1. Introduction
Advanced materials and carbon-fiber composites are used extensively throughout revolutionary aircrafts such as Boeing 787 Dreamliner and Airbus A350 family [
1]. The stiffness, lightness, and toughness of the carbon fiber allowed technologists to create a very-low-drag delta wing body and fuselage. These advanced carbon fiber composites lead to not only lighter aircraft but also lower fatigue sensitivity, which means they require less maintenance. The Boeing 787’s heavy maintenance interval was increased from 6 to 13 years [
1]. Carbon-fiber-reinforced plastics (CFRPs) constitutes more than 50 vol.% of these aerospace mobile structures, as represented in
Figure 1. CFRPs are micro-composites formed from a lightweight polymer binder (e.g., epoxy) with laid carbon fiber to manufacture structures having extraordinarily high stiffness and strength-to-weight ratios.
This revolutionary technology (
Figure 1) relies on the superlative combination properties of CFRPs primarily contributed by carbon fibers [
2,
3,
4,
5,
6]. Carbon fibers, containing more than 92% by weight of carbon, have high strength, low density (1.8 g/cm
3, light weight, high breaking strength (2–7 GP), high tensile modulus (200–500 GPa), and a low thermal expansion coefficient (0.1–1.1 × 10
−6 K
−1) [
3,
7]. They are also characterized by high resistance to acids, alkalis, and organic solvents. Carbon fibers have a low coefficient of thermal expansion and a good electrical conductivity, as well as low
x-ray absorption and nonmagnetic properties [
8,
9,
10]. The as-produced carbon fibers usually have relatively smooth surfaces, low surface energy, low chemical reactivity, and lack of chemically active functional groups, which have a significant effect on their mechanical properties and restrict their extensive applications [
11,
12].
Wing boxes, made of CFRP, are able to support the load imposed during flight and support the whole aircraft aerodynamically while also strategically minimizing their overall contribution to the weight of an aircraft. However, CFRPs, unlike like their aluminum counterparts, do not conduct electricity. This makes them susceptible to lightning strike damage and to mitigate such a drawback, an electrically conductive expanded copper foil layer is usually laid on the outer surface of the composite structure layup [
13]. If a lightning bolt strikes an unprotected composite structure, up to 200,000 amps of electricity seeks the path of least resistance and may vaporize metal control cables and weld hinges on control surfaces or explode fuel vapors within fuel tanks if the current arcs through gaps around fasteners [
1]. High electrical conductivity is, therefore, required here to dissipate the high current and heat generated by a lightning strike. However, expanded copper foil (ECF) layer possess issues of its own. Temperature and atmospheric pressure variations (for instance, 50 °C to −50 °C and 100 to 25 kPa, respectively) during the ground-to-air flight cycle can lead to the expansion and reduction of the protective layer, which can damage the relatively less resilient epoxy matrix of CFRPs, reducing the overall effectiveness of the composite substitution.
Here, we synthesized nano-copper-influenced carbon fibers as an alternate technology for the ECF laid on top of the CFRP wing box. Carbon fibers, generally speaking, have poor wetting behavior with metals such as copper and aluminum [
12,
14,
15,
16,
17].
It is necessary to modify the surfaces of carbon fibers to resolve this key issue. One of the widely researched solutions is to coat the carbon fibers with metal layers. This method also reduces their susceptibility to interfering with the matrix and avoids the interaction of carbon fibers with several metals such as iron [
18]. Metal-coated carbon fibers can also be used as a reinforcement phase in different metal matrix composites for different applications such as electric contact materials and electric brushes [
16,
19], as well as for the fabrication of fiber composites used automotive and aerospace sectors and other electrical equipment [
20]. Materials with high electrical and thermal conductivities in combination with a low coefficient of thermal expansions are currently required for electrical and electronic applications. Carbon fiber/Cu composites possess the properties of copper, i.e., the excellent electrical and thermal conductivities, and the properties of carbon fiber, i.e., small coefficient of thermal expansion. These composites can be used in electrical and electronic applications. The electrical conductivity of carbon fiber/Cu composite materials is very important, particularly if these materials are used for electrical and electronic applications. The materials for this application should possess high electrical and thermal conductivities. Carbon fiber/Cu composites have successfully solved this problem. In this type of material, carbon is utilized because of its good sliding and antifriction properties, whilst copper is used because of its high electrical and thermal conductivities [
16,
19]. Various other studies have been conducted to improve the wetting of carbon fibers to metals. An SnCl
2/PdCl
2 solution is used as activating solution by depositing Pd nanoparticles on the surface of the carbon fibers before coating using the electroless deposition technique [
21,
22]. Electroless deposition can take place after the surface has been activated by Pd particles via the autocatalytic reaction to deposit metal nanoparticles on the surface of carbon fibers [
23].
In another report, deposition of silver nanoparticles or films using electroless silver deposition was used for obtaining surface activity and improving their electrical conductivity and physical properties. Activation by silver aerosols and copper electrolyte deposition was also considered. After annealing, silver-activated carbon fibers were effectively placed in a solution for electroplating copper, to obtain a uniform copper coating on their surface [
24]. Electroplating Cu was utilized to increase the thickness of the interlayer and forming a coating layer with a good adhesion with the surfaces of the carbon fibers. The current work utilizes a superlative combination of properties offered by carbon fiber (PAN: Polyacrylonitrile type) with high electrical conductivities of copper to synthesize a nano-copper-decorated carbon fiber nanocomposite via the coating route. To make these composites suitable for powder technology processing, surface treatment of the carbon fibers was essential via thermal de-binding, acid treatments, and/or a tin/silver metallization process before encapsulating the carbon fibers into the copper matrix using two coating methods, (electroless or electrodeposition) to produce a continuous conductive coating with uniform thickness. The contact electrical resistivity of the produced carbon fiber/Cu nanocomposites using either electroless or electrodeposition techniques was measured as well.
3. Results and Discussion
Figure 4 shows the SEM images of as-received carbon fibers. It was observed that the fibers had diameters of approximately 6.7 µm, as roughly confirmed in
Table 1.
Carbon long fibers were heat-treated to remove some of the volatile organic materials such as the sizing agents which were added to the fibers during the fabrication process.
Figure 5 shows the SEM images with different magnifications and the EDAX compositional analysis of the carbon fibers after heat treatment. Comparing the surface morphology of the untreated carbon fibers (
Figure 4) with the surface morphology of the heat-treated carbon fibers (
Figure 5), it was observed that the layers of the sizing agent were partially removed as the carbon fiber surface in evident. Some other impurities composed of Ba, K, Cr, and oxygen were detected through the EDAX analysis of heat-treated carbon fibers due to the presence of remnants from the sizing agent.
Figure 6 shows SEM images with different magnifications of the treated carbon fibers after washing and treating them with chromic acid. The results reveal that the diameter of the carbon fibers decreased from around 6.7 µm to around 5.1 µm due to heat and chemical treatments. The decrease in the diameter was due to the removal of the binding, sizing, and degreasing agents which adhered to the surface of carbon fibers as a consequence of heat and chemical treatments of the carbon fibers with acetone and chromic acid. A uniform morphological roughening of the carbon fiber was observed (
Figure 6). This process was conducted to increase the bond strength of the applied deposits by increasing the surface roughness of the carbon fiber substrate. However, a very rough surface such as that of etched fibers is not recommended since it affects the smoothness and uniformity of the final deposits. Suitably rough surfaces create a network profile to which the subsequent deposit can be physically anchored and produce a uniform coating thickness.
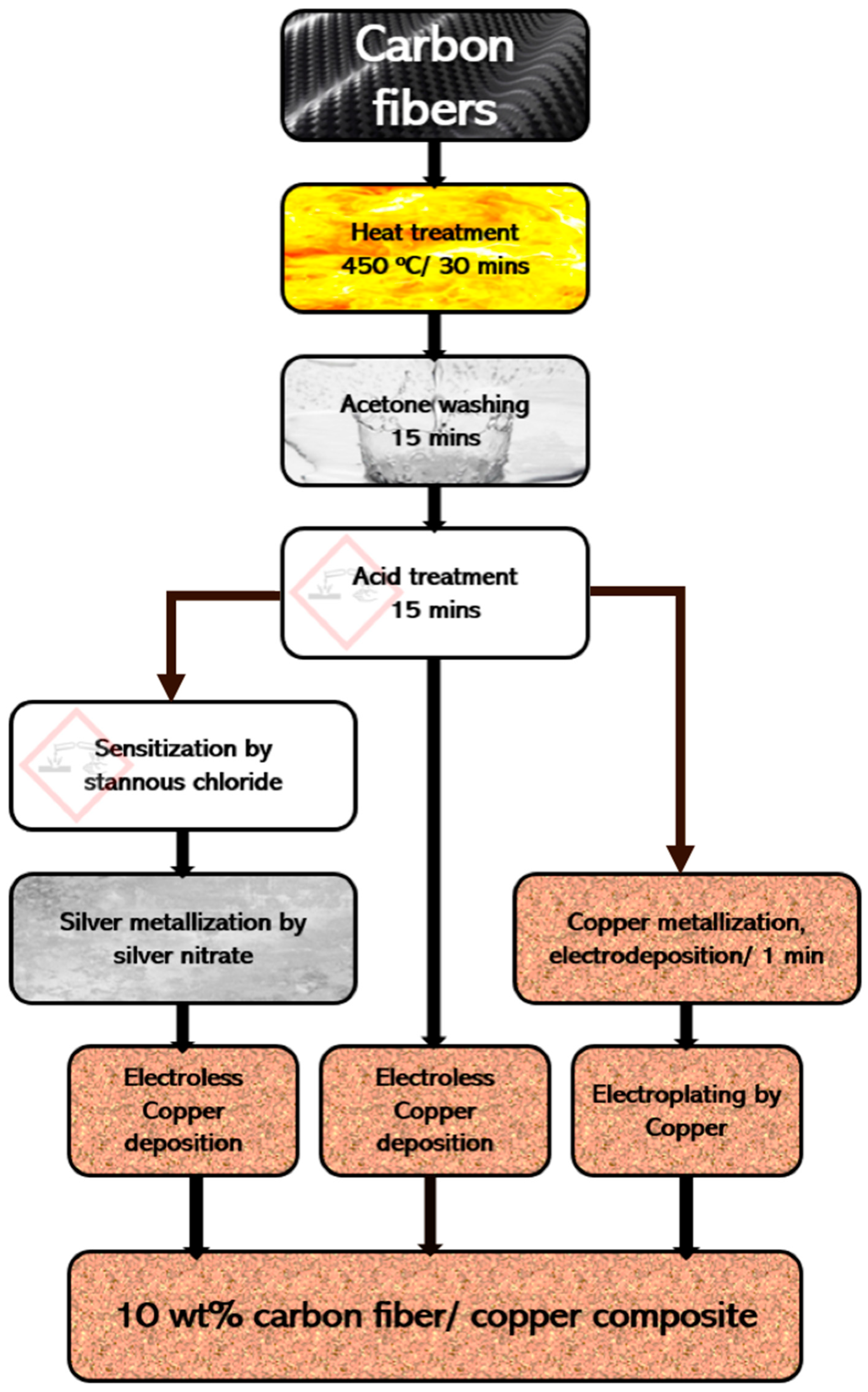
The chemically treated carbon fibers were metallized using three different techniques, namely, electroless tin/silver deposition, electroless copper deposition, and copper electrodeposition.
3.1. Metallization of Carbon Fibers Using Electroless Tin/Silver Deposition
Some of the surface-etched carbon fibers were further sensitized and activated to impart a uniform conducting film on the fiber surfaces, ensuring uniform adhesion of subsequent metallization and further promoting better coating and plating.
Tin/silver sensitization and activation were carried out to deposit nanosized silver particles onto carbon fibers prior to the electroless copper coating operations. Tin(II) ions were adsorbed onto the carbon fiber surfaces and silver(I) ions were reduced to metallic silver nanoparticles. Silver nanoparticles were deposited onto the surface of the carbon fibers in the second step as shown in the chemical reaction below.
Tin(IV) ions were produced from the oxidation of tin(II) ions by silver(I) ions.
Figure 7a,b show the SEM image of the silver-activated carbon fibers. Deposited silver nanoparticles in the range of around 40–130 nm could be observed. These deposited silver nanoparticles decorated and adhered to the surface of the carbon fibers.
Figure 7c,d show the EDAX compositional area analysis of the silver-activated carbon fibers. It was observed from the results that silver particles were composed mainly of 2.04 wt.% Ag and 0.61 wt.% tin, as remnants from the sensitization process.
3.2. Metallization of Carbon Fibers Using Electroless Copper Deposition
The chromic-acid-treated carbon fibers were subjected to autocatalytic electroless copper deposition on its surface in the alkaline tartrate copper sulfate solution.
Figure 8a,b show the SEM images with different magnifications of the deposited copper nanoparticles on the acid-treated carbon fibers. It was observed from the results that the deposited copper nanoparticles had particle size range between 85 and 165 nm. The copper nanoparticles had polygonal particle shapes, and some agglomerated particles were also observed. The deposited copper nanoparticles adhered to the surface of the carbon fibers, imparting a decorative type copper layer. A good uniform deposition can be observed.
Figure 7c,d show the EDAX compositional analysis of the metallized carbon fiber by the deposited copper nanoparticles. It was revealed that the copper nanoparticles were composed mainly of copper metal. The appearance of the small oxygen peak may be due to the oxidation of the surface of some deposited copper nanoparticles which were present in the aqueous solution during the process or due to the technical limitations of the EDAX technology with respect to the detection of oxygen.
The alkaline tartrate electroless copper solution used formaldehyde as a reducing agent of the copper ions to the copper metal. The half-cell reaction for the electroless copper deposition is shown below.
The rate of copper deposition was affected by the variation of the pH of the solution. Electroless copper solutions, using formaldehyde as a reducing agent, employed a high pH above 12. It was reported that the E
0 of formaldehyde depends on the pH of the solution [
22], as shown below.
As copper salts (copper sulfate pentahydrate) are insoluble at pH above 4, the use of alkaline media necessitates the use of a complexing or chelating component, such as tartrate salts and ethylenediaminetetraacetic acid (EDTA) [
22]. The full electroless copper deposition process at pH ~12 can be written according to the redox reaction below.
3.3. Metallization of Carbon Fibers Using Copper Electrodeposition
Electrodeposition is a common way of depositing of metals and its alloys on the surface of conductive materials. Copper is used in the electroplating process with either cyanide or sulfate baths. However, cyanide solutions are hazardous and should be avoided for industrial practices. An alternative approach is to use other acid baths to precipitate Cu. Chloride, oxalate, nitrate, thiosulfate, glycolate, lactate, and acetate have been reported; however, sulfate baths are the most commonly used [
23].
A conventional acid electrochemical copper cell was used to electroplate carbon long fibers. This electrochemical cell consisted of copper sulfate and a sulfuric acid solution as the electrolyte. Two high-purity copper plates were used as anodes. A cathode of carbon fibers was inserted between the anodes in the solution. Copper ions of the copper sulfate were dissolved in the electroplating solution. The remaining SO
42− anion played no part in the reactions and, therefore, does not appear in the equations [
23]. The complete chemical reaction of the electroplating process of the carbon fibers confirmed the transfer of copper ions from the anode to the cathode passing through the electrolyte and depositing copper on the conductive carbon long fibers fixated on the cathode. It was assumed that the total copper ion concentration in the electrolyte remained unchanged.
Figure 9 shows SEM images with different magnifications of the deposited copper nanoparticles on the surface of the carbon long fibers upon passing a current of 8 µA for 1 min through the electrodeposition cell. It was observed that polygonal copper nanoparticles ranging between 85 and 135 nm in size were homogeneously deposited on the surface of the treated carbon fibers, giving a decorative morphology texture (
Figure 9b).
3.4. Syntheses of Carbon Fiber/Copper Composites
The acid-treated, tin/silver-metallized, and copper-metallized carbon fibers were used to fabricate the 10 wt.% carbon fiber/copper composite via electroless coating and copper electroplating.
Figure 10a,b show SEM images with different magnifications of 10 wt.% carbon fibers coated via electroless deposition of copper on silver-metallized carbon fibers. The surface of the fibers was completely covered with multilayers composed of polygonal copper nanoparticles ranging in size from 50–100 nm.
Figure 10c,d show the EDAX semiquantitative analysis of the produced 10 wt.% carbon long fiber/copper composite via electroless deposition in the alkaline potassium sodium tartrate bath using formaldehyde as a reducing agent. A compositional analysis of the copper-coated silver-metallized carbon fibers showed mainly copper and carbon. In addition, some silver and tin remained in the copper-coated layers from the earlier tin/silver metallization process.
Figure 11 shows SEM images with different magnifications for carbon fiber/copper composites prepared via the electroplating method. The morphology (
Figure 11) was achieved using 12 µA/cm
2 current density for 5 min. Carbon fibers were completely coated and covered by a dense copper layer. Fine copper particles were deposited on the surface of the carbon fibers. These particles appeared to be growing laterally, simultaneously forming a network and eventually becoming a layer of bulky copper clusters of particles. It was also noticed that the Cu deposits on the carbon fibers adhered very well to the carbon fibers, and the degree of tightness was higher than the Cu-coated carbon fibers fabricated via the electroless deposition method due to the absence of pores in the deposited layers. However,
Figure 12 shows that there were some regions where there was no coating. Such regions could create some interfacial regions for the application discussed in this work. The EDAX compositional analysis of the prepared carbon fiber/copper composites via the electrodeposition method with 12 µA/cm
2 current density is also shown in
Figure 12. It was observed that the electrodeposited layer was composed mainly of copper on the surface of the carbon fibers.
3.5. Electrical Resistivity of Carbon Fiber/Copper Composites
The contact electrical resistivity for the fiber/deposit interface following electroless and electrolytic deposition for each Cu deposit condition was measured. The correlation provided qualitative analysis on the bonding and adhesion of the deposit to the carbon fibers. For example, a higher void content at the interface would result higher contact resistivity and lower adhesion. When measuring the contact electrical resistivity of the carbon fiber/deposit interfaces of the investigated Cu-coated fibers, as shown in
Figure 13, it was found that the contact electrical resistivity of the carbon fiber/copper composites fabricated via electrodeposition was lower than that of the carbon fiber/copper composites fabricated via silver metallization and that of the non-metallized carbon fiber/copper composites fabricated via electroless deposition. This was probably due to the high purity of the deposited copper when using the electrodeposition method than the electroless one, as shown from
Figure 10d and
Figure 12b,d. Copper and carbon fibers have no mutual wettability and solubility. Accordingly, carbon fiber is mechanically bonded to copper matrix solely due to the fiber’s roughness. Thus, the interfacial adhesion between carbon fibers and the copper matrix remains weak. When carbon fibers are oxidized with CrO
3, greater surface roughness is produced, resulting in better adhesion properties between the carbon fibers and Cu and leading to improved electrical conductivity. When the oxidized carbon is surface-activated and metallized with Sn/Ag solution, Ag acts as the active center for Cu deposition, consequently improving the carbon fiber/copper matrix interface and decreasing the percentage porosity, thereby resulting in a higher electrical conductivity. In addition, the resistivity of all the produced carbon fibers/copper composites was lower than that of the uncoated fibers (
Figure 13). This means that the conductivity of carbon fibers was improved by the contribution of copper in the composite. Since copper is a face-centered cubic (FCC) crystalline material, it theoretically contributes a free electron per atom to the conduction bond where it is available for conducting electrical current. At any time, a certain number of free electrons can be at any given distance outside of the copper surface. Carbon fibers, similar to copper, also possess free electrons, which are available for conduction. In the case of two clean surfaces (i.e., carbon fiber and copper) placed together closely in an intimate contact, the free electrons are able to exchange positions without interference [
25].