Synthesis and Visualization of a Novel Fluorescent-Tagged Polymeric Antiscalant during Gypsum Crystallization in Combination with Bisphosphonate Fluorophore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
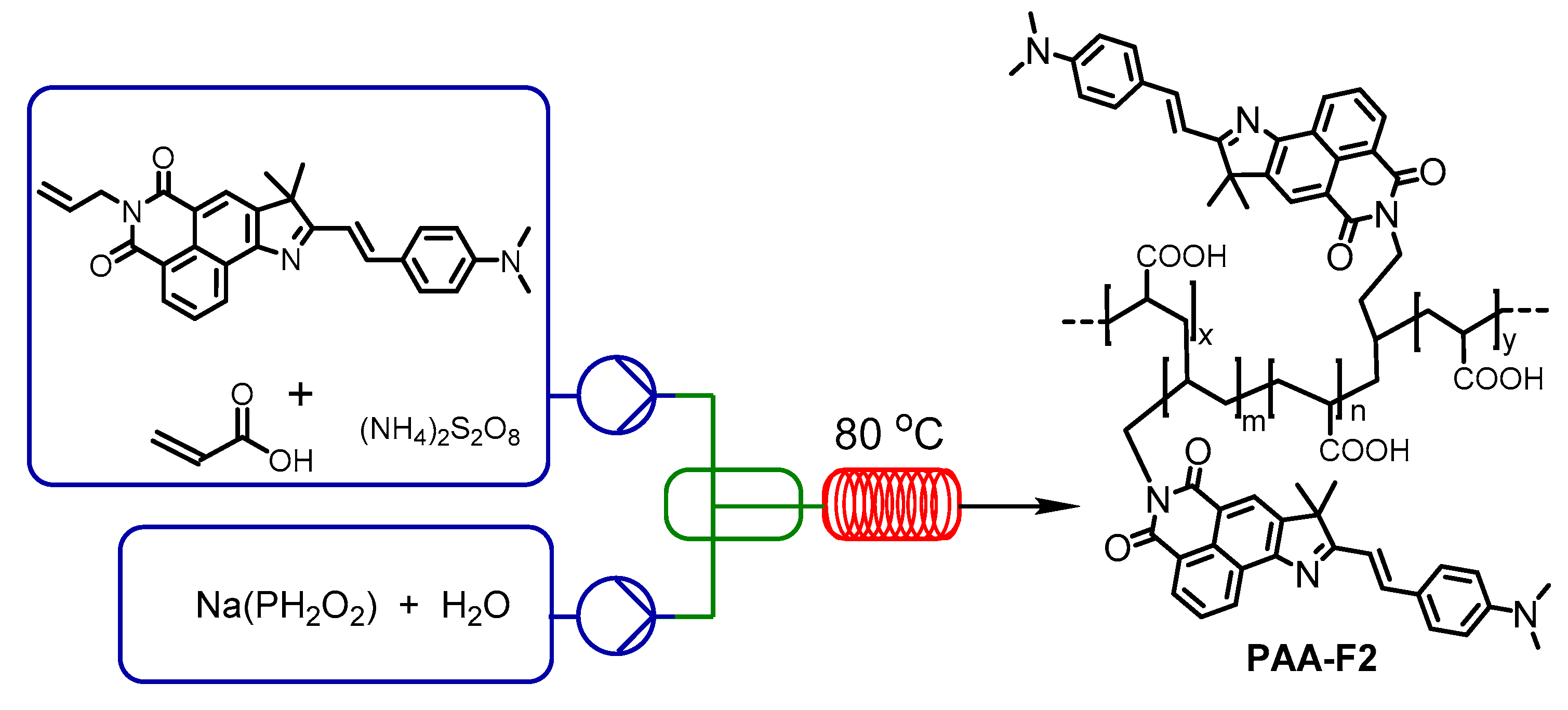
2.2. PAA-F2 Synthesis
2.3. Gypsum Scale Formation Procedure and Antiscalant Efficacy Testing
2.4. Methods
3. Results and Discussion
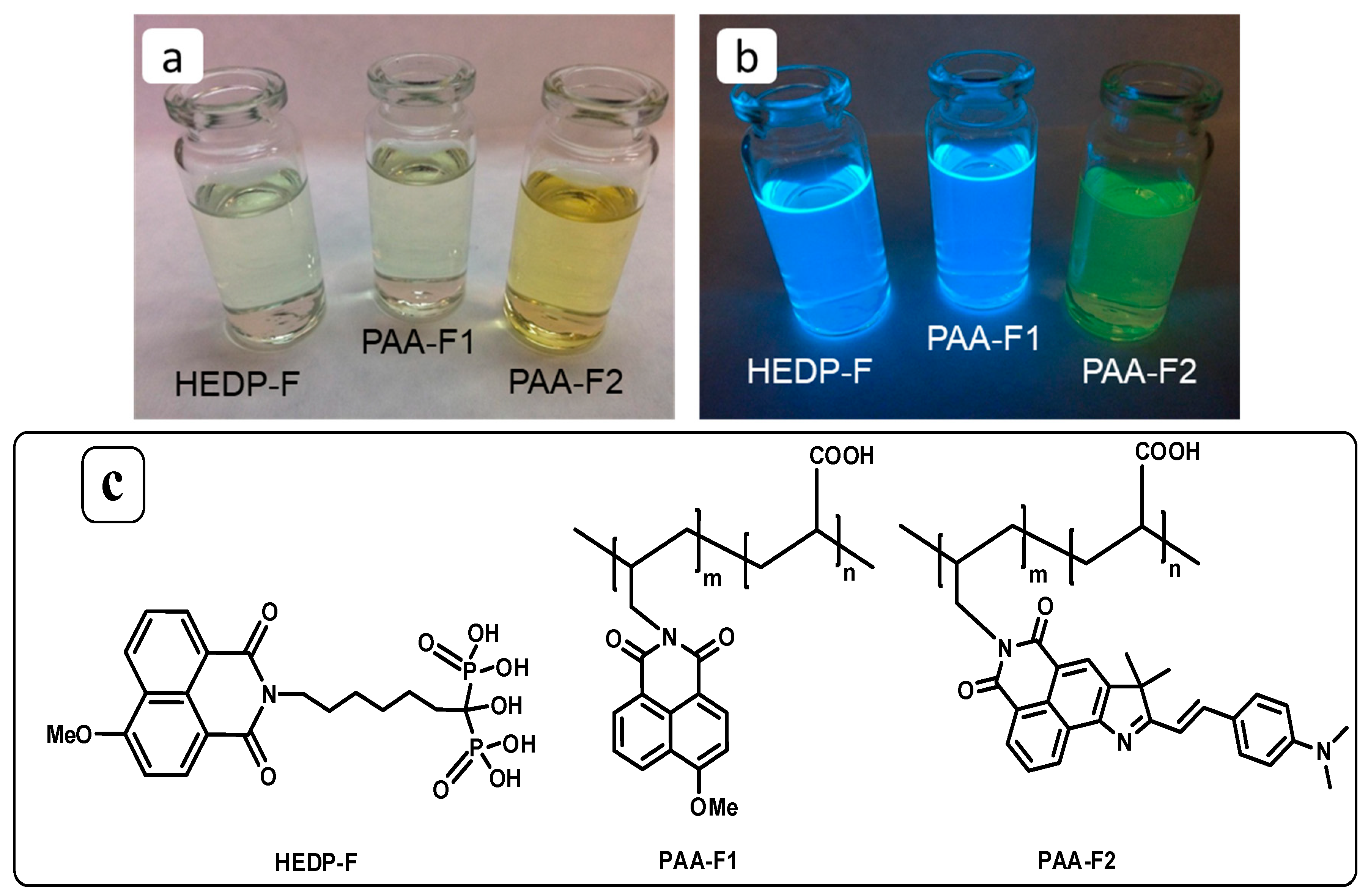
3.1. Synthesis of the PAA-F2
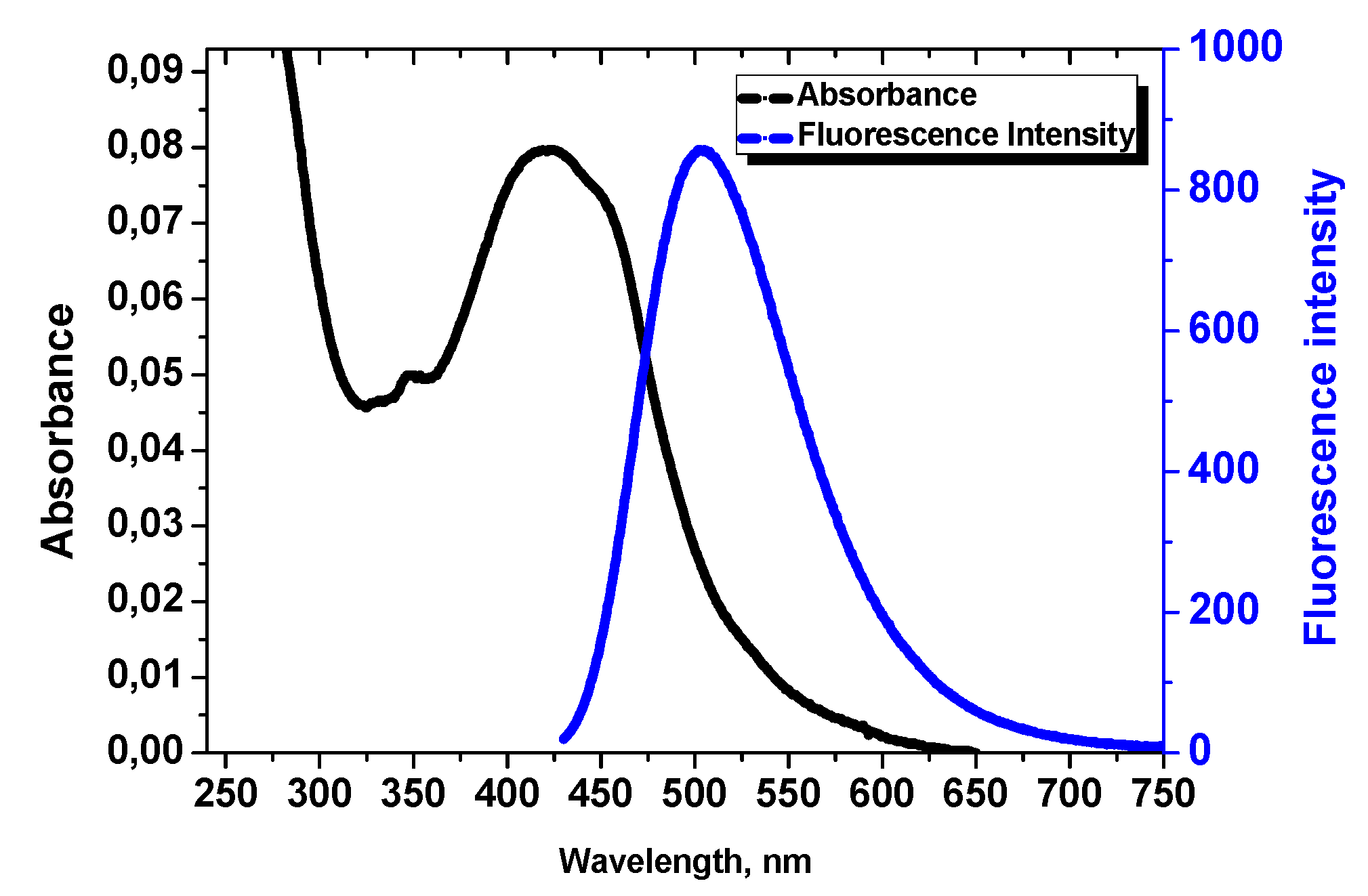
3.2. Excitation and Emission Properties of PAA-F2
3.3. Scale Inhibition Performance of PAA-F2, HEDP-F, and of a PAA-F2/HEDP-F Blend
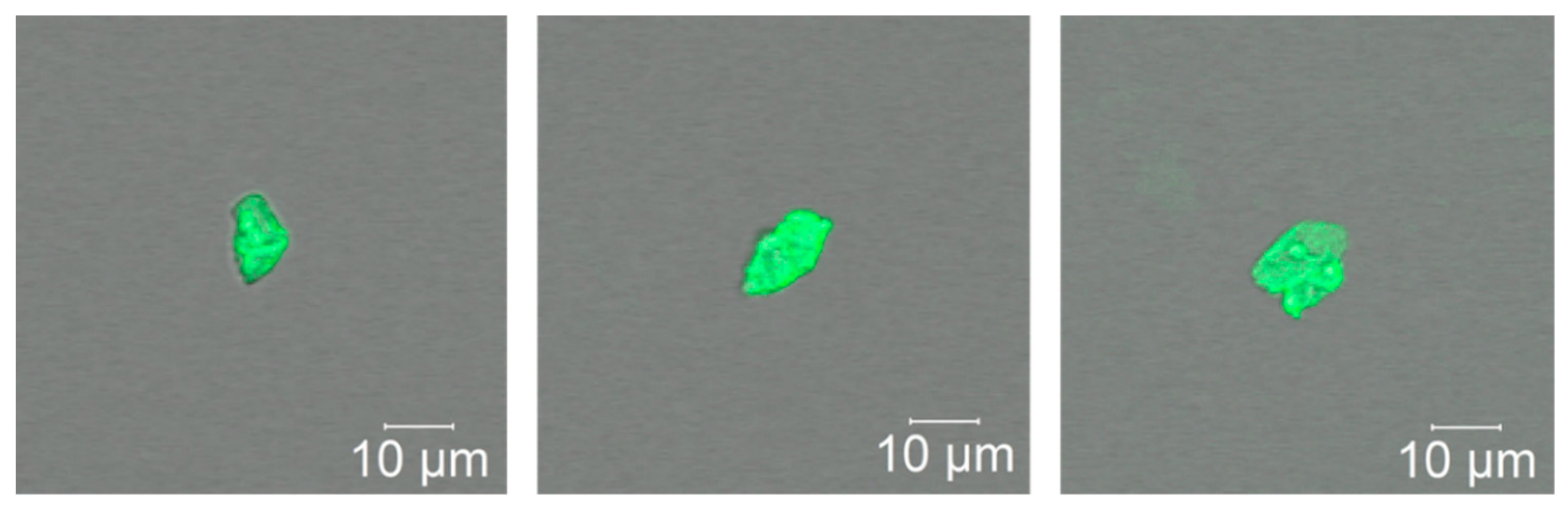
3.4. Visualization of PAA-F2 in Calcium Brine
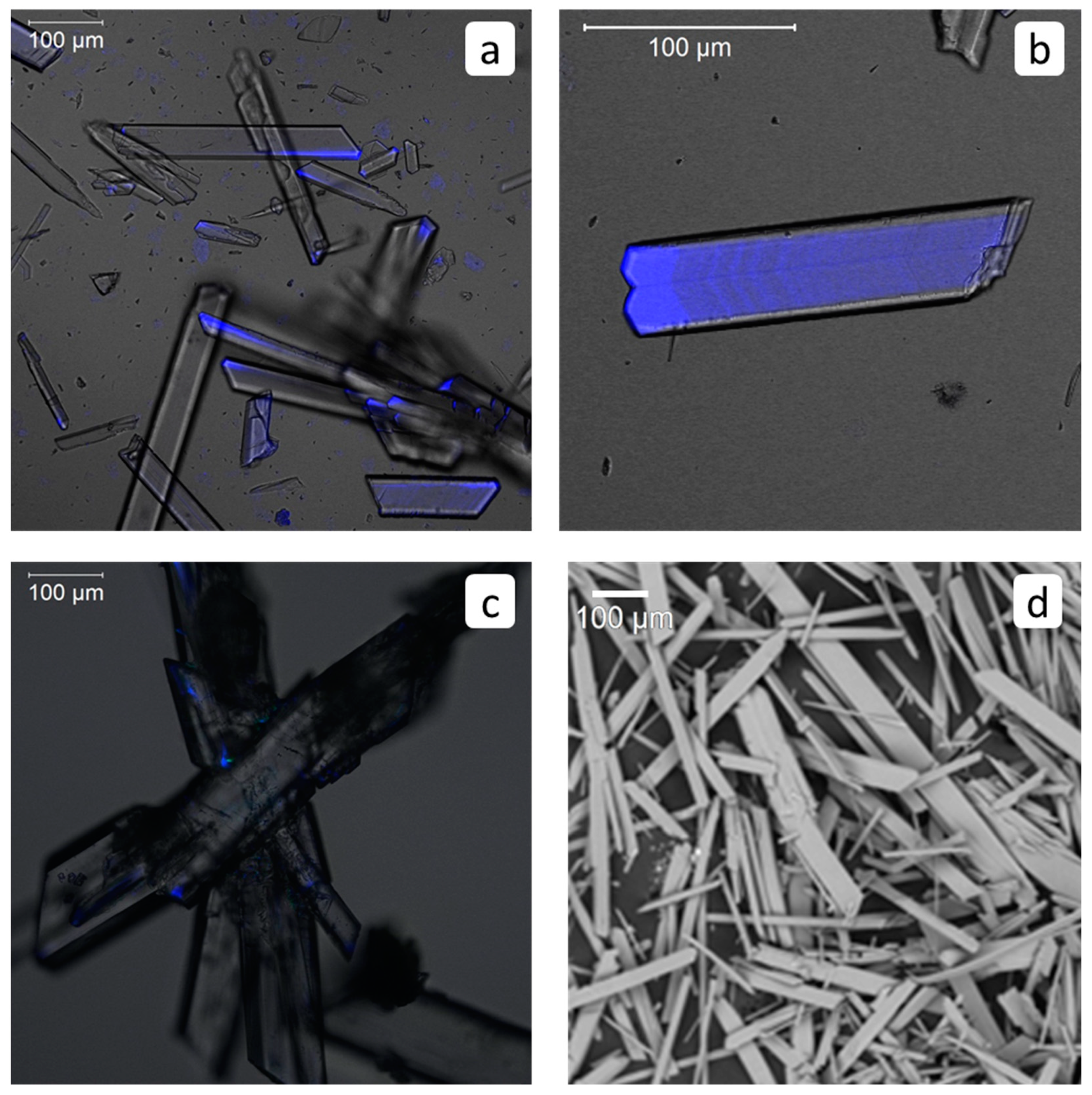
3.5. Visualization of HEDP-F in a Binary Gypsum-HEDP-F System
3.6. Visualization of PAA-F2 in a Binary Gypsum-PAA-F2 System
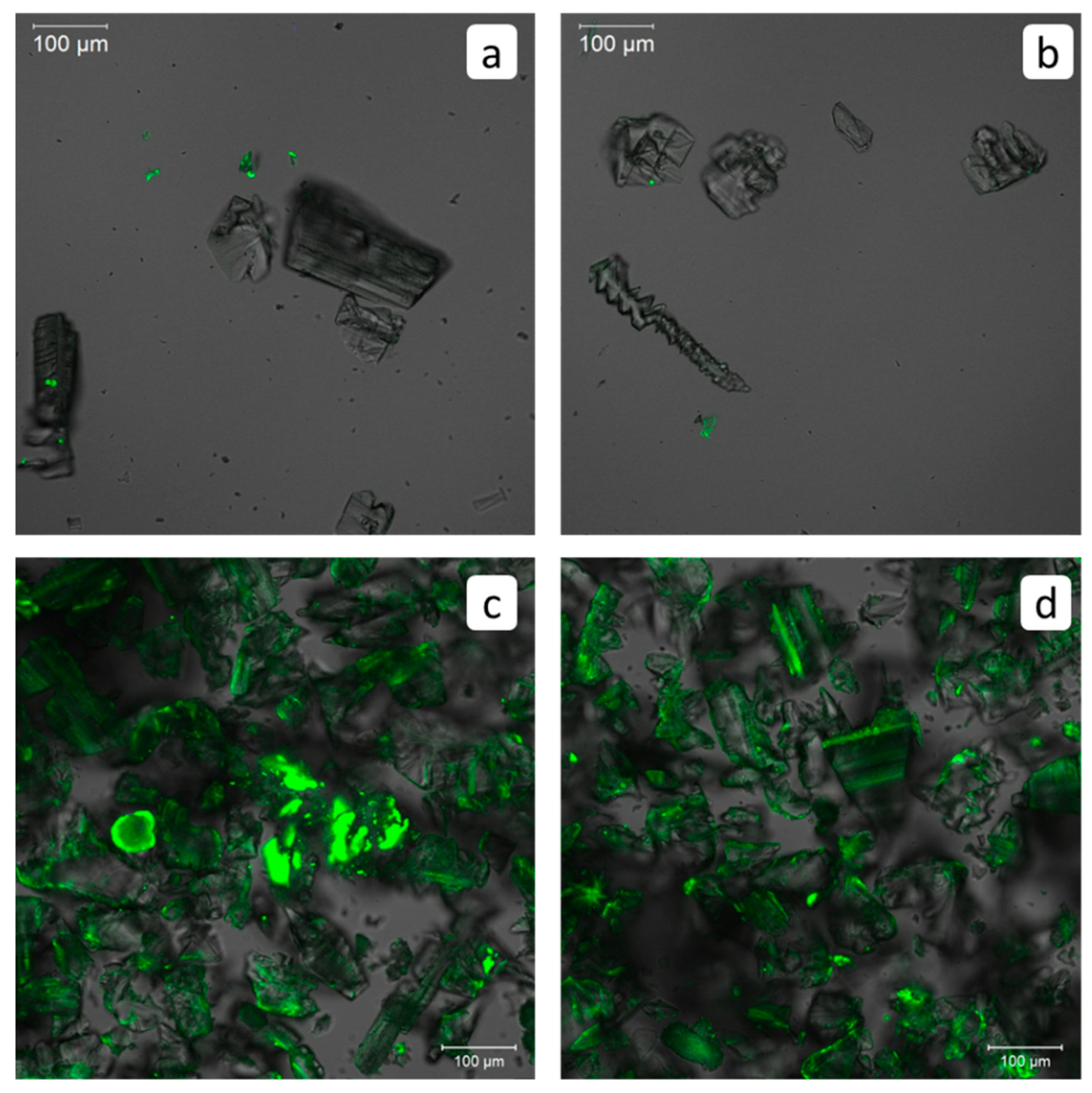
3.7. Visualization of PAA-F2/HEDP-F Blend after Gypsum Scale Formation in a Triple System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Shemer, H.; Hasson, D.; Semiat, R. Characterization of the effectiveness of anti-scalants in suppressing scale deposition on a heated surface. Desalination 2016, 397, 38–42. [Google Scholar] [CrossRef]
- Matin, A.; Rahman, F.; Shafi, H.Z.; Zubair, S.M. Scaling of reverse osmosis membranes used in water desalination: Phenomena, impact, and control; future directions. Desalination 2019, 455, 135–157. [Google Scholar] [CrossRef]
- Mady, M.F.; Fevang, S.; Kelland, M.A. Study of Novel Aromatic Aminomethylenephosphonates as Oilfield Scale Inhibitors. Energy Fuels 2018, 33, 228–237. [Google Scholar] [CrossRef]
- Hoang, T.A. Mechanisms of Scale Formation and Inhibition. Mineral Scales and Deposits, Scientific and Technological Approaches, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 47–83. [Google Scholar]
- Amjad, Z. Scale inhibitor blends for industrial water systems. Mater. Perform. 2019, 58, 38–41. [Google Scholar]
- Amjad, Z.; Landgraf, R.; Penn, J. Calcium sulfate dihydrate (gypsum) scale inhibition by PAA, PAPEMP, and PAA/PAPEMP blend. Int. J. Corros. Scale Inhib. 2014, 3, 35–47. [Google Scholar] [CrossRef]
- Shaw, S.S.; Sorbie, K. Synergistic Properties of Phosphonate and Polymeric Scale-Inhibitor Blends for Barium Sulfate Scale Inhibition. SPE Prod. Oper. 2015, 30, 16–25. [Google Scholar] [CrossRef]
- Ou, H.-H.; Hsieh, L.-H.C. A synergistic effect of sodium gluconate and 2-phosphonobutane-1,2,4-tricarboxylic acid on the inhibition of CaCO 3 scaling formation. Powder Technol. 2016, 302, 160–167. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Kamagurov, S.; Tkachenko, S.; Popov, K.; Ryabova, A. An Insight into the Mechanisms of the Scale Inhibition. A Case Study of a Novel Task-specific Fluorescent-tagged Scale Inhibitor Location on Gypsum Crystals. ChemNanoMat 2019, 5, 586–592. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Golovesov, V.; Ryabova, A.; Tkachenko, S.; Redchuk, A.; Rönkkömäki, H.; Rudakova, G.; Pervov, A.; Popov, K. Visualization of a Novel Fluorescent-tagged Bisphosphonate in Reverse Osmosis Facility During Gypsum Brine Desalination. Sep. Sci. Technol. 2020, 255, 117382. [Google Scholar]
- Zhang, Z.; Lu, M.; Liu, J.; Chen, H.; Chen, Q.; Wang, B. Fluorescent-tagged hyper-branched polyester for inhibition of CaSO4 scale and the scale inhibition mechanism. Mater. Today Commun. 2020, 25, 101359. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Golovesov, V.; Ryabova, A.; Redchuk, A.; Tkachenko, S.; Pervov, A.; Popov, K. Gypsum Crystallization during Reverse Osmosis Desalination of Water with High Sulfate Content in Presence of a Novel Fluorescent-Tagged Polyacrylate. Crystals 2020, 10, 309. [Google Scholar] [CrossRef] [Green Version]
- Asraf-Snir, M.; Gilron, J.; Oren, Y. Scaling of cation exchange membranes by gypsum during Donnan exchange and electrodialysis. J. Membr. Sci. 2018, 567, 28–38. [Google Scholar] [CrossRef]
- Rahman, F. Calcium sulfate precipitation studies with scale inhibitors for reverse osmosis desalination. Desalination 2013, 319, 79–84. [Google Scholar] [CrossRef]
- Akyol, E.; Oner, M.; Barouda, E.; Demadis, K.D. Systematic Structural Determinants of the Effects of Tetraphosphonates on Gypsum Crystallization. Cryst. Growth Des. 2009, 9, 5145–5154. [Google Scholar] [CrossRef]
- Benecke, J.; Rozova, J.; Ernst, M. Anti-scale effects of select organic macromolecules on gypsum bulk and surface crystallization during reverse osmosis desalination. Sep. Purif. Technol. 2018, 198, 68–78. [Google Scholar] [CrossRef]
- Van Driessche, A.; Stawski, T.; Kellermeier, M. Calcium sulfate precipitation pathways in natural and engineered environments. Chem. Geol. 2019, 530, 119274. [Google Scholar] [CrossRef]
- Prisciandaro, M.; Lancia, A.; Musmarra, D. Gypsum nucleation into sodium chloride solutions. AIChE J. 2001, 47, 929–934. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Peacock, C.L.; Benning, L.G. Investigating the Effectiveness of Phosphonate Additives in Hindering the Calcium Sulfate Dihydrate Scale Formation. Ind. Eng. Chem. Res. 2020, 59, 14970–14980. [Google Scholar] [CrossRef]
- Konstantinova, T.N.; Miladinova, P.M. Synthesis and properties of some fluorescent 1,8-naphthalimide derivatives and their copolymers with methyl methacrylate. J. Appl. Polym. Sci. 2009, 111, 1991–1998. [Google Scholar] [CrossRef]
- Murakami, Y.; Yokoyama, Y.; Miura, T.; Hirasawa, H.; Kamimura, Y.; Izaki, M. p-Toluenesulfonic acid and cation exchange resin in aprotic Solvent: Valuable catalysts for fischer indolization. Heterocycles 1984, 22, 5. [Google Scholar] [CrossRef]
- Chen, H.; Tang, Y.; Shang, H.; Kong, X.; Guo, R.; Lin, W. Development of a Unique Family of Two-photon Full-colortunable Fluorescent Materials for Imaging in Live Subcellular Organelles, Cells, and Tissues. J. Mater. Chem. B 2017, 5, 2436–2444. [Google Scholar] [CrossRef] [PubMed]
- Oshchepkov, M.; Solovieva, I.; Menkov, A.; Tkachenko, S.; Udovenko, V.; Popov, K. Continuous flow microfluidic implementation of a fluorescent marker into a polyacrylate moiety. J. Flow Chem. 2020, 10, 545–550. [Google Scholar] [CrossRef]
- NACE TM0374-HD2001-SG, Laboratory Screening Tests to Determine the Ability of Scale Inhibitors to Prevent the Precipitation of Calcium Sulfate and Calcium Carbonate From Solution (For Oil And Gas Production Systems). Available online: https://www.onepetro.org/standard/NACE-TM0374-2015 (accessed on 1 November 2020).
- Raju, K.U.G.; Atkinson, G. The thermodynamics of “scale” mineral solubilities. 3. Calcium sulfate in aqueous sodium chloride. J. Chem. Eng. Data 1990, 35, 361–367. [Google Scholar] [CrossRef]
- Pettit, L.D. Ionic Strength Corrections for Stability Constants using Specific Interaction Theory (SIT) for Windows 9x, NT, 2000, and xp, Academic Software; IUPAC: Zürich, Switzerland, 2003. [Google Scholar]
- Popov, K.I.; Oshchepkov, M.S.; Kamagurov, S.; Tkachenko, S.; Dikareva, J.; Rudakova, G. Synthesis and properties of novel fluorescent-tagged polyacrylate-based scale inhibitors. J. Appl. Polym. Sci. 2017, 134, 159. [Google Scholar] [CrossRef]
- Popov, K.I.; Rudakova, G.; Larchenko, V.; Tusheva, M.; Kamagurov, S.; Dikareva, J.; Kovaleva, N. A Comparative Performance Evaluation of Some Novel “Green” and Traditional Antiscalants in Calcium Sulfate Scaling. Adv. Mater. Sci. Eng. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Popov, K.; Oshchepkov, M.; Kovalenko, A.; Redchuk, A.; Dikareva, J.; Pochitalkina, I. The Scale Nucleation Natural Precursors: A Case Study of “Micro/Nanodust” Impurities Nature in Laboratory Water Samples, obtained from Moscow TAP Water. Int. J. Corros. Scale Inhib. 2020, 9, 1097–1112. [Google Scholar]
- Antony, A.; Low, J.H.; Gray, S.; Childress, A.E.; Le-Clech, P.; Leslie, G. Scale formation and control in high pressure membrane water treatment systems: A review. J. Membr. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Ang, H.M.; Muryanto, S.; Hoang, T. Gypsum Scale Formation Control in Pipe Flow Systems: A Systematic Study on the Effects of Process Parameters and Additives. In Gypsum: Properties, Production and Applications; Sampson, D.H., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 1–33. [Google Scholar]
- Sheng, K.; Ge, H.; Huang, X.; Zhang, Y.; Song, Y.; Ge, F.; Zhao, Y.; Meng, X. Formation and inhibition of calcium carbonate crystals under cathodic polarization conditions. Crystals 2020, 10, 275. [Google Scholar] [CrossRef] [Green Version]




| Antiscalant | λmaxabs, nm | λmaxfl, nm | Φfl | Ref |
|---|---|---|---|---|
| HEDP-F | 375 | 460 | 0.86 | [9] |
| PAA-F1 | 375 | 465 | 0.69 | [27] |
| PAA-F2 | 422 | 505 | 0.47 | Present work |
| Antiscalant | Dosage, mg·dm−3 | Gypsum Scale Inhibition, % | Ref. |
|---|---|---|---|
| HEDP-F | 25 | 78 ± 9 | [9] |
| 10 | 32 ± 8 | Present work | |
| PAA-F2 | 10 | 96 ± 4 | Present work |
| PAA-F2/HEDP-F Blend | 10 | 66 ± 5 | Present work |
| HEDP-F | 5 | ||
| PAA-F2 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshchepkov, M.; Golovesov, V.; Ryabova, A.; Frolova, S.; Tkachenko, S.; Kamagurov, S.; Rudakova, G.; Popov, K. Synthesis and Visualization of a Novel Fluorescent-Tagged Polymeric Antiscalant during Gypsum Crystallization in Combination with Bisphosphonate Fluorophore. Crystals 2020, 10, 992. https://doi.org/10.3390/cryst10110992
Oshchepkov M, Golovesov V, Ryabova A, Frolova S, Tkachenko S, Kamagurov S, Rudakova G, Popov K. Synthesis and Visualization of a Novel Fluorescent-Tagged Polymeric Antiscalant during Gypsum Crystallization in Combination with Bisphosphonate Fluorophore. Crystals. 2020; 10(11):992. https://doi.org/10.3390/cryst10110992
Chicago/Turabian StyleOshchepkov, Maxim, Vladimir Golovesov, Anastasia Ryabova, Svetlana Frolova, Sergey Tkachenko, Semen Kamagurov, Galina Rudakova, and Konstantin Popov. 2020. "Synthesis and Visualization of a Novel Fluorescent-Tagged Polymeric Antiscalant during Gypsum Crystallization in Combination with Bisphosphonate Fluorophore" Crystals 10, no. 11: 992. https://doi.org/10.3390/cryst10110992
APA StyleOshchepkov, M., Golovesov, V., Ryabova, A., Frolova, S., Tkachenko, S., Kamagurov, S., Rudakova, G., & Popov, K. (2020). Synthesis and Visualization of a Novel Fluorescent-Tagged Polymeric Antiscalant during Gypsum Crystallization in Combination with Bisphosphonate Fluorophore. Crystals, 10(11), 992. https://doi.org/10.3390/cryst10110992









