan, S. Field Validation of Concrete Transport Property Measurement Methods. Materials 2020, 13, 1166. [Google Scholar] [CrossRef] [PubMed]
- Abd El Fattah, A.; Al-Duais, I.; Riding, K.; Thomas, M. Field evaluation of corrosion mitigation on reinforced concrete in marine exposure conditions. Constr. Build. Mater. 2018, 165, 663–674. [Google Scholar] [CrossRef]
- ASTM. Standard C150, 2020, Standard Specification for Portland Cement; ASTM International: West Conshohocken, PA, USA, 2020; Available online: https://www.astm.org/ (accessed on 10 September 2020).
- ASTM. Standard C1240, 2020, Standard Specification for Silica Fume Used in Cementitious Mixtures; ASTM International: West Conshohocken, PA, USA, 2020; Available online: https://www.astm.org/ (accessed on 10 September 2020).
- ASTM. Standard C618, 2019, Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2019; Available online: https://www.astm.org/ (accessed on 7 September 2020).
- ASTM. Standard C989, 2018, Standard Specification for Slag Cement for Use in Concrete and Mortars; ASTM International: West Conshohocken, PA, USA, 2018; Available online: https://www.astm.org/ (accessed on 7 September 2020).
- Thomas, M.D.; Scott, A.; Bremner, T.; Bilodeau, A.; Day, D. Performance of slag concrete in marine environment. ACI Mater. J. 2008, 105, 628. [Google Scholar]
- GAMRY. Referene 3000 Potentiostat. GAMRY Webpage. Available online: https://www.gamry.com/potentiostats/reference-3000/ (accessed on 12 September 2020).
- ASTM. Standard C1556, 2016 Standard Test Method for Determining the Apparent Chloride Diffusion Coefficient of Cementitious Mixtures by Bulk Diffusion; ASTM International: West Conshohocken, PA, USA, 2016; Available online: https://www.astm.org/ (accessed on 10 September 2020).
- Ngala, V.T.; Page, C.L. Effects of Carbonation on Pore Structure and Diffusional Properties of Hydrated Cem. Pastes 1997, 27, 995–1007. [Google Scholar]
- Moffatt, E.T.G.; Thomas, M.D. Effect of Carbonation on the Durability and Mechanical Performance of Ettringite-Based Binders. ACI Mater. J. 2019, 116. [Google Scholar] [CrossRef]
- Mangat, P.S.; Molloy, B.T. Prediction of long term chloride concentration in concrete. Mater. Struct. 1994, 27, 338–346. [Google Scholar] [CrossRef]
- Bamforth, P.B. CONCRETE DURABILIITY BY DESIGN: Limitations of the current prescriptive approach and alternative methods for durability design. In Proceedings of the NZ Concrete Society Conference, Taupo, New Zealand, 4–6 October 2002. [Google Scholar]
- Maage, M.; Helland, S.; Carlsen, J.E. Practical non-steady state chloride transport as a part of a model for predicting the initiation period. In Proceedings of the RILEM International Workshop on Chloride Penetration into Concrete, Saint-Rémy-lès-Chevreuse, France, 15–18 October 1995; pp. 398–406. [Google Scholar]
- Thomas, M.D.A.; Bamforth, P.B. Modelling chloride diffusion in concrete effect of fly ash and slag. Cem. Concr. Res. 1999, 29, 487–495. [Google Scholar] [CrossRef]
- Ehlen, M.; Thomas, M.D.; Bentz, E.C. Life-365 Service Life Prediction Model. Concr. Int. 2009, 31, 41–46. [Google Scholar]
- Thomas, M.D.A.; Hooton, R.D.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Zibara, H. Binding of External Chlorides by Cement Pastes; University of Toronto: Toronto, ON, Canada, 2001. [Google Scholar]
- Delagrave, A.; Marchand, J.; Ollivier, J.; Julien, S.; Hazrati, K. Chloride Binding Capacity of Various Hydrated Cement Paste Systems. Adv. Cem. Based Mater. 1997, 6, 28–35. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Tian, W.; Wei, J.; Yu, Q. Physically and chemically bound chlorides in hydrated cement pastes: A comparison study of the effects of silica fume and metakaolin. J. Mater. Sci. 2019, 54, 2152–2169. [Google Scholar] [CrossRef]
- Saillio, M.; Baroghel-Bouny, V.; Barberon, F. Chloride binding in sound and carbonated cementitious materials with various types of binder. Constr. Build. Mater. 2014, 68, 82–91. [Google Scholar] [CrossRef]
- Ogirigbo, O.R.; Black, L. Chloride binding and diffusion in slag blends: Influence of slag composition and temperature. Constr. Build. Mater. 2017, 149, 816–825. [Google Scholar] [CrossRef]
- Saillio, M.; Baroghel-Bouny, V.; Pradelle, S. Effect of carbonation and sulphate on chloride ingress in cement pastes and concretes with supplementary cementitious materials. In Proceedings of the 8th International Conference on Concrete under Severe Conditions–Environment and Loading, Lecco, Italy, 12–14 September 2016; Volume 711, pp. 241–248. [Google Scholar]
- Song, H.W.; Lee, C.H.; Jung, M.S.; Ann, K.Y. Development of chloride binding capacity in cement pastes and influence of the pH of hydration products. Can. J. Civ. Eng. 2008, 35, 1427–1434. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, Y.; Mu, S.; Chang, H.; Sun, W.; Jiang, J. Improving the chloride binding capacity of cement paste by adding nano-Al2O3. Constr. Build. Mater. 2019, 195, 415–422. [Google Scholar] [CrossRef]
- Ann, K.Y.; Hong, S.I. Modeling chloride transport in concrete at pore and chloride binding. ACI Mater. J. 2018, 115, 595–604. [Google Scholar] [CrossRef]
- Jung, M.S.; Kim, K.B.; Lee, S.A.; Ann, K.Y. Risk of chloride-induced corrosion of steel in SF concrete exposed to a chloride-bearing environment. Constr. Build. Mater. 2018, 166, 413–422. [Google Scholar] [CrossRef]
- Ann, K.Y.; Kim, T.S.; Kim, J.H.; Kim, S.H. The resistance of high alumina cement against corrosion of steel in concrete. Constr. Build. Mater. 2010, 24, 1502–1510. [Google Scholar] [CrossRef]
- Ramírez-Ortíz, A.E.; Castellanos, F.; de Cano-Barrita, P.F. Ultrasonic Detection of Chloride Ions and Chloride Binding in Portland Cement Pastes. Int. J. Concr. Struct. Mater. 2018, 12, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Gao, X.; Yu, R.; Huang, Y.; Cheng, S. Understanding the chloride binding and diffusion behaviors of marine concrete based on Portland limestone cement-alumina enriched pozzolans. Constr. Build. Mater. 2019, 198, 207–217. [Google Scholar] [CrossRef]
- Qiao, C.; Suraneni, P.; Ying, T.N.W.; Choudhary, A.; Weiss, J. Chloride binding of cement pastes with fly ash exposed to CaCl2 solutions at 5 and 23 °C. Cem. Concr. Compos. 2019, 97, 43–53. [Google Scholar] [CrossRef]
- Freundlich, H. Kapillarchemie: Eine Darstellung der Chemie der Kolloide und verwandter Gebiete; Akademische Verlagsgesellschaf: Leipzig, Germanny, 1909. [Google Scholar]
- Lee, J.S.; Ray, R.I.; Little, B.J. Comparison of Key West and Persian Gulf Seawaters. In Proceedings of the NACE–International Corrosion Conference Series, Nashville, TN, USA, 11–15 March 2007. [Google Scholar]
- Liu, Q.F.; Hu, Z.; Y, X.L.; Yang, J.; Azim, I.; Sun, W. Prediction of chloride distribution for offshore concrete based on statistical analysis. Materials 2020, 13, 174. [Google Scholar] [CrossRef] [PubMed]











| Mix | W/C | Cement kg/m3 | Coarse Aggregate kg/m3 | Sand kg/m3 | Water kg/m3 | Silica Fume kg/m3 | Fly Ash kg/m3 | Slag Cement kg/m3 | Notes |
|---|---|---|---|---|---|---|---|---|---|
| I | 0.4 | 340 | 1070 | 775 | 136 | - | - | - | Type OP/CEM 1 |
| V | 340 | 1070 | 775 | 136 | - | - | - | Type V | |
| SF | 320 | 1100 | 735 | 136 | 21 | - | - | OP + SF | |
| FA | 255 | 1090 | 735 | 136 | - | 85 | - | OP + FA | |
| SC | 100 | 1095 | 735 | 136 | - | - | 240 | OP + SC |
| Coastal | ||||||
|---|---|---|---|---|---|---|
| 6 Months | 12 Months | 24 Months | ||||
| β-Coefficient | R2 | β-Coefficient | R2 | β-Coefficient | R2 | |
| Mix 1 | 0.693 | 0.918 | 0.713 | 0.969 | 0.928 | 0.988 |
| Mix 2 | 0.737 | 0.990 | 0.905 | 0.996 | 0.937 | 0.994 |
| Mix 3 | 0.979 | 0.914 | 0.816 | 0.968 | 0.968 | 0.989 |
| Mix 4 | 0.701 | 0.992 | 0.837 | 0.991 | 0.941 | 0.996 |
| Mix 5 | 0.714 | 0.850 | 0.776 | 0.990 | 0.962 | 0.980 |
| Tidal | ||||||
| 6 Months | 12 Months | 24 Months | ||||
| β-Coefficient | R2 | β-Coefficient | R2 | β-Coefficient | R2 | |
| Mix 1 | 0.789 | 0.974 | 0.806 | 0.983 | 0.895 | 0.972 |
| Mix 2 | 0.612 | 0.957 | 0.851 | 0.985 | 0.920 | 0.988 |
| Mix 3 | 0.864 | 0.978 | 0.959 | 0.978 | 0.965 | 0.980 |
| Mix 4 | 0.650 | 0.978 | 0.725 | 0.994 | 0.779 | 0.966 |
| Mix 5 | 0.758 | 0.955 | 0.795 | 0.965 | 0.761 | 0.907 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oluwaseun Azeez, M.; Abd El Fattah, A. Service Life Modeling of Concrete with SCMs Using Effective Diffusion Coefficient and a New Binding Model. Crystals 2020, 10, 967. https://doi.org/10.3390/cryst10110967
Oluwaseun Azeez M, Abd El Fattah A. Service Life Modeling of Concrete with SCMs Using Effective Diffusion Coefficient and a New Binding Model. Crystals. 2020; 10(11):967. https://doi.org/10.3390/cryst10110967
Chicago/Turabian StyleOluwaseun Azeez, Mukhtar, and Ahmed Abd El Fattah. 2020. "Service Life Modeling of Concrete with SCMs Using Effective Diffusion Coefficient and a New Binding Model" Crystals 10, no. 11: 967. https://doi.org/10.3390/cryst10110967
APA StyleOluwaseun Azeez, M., & Abd El Fattah, A. (2020). Service Life Modeling of Concrete with SCMs Using Effective Diffusion Coefficient and a New Binding Model. Crystals, 10(11), 967. https://doi.org/10.3390/cryst10110967





