Comparative Analysis of Hg2Br2 and Hg2BrxCl2-x Crystals Grown via PVT
Abstract
:1. Introduction
2. Materials and Methods
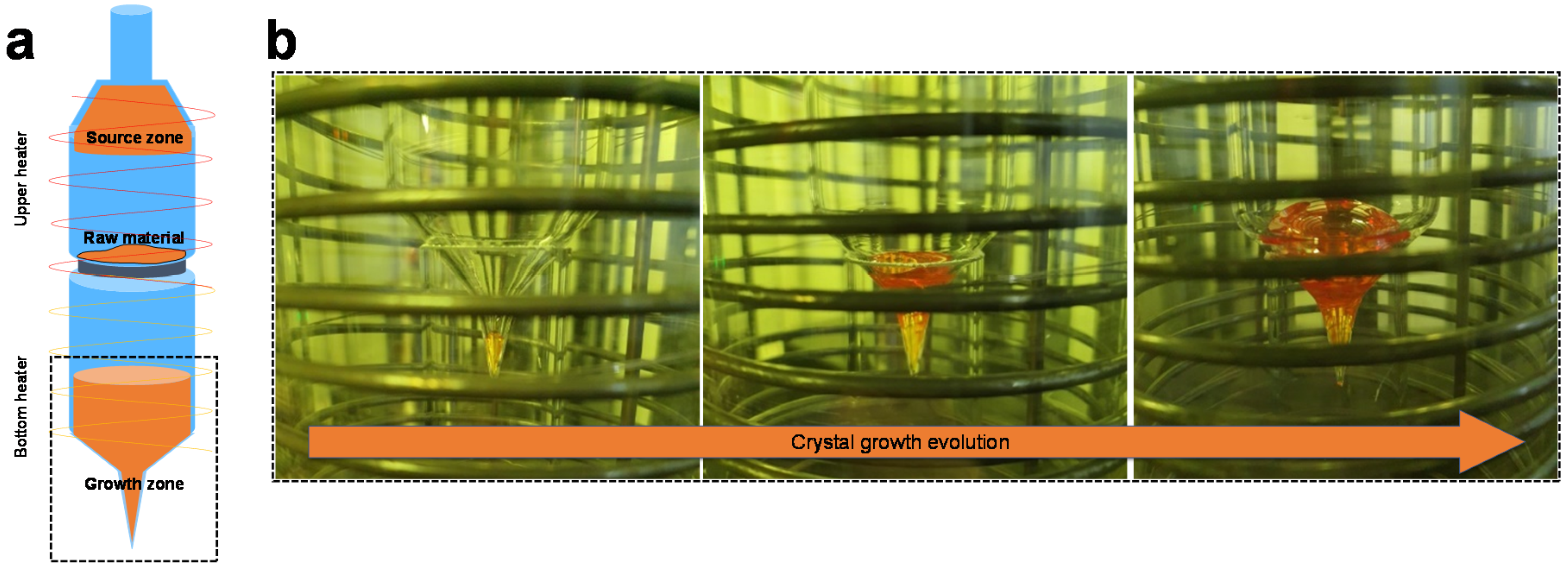
2.1. Purification and Crystal Growth Process of Mercury Halide Powders
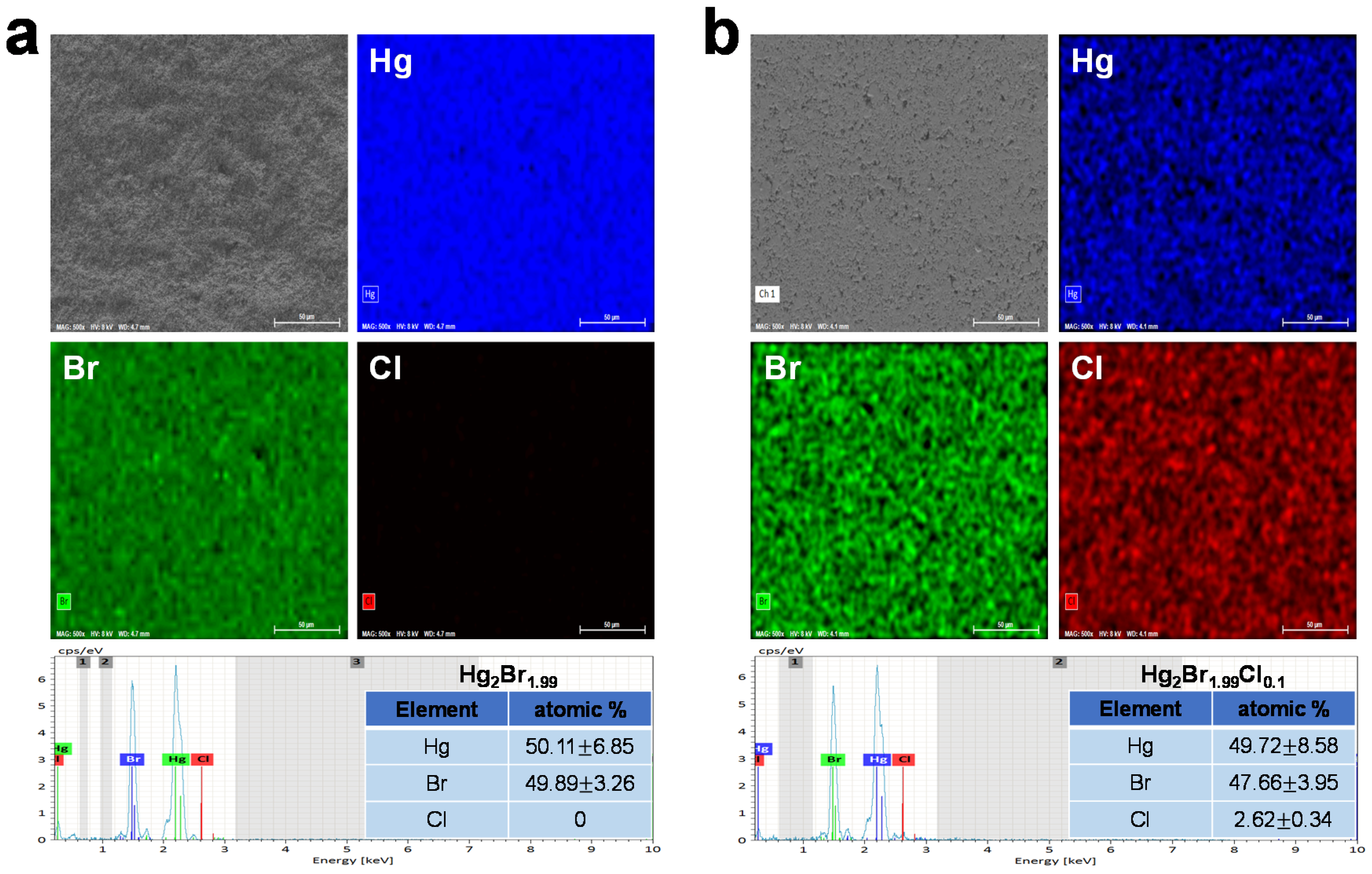
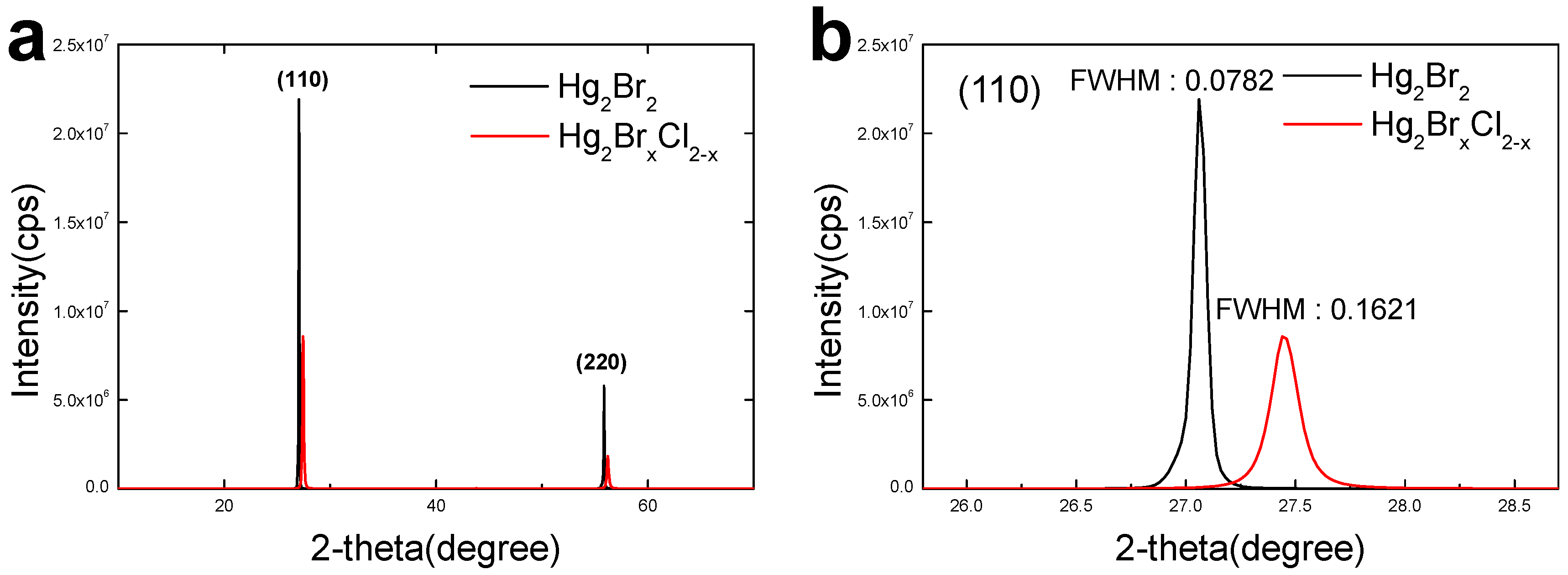
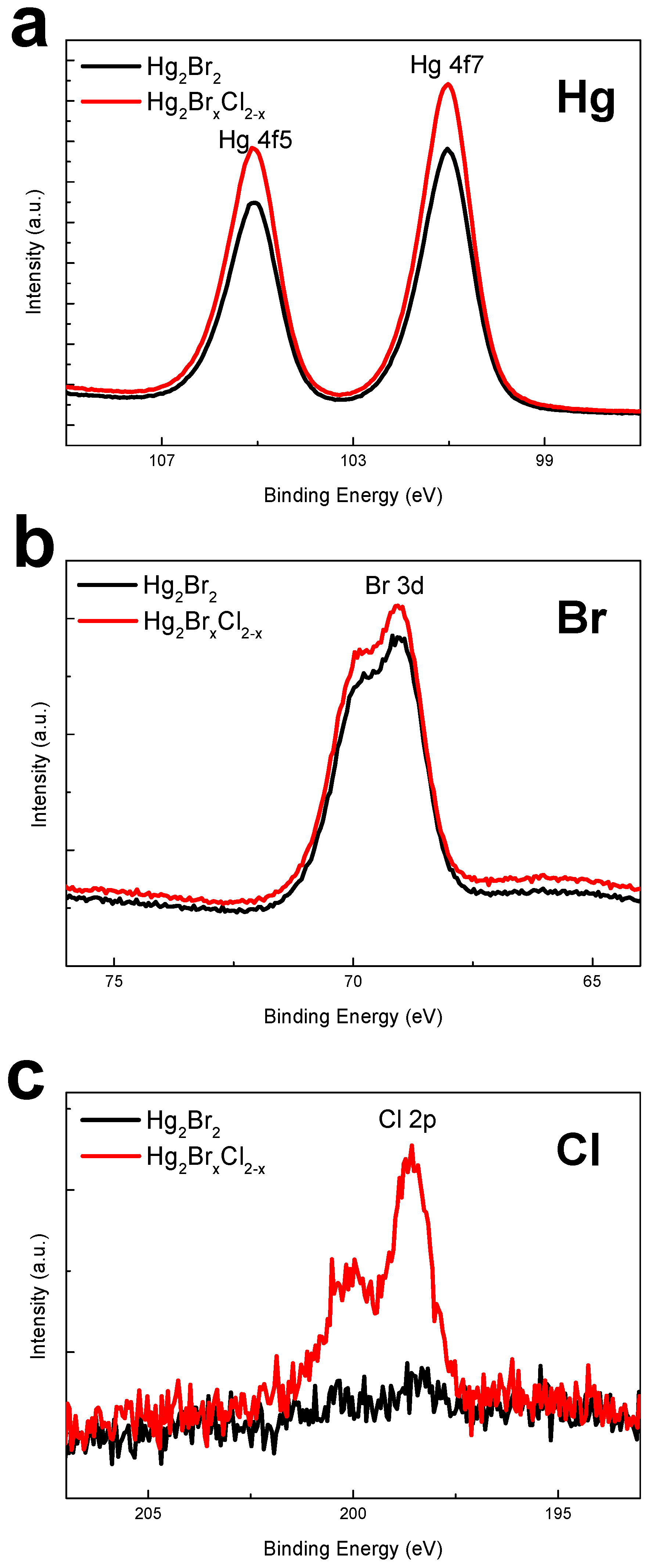
2.2. Material Characterization of Mercury Halide
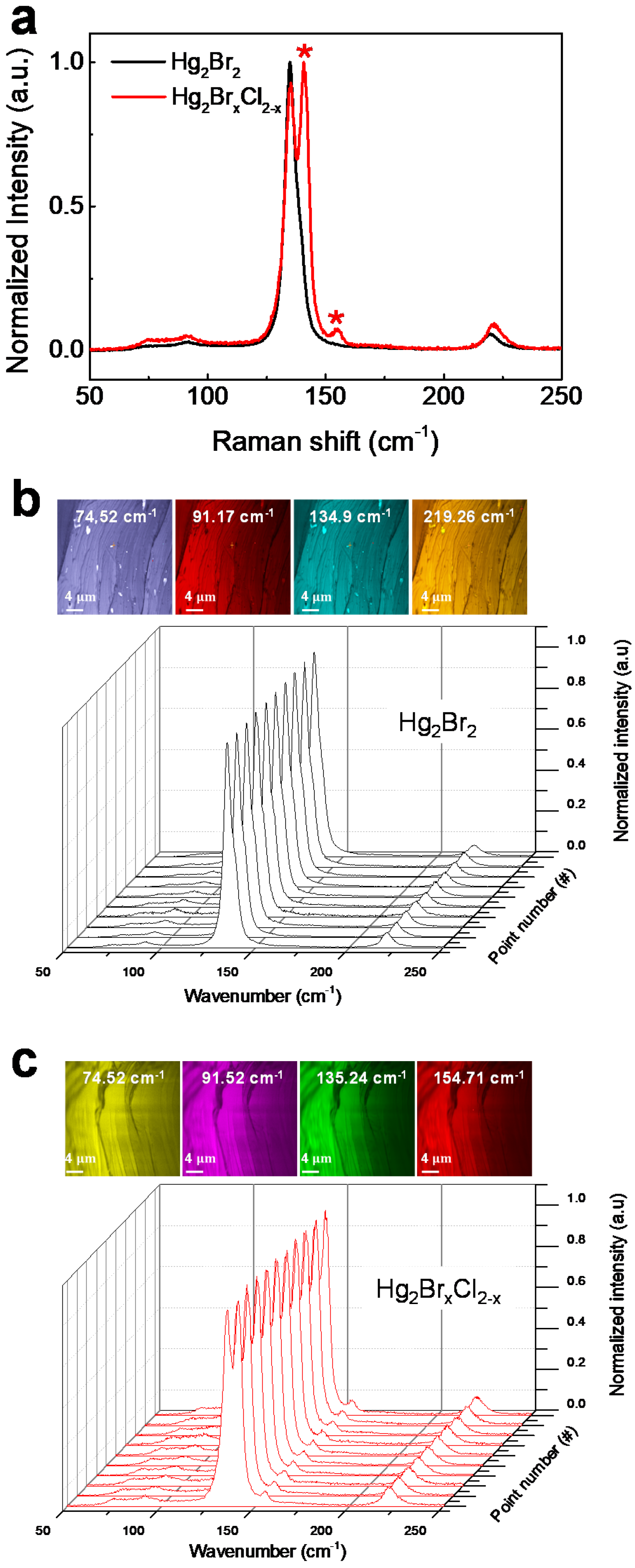
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, N. Acousto-optic tunable filters for infrared imaging. In Proceedings of the the Acousto-Optics and Photoacoustics, Warsaw, Poland, 30 August–2 September 2005; Volume 5953. [Google Scholar]
- Amarasinghe, P.M.; Kim, J.S.; Chen, H.; Trivedi, S.; Qadri, S.B.; Soos, J.; Diestler, M.; Zhang, D.; Gupta, N.; Jensen, J.L.; et al. Growth of high quality mercurous halide single crystals by physical vapor transport method for AOM and radiation detection applications. J. Cryst. Growth 2016, 450, 96. [Google Scholar] [CrossRef]
- Gupta, N.; Dahmani, R.; Choy, S. Acousto-optic tunable filter based visible- to near-infrared spectropolarimetric imager. Opt. Eng. 2002, 41, 1033–1038. [Google Scholar] [CrossRef]
- Kim, J.; Trivedi, S.B.; Soos, J.; Gupta, N.; Palosz, W. Development of mercurous halide crystals for acousto-optic devices. In Proceedings of the Imaging Spectrometry XII, San Diego, CA, USA, 28–29 August 2007; Volume 6661. [Google Scholar]
- Harris, S.E.; Wallace, R.W. Acousto-optic tunable filter. J. Opt. Soc. Am. 1969, 59, 744. [Google Scholar] [CrossRef]
- Chang, I.C. Tunable acousto-optic filters: An overview. In Proceedings of the Acousto-Optics, San Diego, CA, USA, 26–27 August 1976; Volume 90, pp. 12–22. [Google Scholar]
- Gupta, N.; Voloshinov, V. Hyperspectral imaging performance of a TeO2 acousto-optic tunable filter in the ultraviolet region. Opt. Soc. Am. 2005, 30, 985. [Google Scholar]
- Xu, J.; Stroud, R. Acousto-Optic Devices: Princioles, Design and Applications, 1st ed.; Wiley-Interscience: New York, NY, USA, 1992; Volume 12. [Google Scholar]
- Kim, J.; Trivedi, S.B.; Soos, J.; Gupta, N.; Palosz, W. Growth of Hg2Cl2 and Hg2Br2 single crystals by physical vapor transport. J. Cryst. Growth 2008, 310, 2457–2463. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, H.T.; Kwon, I.H.; Kang, Y.; Woo, S.; Jang, G.; Cho, B. High Purification of Hg2Br2 Powder for Acousto-Optic Tunable Filters Utilizing a PVT Process. Korean J. Mater. Res. 2018, 28, 732–737. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, H.T.; Kang, Y.M.; Jang, G.E.; Kwon, I.H.; Cho, B. In-depth Investigation of Hg2Br2 Crystal Growth and Evolution. Materials 2019, 12, 4224. [Google Scholar] [CrossRef] [Green Version]
- Thirsk, H.R. The structure and orientation of calomel formed on liquid mercury by anodic polarization. Proc. Phys. Soc. Sect. B 1953, 66, 129. [Google Scholar] [CrossRef]
- Maxwell, L.R.; Mosley, V.M. Internuclear Distances in Se2, Te2, and HgCl by Electron Diffraction. Phys. Rev. 1940, 57, 21. [Google Scholar] [CrossRef]
- Chung, C. Crystal structure of mercury(II) bromide dithiocarbamate, [HgBr2(S2CNEt2)Hg(S2CNEt2)]n, an inorganic polymer. Can. J. Chem. 1978, 56, 564–566. [Google Scholar]
- Ding, T.; Zhang, J.; Hong, J.; Zhu, J.; Chen, H. Sonochemical synthesis of taper shaped HgSe nanorods in polyol solvent. J. Cryst. Growth 2004, 260, 527. [Google Scholar] [CrossRef]
- Rahane, S.B.; Hensarling, R.M.; Sparks, B.J.; Stafford, M.; Patton, D.L. Synthesis of multifunctional polymer brush surfaces via sequential and orthogonal thiol-click reactions. J. Mater. Chem. 2012, 22, 932. [Google Scholar] [CrossRef]
- Li, B.; Zhou, L.; Wu, D.; Peng, H.; Yan, K.; Zhou, Y.; Liu, Z. Photochemical Chlorination of Graphene. ACS Nano 2011, 5, 5957–5961. [Google Scholar] [CrossRef] [PubMed]
- Roginskii, E.M.; Krylov, A.S.; Markov, Y.F. Lattice dynamics and baric behavior of phonons in the Hg2Br2 model ferroelastics. Phys. Solid State 2019, 61, 325. [Google Scholar] [CrossRef]
- Barta, C.; Kaplyanskii, A.A.; Markov, Y.F. The Raman scattering spectra of the Hg2Cl2 and Hg2Br2 single crystals. Sov. Phys. Solid State 1973, 15, 1896. [Google Scholar]
- Barta, C.; Kaplyanskii, A.A.; Kulakov, V.V.; Markov, Y.F. Raman-spectra of I and II orders of Hg2Cl2, Hg2Br2, Hg2J2 single-crystals. Opt. Spectrosk. 1974, 37, 95. [Google Scholar]
- Papatheodorou, G.N.; Boviatsis, I.V.; Voyiatzis, G.A. In situ Raman spectra of electrode products during electrolysis of HgCl2 in molten LiCl-KCl eutectic. J. Appl. Electrochem. 1992, 22, 517–521. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, O.; Kim, K.; Woo, S.-G.; Jang, G.-E.; Cho, B. Comparative Analysis of Hg2Br2 and Hg2BrxCl2-x Crystals Grown via PVT. Crystals 2020, 10, 1096. https://doi.org/10.3390/cryst10121096
Kwon O, Kim K, Woo S-G, Jang G-E, Cho B. Comparative Analysis of Hg2Br2 and Hg2BrxCl2-x Crystals Grown via PVT. Crystals. 2020; 10(12):1096. https://doi.org/10.3390/cryst10121096
Chicago/Turabian StyleKwon, Ojun, Kyoungah Kim, Shi-Gwan Woo, Gun-Eik Jang, and Byungjin Cho. 2020. "Comparative Analysis of Hg2Br2 and Hg2BrxCl2-x Crystals Grown via PVT" Crystals 10, no. 12: 1096. https://doi.org/10.3390/cryst10121096





