Preparation of Cellulose Nanocrystal-Reinforced Physical Hydrogels for Actuator Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose Nanocrystal
2.3. Preparation of Cellulose and PVA Solutions
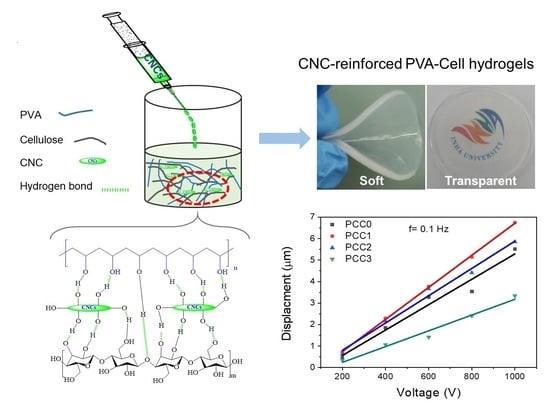
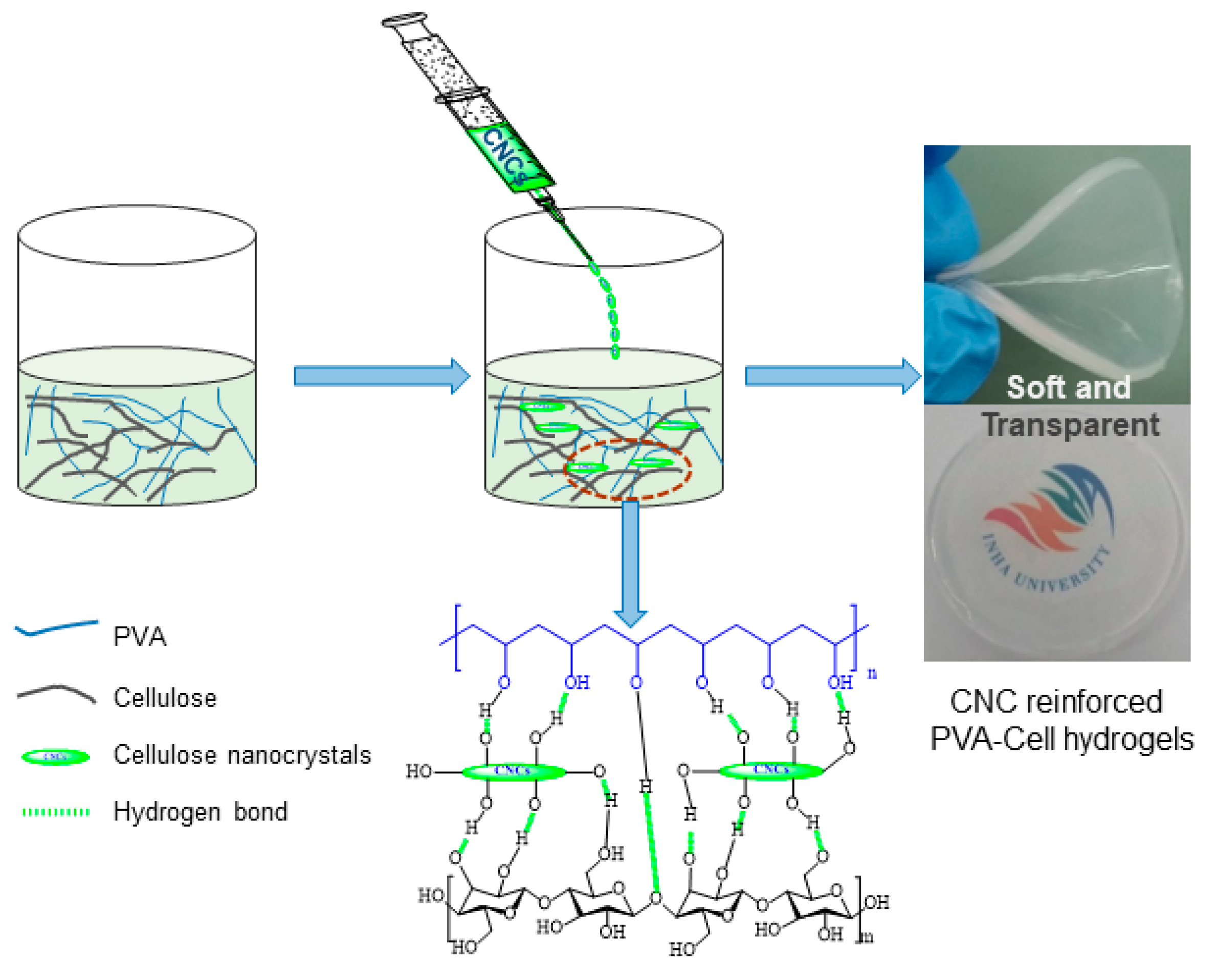
2.4. Preparation of CNC-Reinforced PVA-Cell Hydrogels
3. Characterization
Actuation Test
4. Results and Discussion
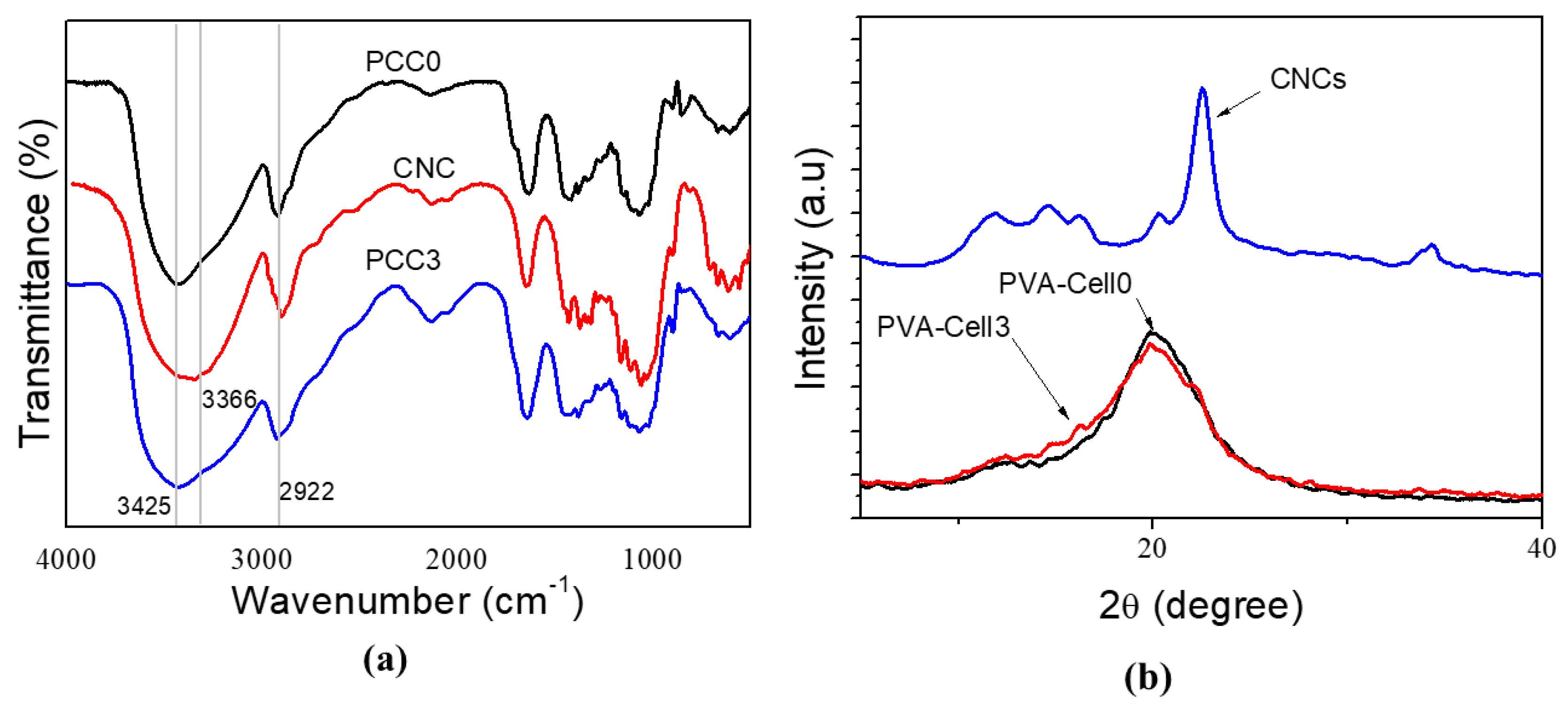
4.1. FTIR Analysis
4.2. XRD Studies
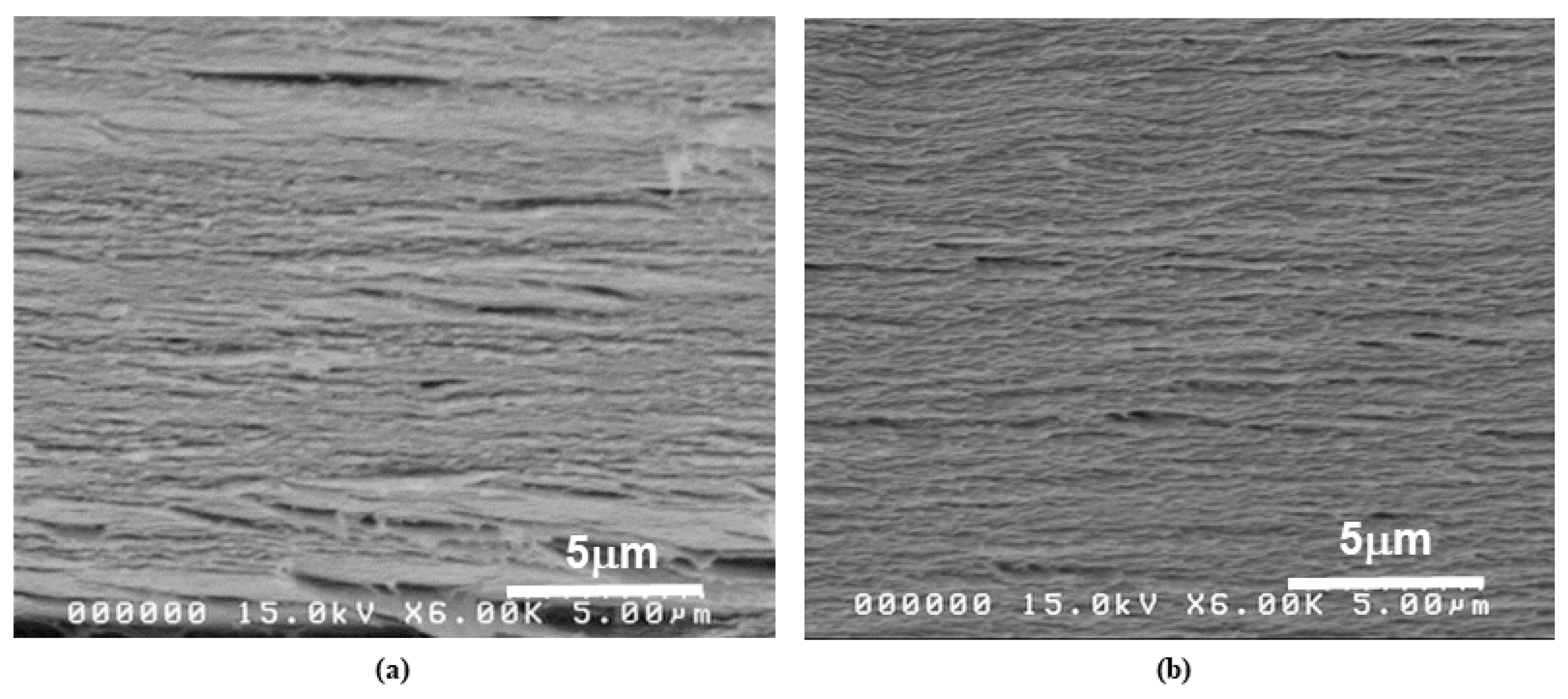
4.3. Surface Morphology
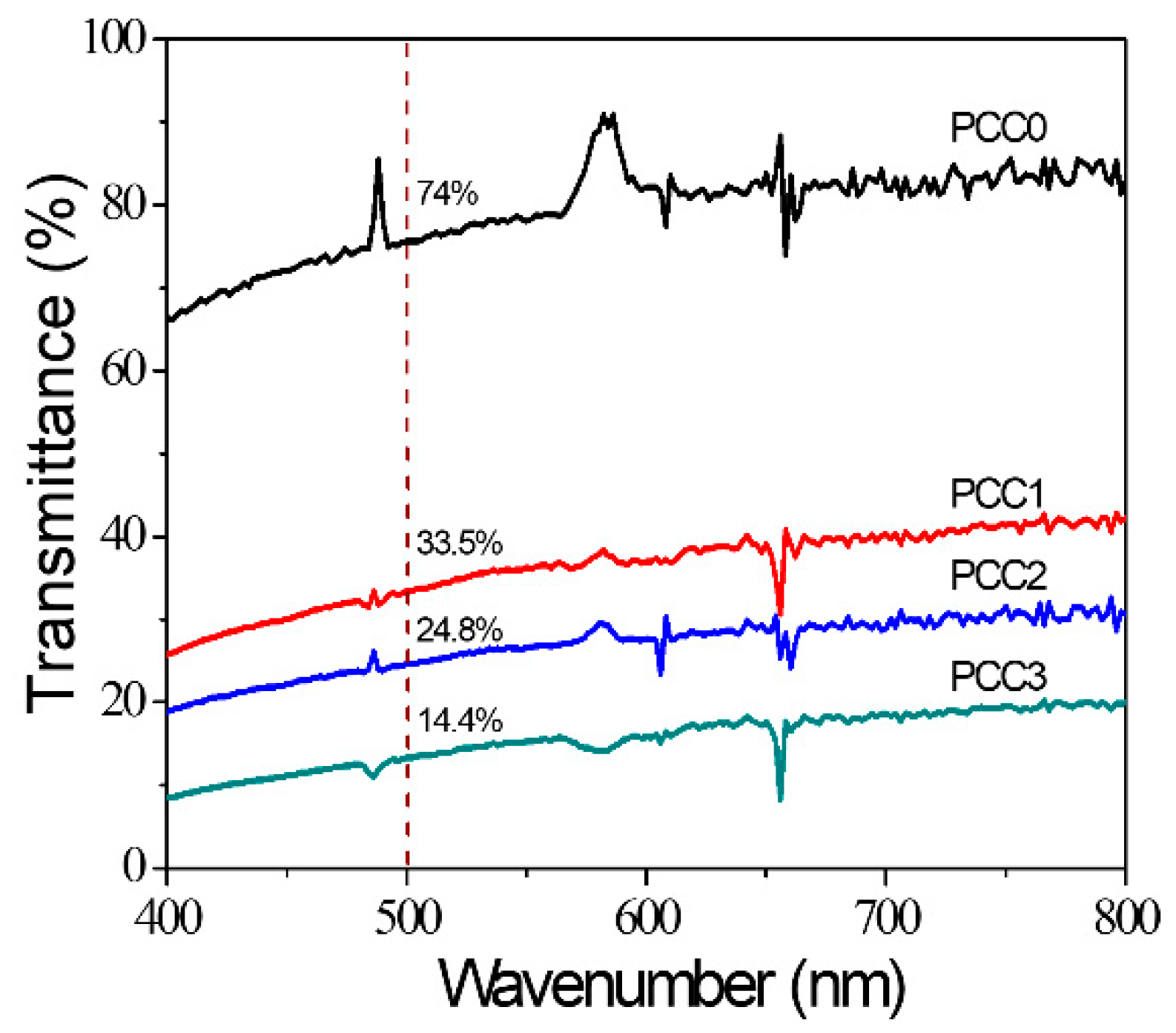
4.4. Optical Transparency
4.5. Mechanical Properties and Swelling Behaviors
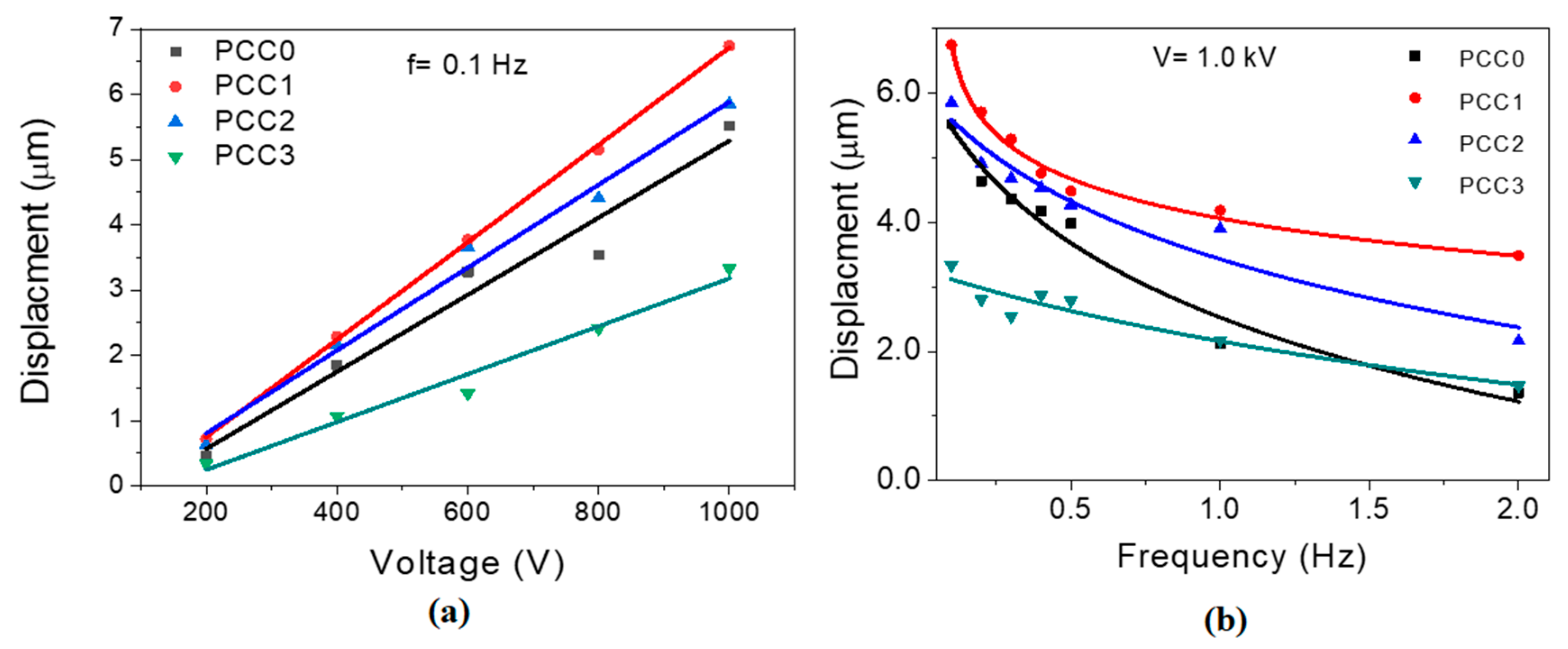
4.6. Actuation Test
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reddy, N.N.; Mohan, Y.M.; Varaprasad, K.; Ravindra, S.; Joy, P.A.; Raju, K.M. Magnetic and electric responsive hydrogel–magnetic nanocomposites for drug-delivery application. J. Appl. Polym. Sci. 2011, 122, 1364–1375. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.W.; Kim, H.C.; Zhai, L.; Ko, H.-U. Review of Soft Actuator Materials. Int. J. Precis. Eng. Manuf. 2019, 20, 2221–2241. [Google Scholar] [CrossRef] [Green Version]
- Belviso, B.D.; Caliandro, R.; Salehi, S.M.; Di Profio, G.; Caliandro, R. Protein Crystallization in Ionic-Liquid Hydrogel Composite Membranes. Crystals 2019, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Kruusamäe, K.; Punning, A.; Aabloo, A.; Asaka, K. Self-Sensing Ionic Polymer Actuators: A Review. Actuators 2015, 4, 17–38. [Google Scholar] [CrossRef]
- Bounabi, L.; Mokhnachi, N.B.; Haddadine, N.; Ouazib, F.; Barille, R. Development of poly(2-hydroxyethyl methacrylate)/clay composites as drug delivery systems of paracetamol. J. Drug Deliv. Sci. Technol. 2016, 33, 3358–3365. [Google Scholar] [CrossRef] [Green Version]
- Cancian, G.; Tozzi, G.; Hussain, A.; De Mori, A.; Roldo, M. Carbon nanotubes play an important role in the spatial arrangement of calcium deposits in hydrogels for bone regeneration. J. Mater. Sci. Mater. Med. 2016, 27, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaramudu, T.; Raghavendra, G.M.; Varaprasad, K.; Sadiku, R.; Raju, K.M. Development of novel biodegradable Au nanocomposite hydrogels based on wheat: For inactivation of bacteria. Carbohydr. Polym. 2013, 92, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Jayaramudu, T.; Raghavendra, G.M.; Varaprasad, K.; Raju, K.M.; Sadiku, R.; Kim, J. 5-Fluorouracil encapsulated magnetic nanohydrogels for drug-delivery applications. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Kim, J.-H.; Shim, B.S.; Kim, H.S.; Lee, Y.-J.; Min, S.-K.; Jang, D.; Abas, Z.; Kim, J. Review of nanocellulose for sustainable future materials. Int. J. Precis. Eng. Manuf. Green Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Palomero, C.; Soriano, M.L.; Valcárcel, M. Nanocellulose as analyte and analytical tool: Opportunities and challenges. TrAC Trends Anal. Chem. 2017, 87, 1–18. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Hou, Q.; Liu, R.; Wu, T.; Si, C. Further characterization of cellulose nanocrystal (CNC) preparation from sulfuric acid hydrolysis of cotton fibers. Cellulose 2016, 23, 439–450. [Google Scholar] [CrossRef]
- Abitbol, T.; Kloser, E.; Gray, D.G. Estimation of the surface sulfur content of cellulose nanocrystals prepared by sulfuric acid hydrolysis. Cellulose 2013, 20, 785–794. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nature Rev. Mater. 2020. [Google Scholar] [CrossRef]
- Xu, S.; Girouard, N.; Schueneman, G.; Shofner, M.L.; Meredith, J.C. Mechanical and thermal properties of waterborne epoxy composites containing cellulose nanocrystals. Polymers 2013, 54, 6589–6598. [Google Scholar] [CrossRef]
- Ooi, S.Y.; Ahmad, I.; Amin, M.C.I.M. Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatin hydrogels for use in controlled drug delivery systems. Ind. Crops Prod. 2016, 93, 227–234. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.-M.; Xu, F. Design of cellulose nanocrystals template-assisted composite hydrogels: Insights from static to dynamic alignment. Macromolecules 2015, 48, 1231–1239. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Waeijen, H.A.; Berry, R.M.; Tam, K.C. Continuous flow adsorption of methylene blue by cellulose nanocrystal-alginate hydrogel beads in fixed bed columns. Carbohydr. Polym. 2016, 136, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Sadasivuni, K.K.; Ponnamma, D.; Ko, H.-U.; Zhai, L.; Kim, H.-C.; Kim, J. Electroactive and optically adaptive bionanocomposite for reconfigurable microlens. J. Phys. Chem. B 2016, 120, 4699–4705. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-U.; Kim, H.C.; Kim, J.W.; Zhai, L.; Jayaramudu, T.; Kim, J. Fabrication and characterization of cellulose nanocrystal based transparent electroactive polyurethane. Smart Mater. Struct. 2017, 26, 085012. [Google Scholar] [CrossRef]
- Gao, X.; Sasasivuni, K.K.; Kim, H.-C.; Min, S.-K.; Kim, J. Designing pH-responsive and dielectric hydrogels from cellulose nanocrystals. J. Chem. Sci. 2015, 127, 1119–1125. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Ko, H.-U.; Zhai, L.; Li, Y.; Kim, J. Preparation and characterization of hydrogels from polyvinyl alcohol and cellulose and their electroactive behavior. Soft Mater. 2017, 15, 64–72. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Ko, H.-U.; Kim, H.C.; Kim, J.W.; Muthoka, R.M.; Kim, J. Electroactive hydrogels made with polyvinyl alcohol/cellulose nanocrystals. Materials 2018, 11, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, M.; Mun, S.; Hyun, J.; Kim, J. Synthesis and characterization of iron oxide/cellulose nanocomposite film. Int. J. Biol. Macromol. 2015, 74, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Jayaramudu, T.; Li, Y.; Ko, H.-U.; Shishir, I.R.; Kim, J. Poly(acrylic acid)-poly(vinyl alcohol) hydrogels for reconfigurable lens actuators. Int. J. Precis. Eng. Manuf. Green Technol. 2016, 3, 375–379. [Google Scholar] [CrossRef]
- Raghunathan, S.P.; Narayanan, S.; Poulose, A.C.; Joseph, R. Flexible regenerated cellulose/polypyrrole composite films with enhanced dielectric properties. Carbohydr. Polym. 2016, 157, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis.; functional properties and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Murthy, P.S.K.; Murali Mohan, Y.; Varaprasad, K.; Sreedhar, B.; Mohana Raju, K. First successful design of semi-IPN hydrogel–silver nanocomposites: A facile approach for antibacterial application. J. Colloid Interface Sci. 2008, 318, 217–224. [Google Scholar] [CrossRef]
- Qi, X.; Hu, X.; Wei, W.; Yu, H.; Li, J.; Zhang, J.; Dong, W. Investigation of Salecan/poly (vinyl alcohol) hydrogels prepared by freeze/thaw method. Carbohydr. Polym. 2015, 118, 60–69. [Google Scholar] [CrossRef]
- Tanpichai, S.; Oksman, K. Cross-linked nanocomposite hydrogels based on cellulose nanocrystals and PVA: Mechanical properties and creep recovery. Compos. Part A Appl. Sci. Manuf. 2016, 88, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Shin, W.H.; Min, M.S.; Lee, T.H.; Jho, J.Y.; Rhee, K. Computational simulation of spirally coiled deformation of a Bi-Layered hydrogel strip induced by swelling. Int. J. Precis. Eng. Manuf. 2015, 16, 409–412. [Google Scholar] [CrossRef]

| Hydrogel Ccode | PVA Solution (3 wt.%) g | Cellulose Solution (1.5 wt.%) g | 1 wt.% CNC mL | Swelling Ratio (Sg/g) | Compressive Modulus kPa |
|---|---|---|---|---|---|
| PCC0 | 50 | 50 | - | 13.2 | 99.1 |
| PCC1 | 50 | 50 | 1 | 14.8 | 65.8 |
| PCC2 | 50 | 50 | 2 | 14.6 | 66.3 |
| PCC3 | 50 | 50 | 3 | 13.4 | 88.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Jayaramudu, T.; Zhai, L.; Kim, H.C.; Agumba, D.O. Preparation of Cellulose Nanocrystal-Reinforced Physical Hydrogels for Actuator Application. Crystals 2020, 10, 969. https://doi.org/10.3390/cryst10110969
Kim J, Jayaramudu T, Zhai L, Kim HC, Agumba DO. Preparation of Cellulose Nanocrystal-Reinforced Physical Hydrogels for Actuator Application. Crystals. 2020; 10(11):969. https://doi.org/10.3390/cryst10110969
Chicago/Turabian StyleKim, Jaehwan, Tippabattini Jayaramudu, Lindong Zhai, Hyun Chan Kim, and Dickens Owino Agumba. 2020. "Preparation of Cellulose Nanocrystal-Reinforced Physical Hydrogels for Actuator Application" Crystals 10, no. 11: 969. https://doi.org/10.3390/cryst10110969
APA StyleKim, J., Jayaramudu, T., Zhai, L., Kim, H. C., & Agumba, D. O. (2020). Preparation of Cellulose Nanocrystal-Reinforced Physical Hydrogels for Actuator Application. Crystals, 10(11), 969. https://doi.org/10.3390/cryst10110969