The Influence of Deposition Time on the Structural, Morphological, Optical and Electrical Properties of ZnO-rGO Nanocomposite Thin Films Grown in a Single Step by USP
Abstract
:1. Introduction
2. Experimental
2.1. Materials
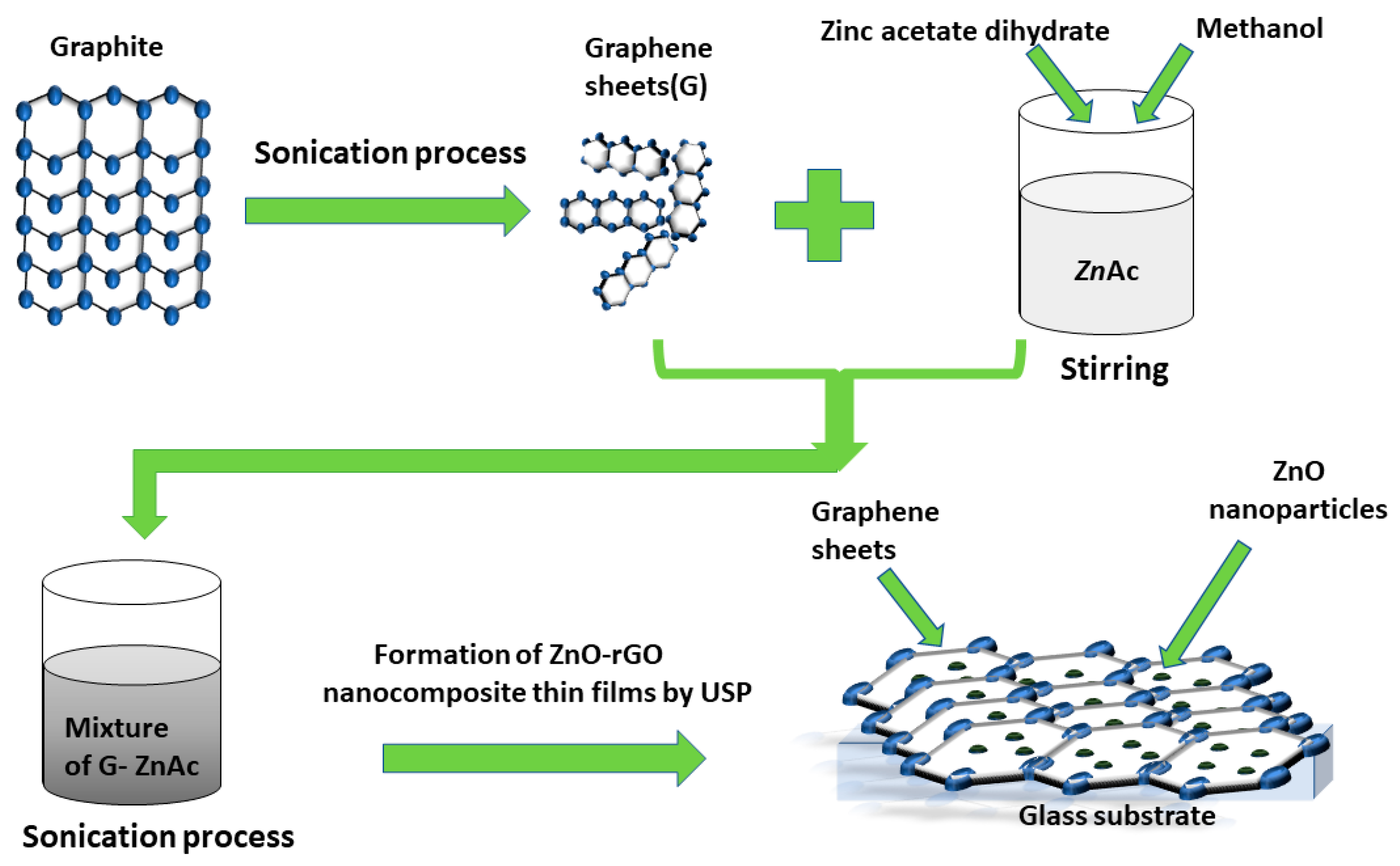
2.2. Synthesis of Graphene
2.3. Deposition Process of ZnO-rGO Nanocomposite Thin Films by USP
2.4. Characterization
3. Results and Discussion
3.1. XRD Analysis
3.2. HR-TEM, SEM and AMF Analyses
3.3. Elemental Mapping Composition (EDS)
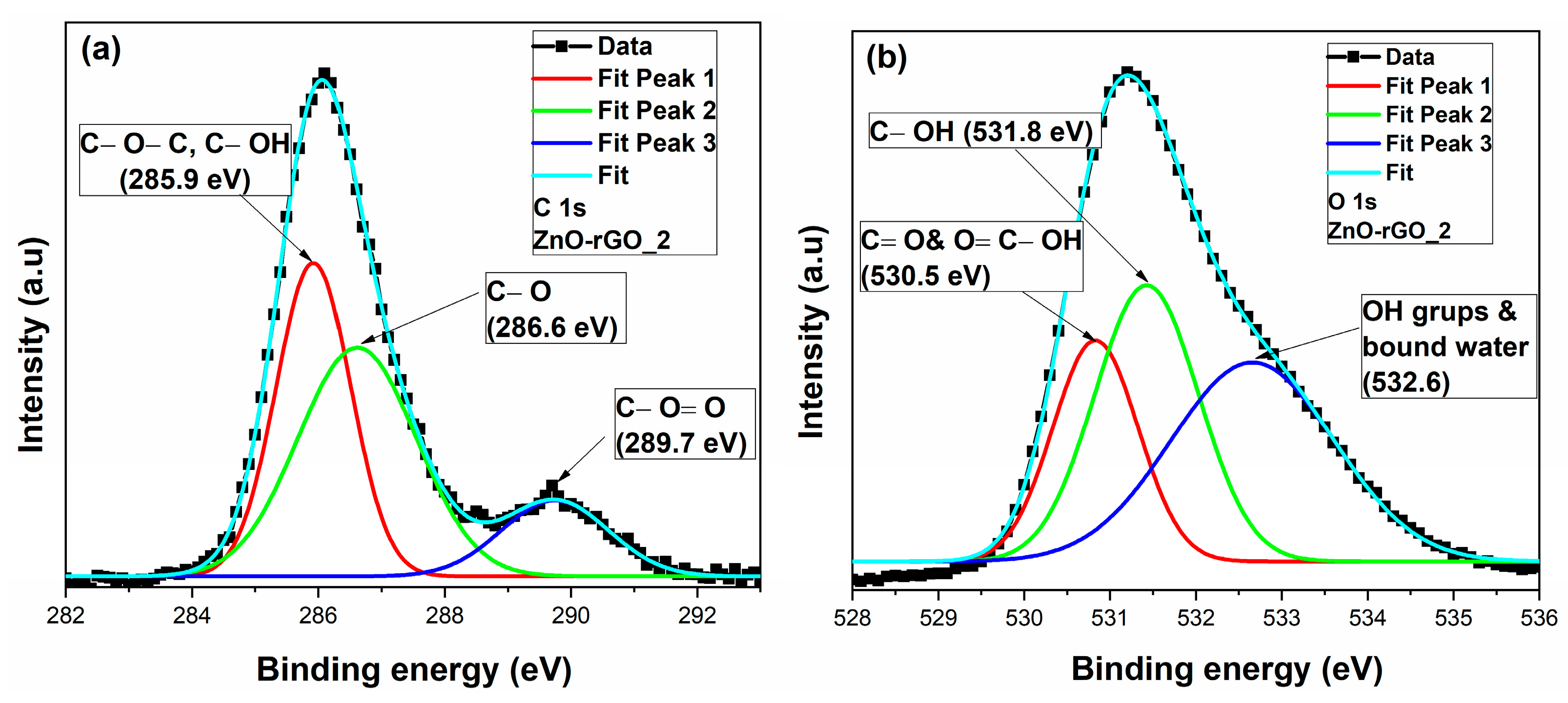
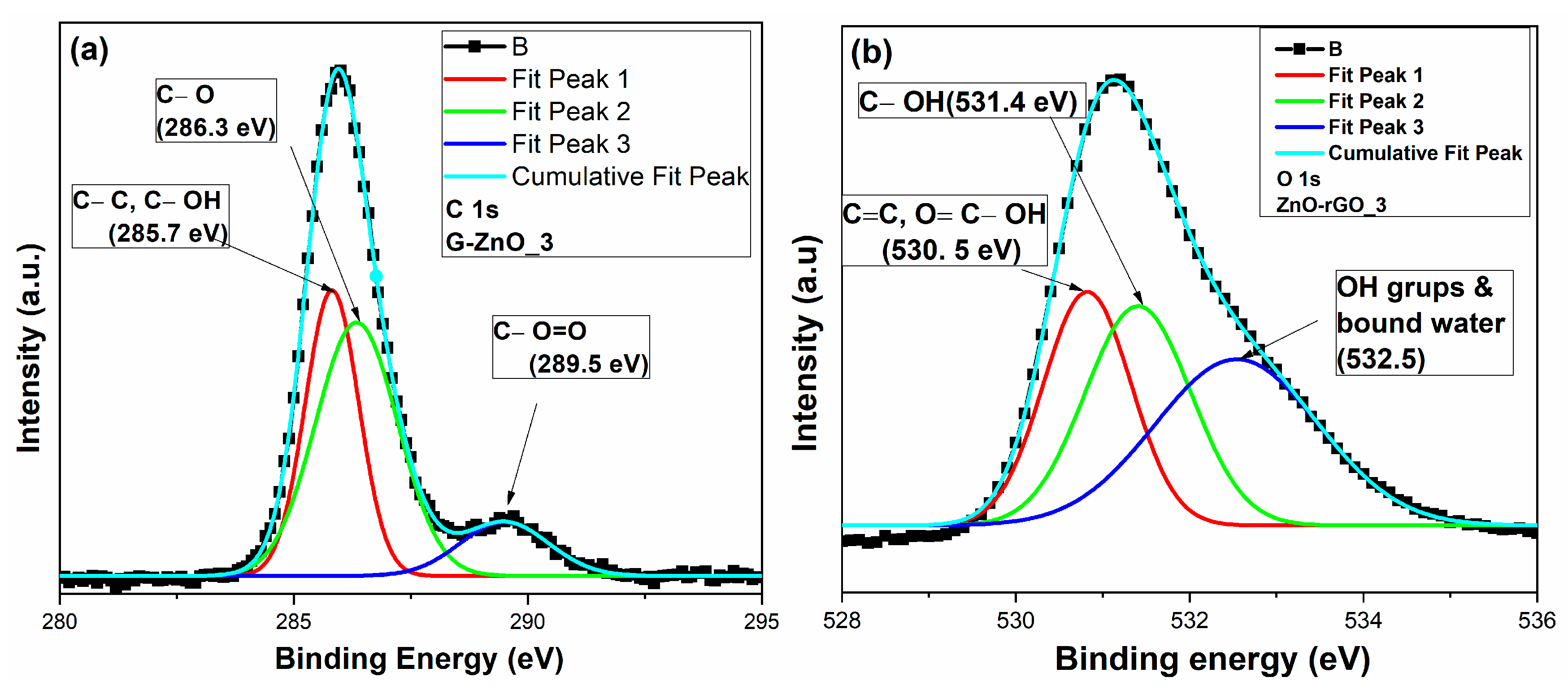
3.4. X-ray Photoelectron Spectroscopy (XPS) Analysis
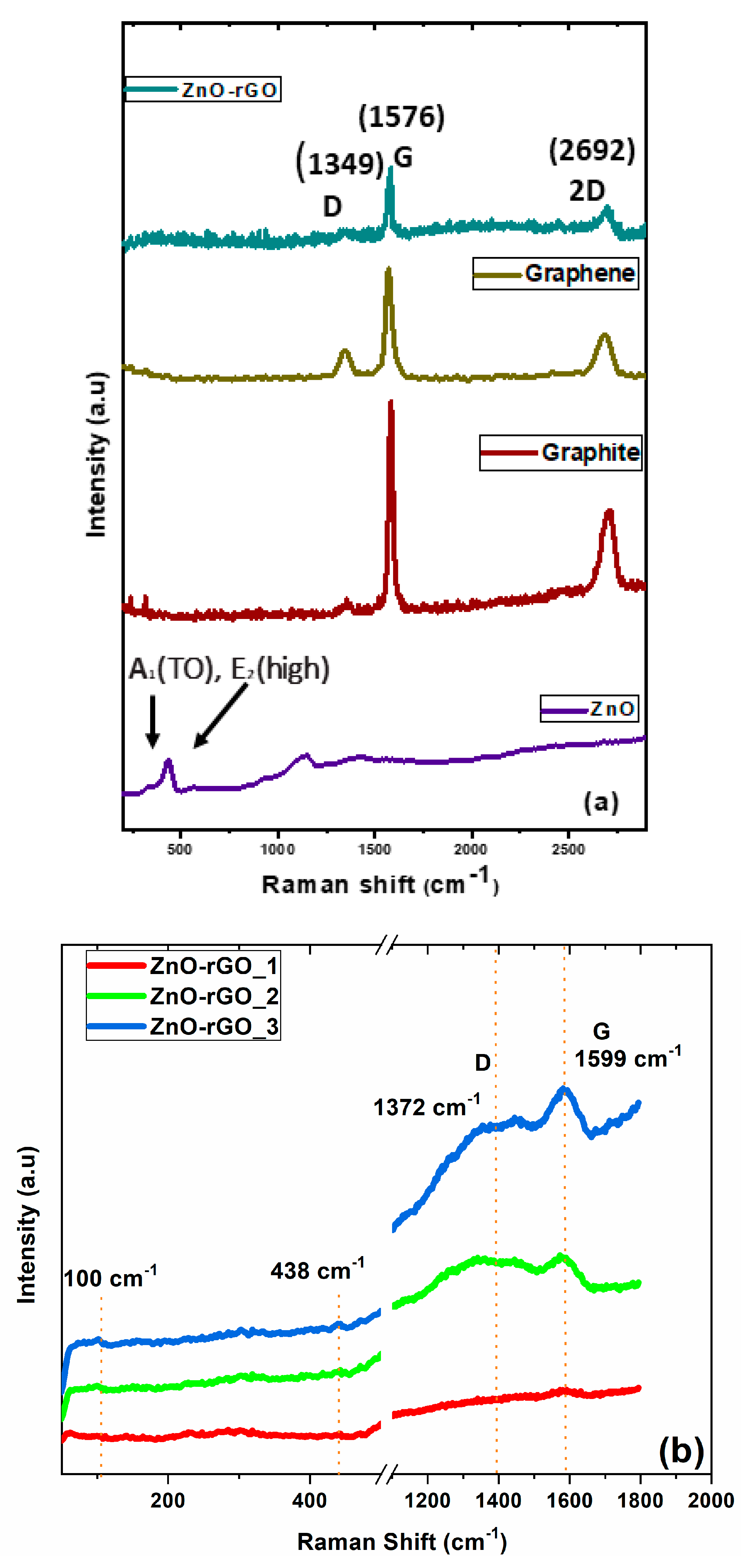
3.5. Raman Spectroscopy
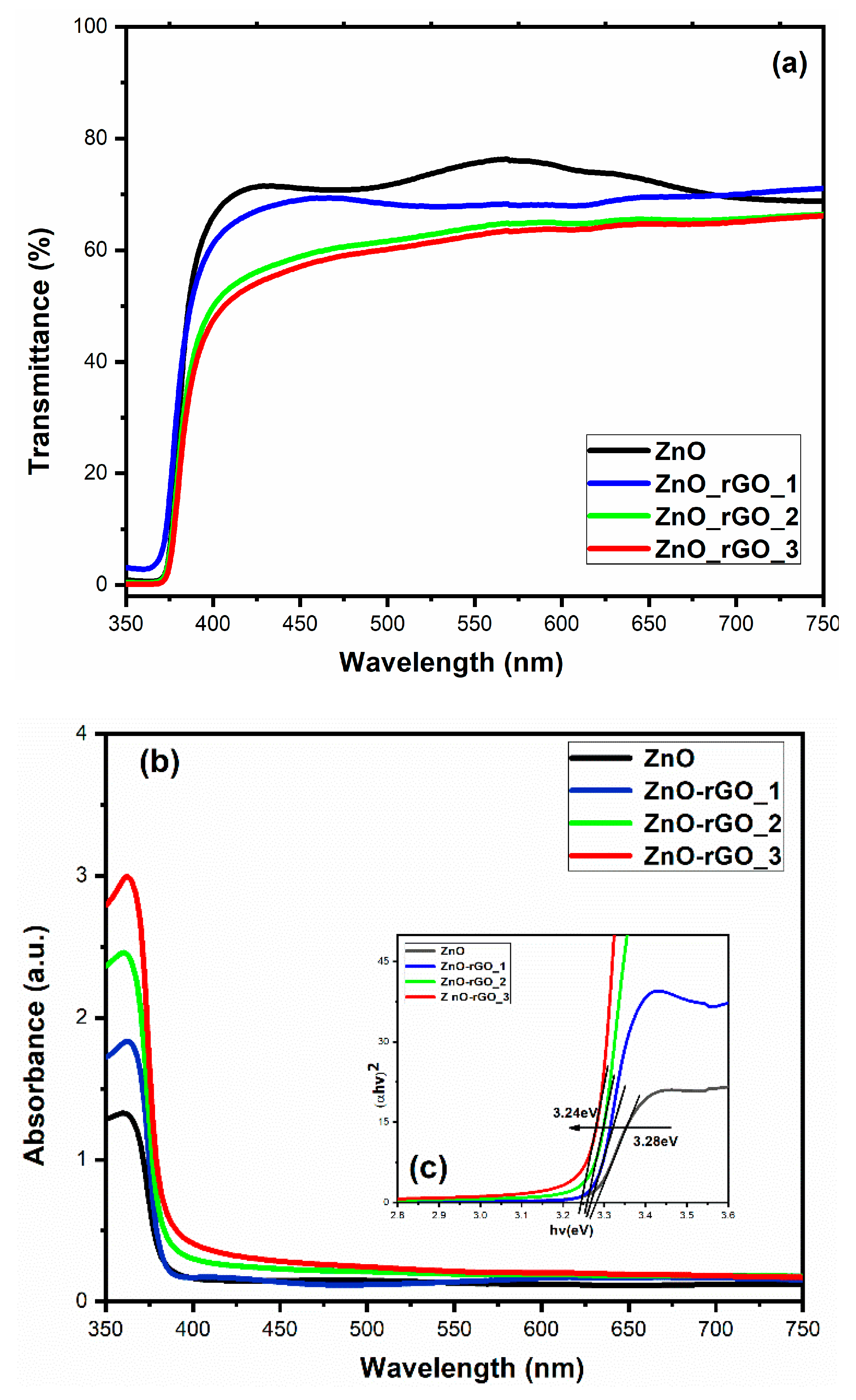
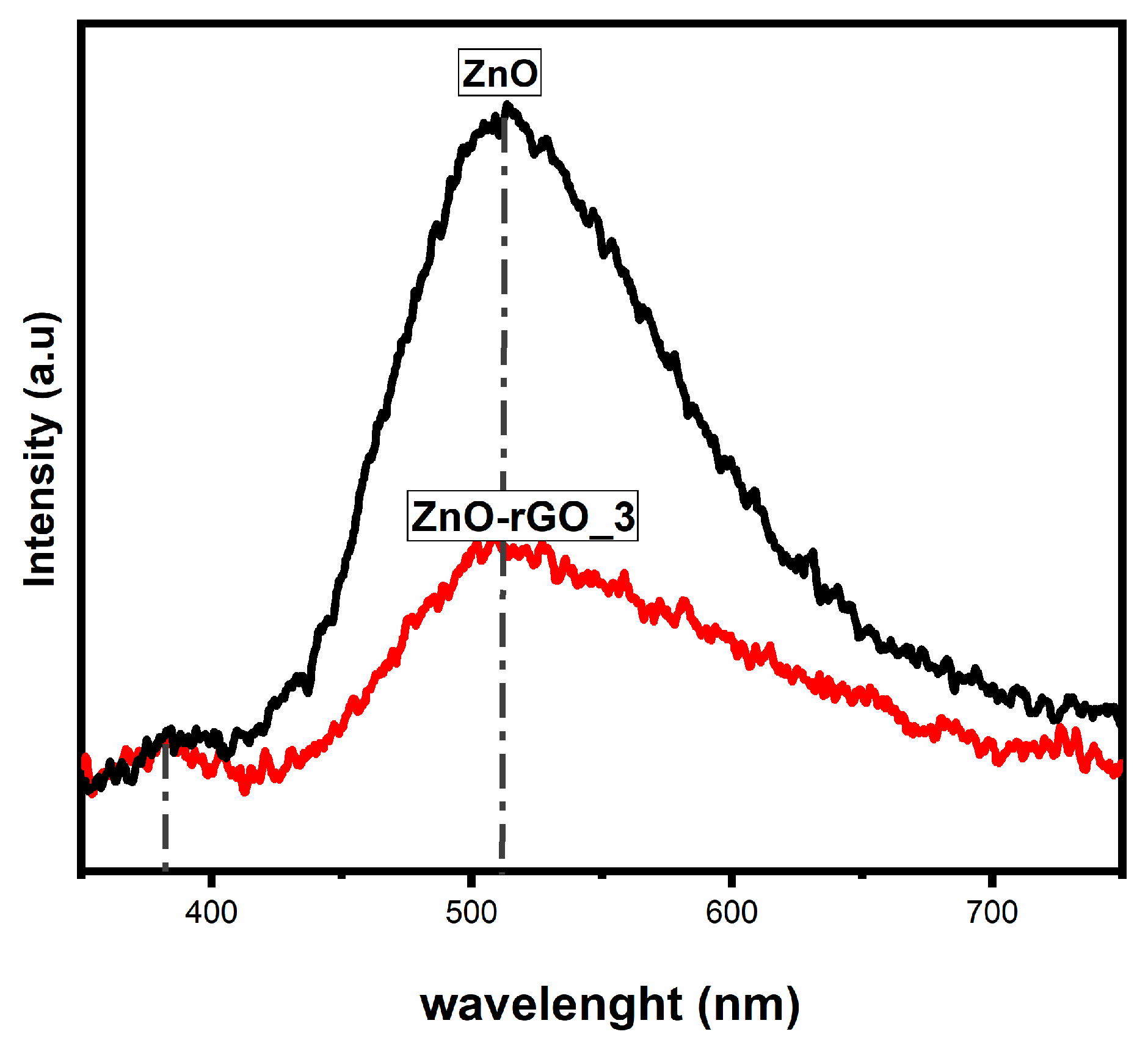
3.6. Optical Measurements
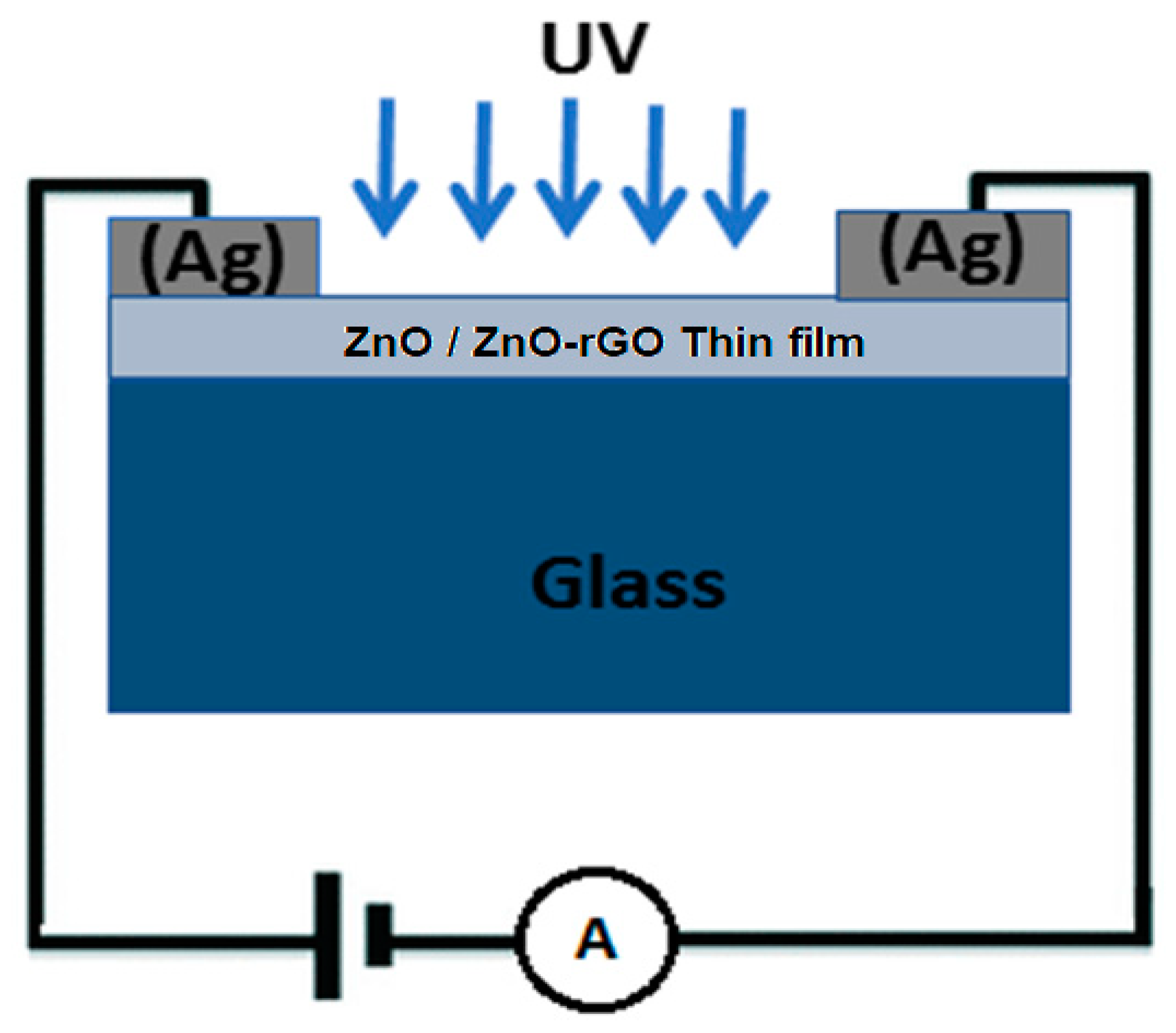
3.7. Electrical Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2015, 41, 666–686. [Google Scholar] [CrossRef]
- Neto, A.C.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Son, D.I.; Kwon, B.W.; Park, D.H.; Seo, W.S.; Yi, Y.; Angadi, B.; Choi, W.K. Emissive ZnO–graphene quantum dots for white-light-emitting diodes. Nat. Nanotechnol. 2012, 7, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, E.; Kavitha, G.; Kennedy, J. Epitaxial zinc oxide, graphene oxide composite thin-films by laser technique for micro-Raman and enhanced field emission study. Ceram. Int. 2014, 40, 16065–16070. [Google Scholar] [CrossRef]
- Zhong, L.; Samal, M.; Yun, K. Synthesis, characterization and electrochemical properties of different morphological ZnO anchored on graphene oxide sheets. Mater. Chem. Phys. 2018, 204, 315–322. [Google Scholar] [CrossRef]
- Paul, R.; Gayen, R.N.; Biswas, S.; Bhat, S.V.; Bhunia, R. Enhanced UV detection by transparent graphene oxide/ZnO composite thin films. RSC Adv. 2016, 6, 61661–61672. [Google Scholar] [CrossRef]
- Yin, Z.; Wu, S.; Zhou, X.; Huang, X.; Zhang, Q.; Boey, F.; Zhang, H. Electrochemical deposition of ZnO nanorods on transparent reduced graphene oxide electrodes for hybrid solar cells. Small 2010, 6, 307–312. [Google Scholar] [CrossRef]
- Teh, S.J.; Yeoh, S.L.; Lee, K.M.; Lai, C.W.; Hamid, S.B.A.; Thong, K.L. Effect of reduced graphene oxide-hybridized ZnO thin films on the photoinactivation of Staphylococcus aureus and Salmonella enterica serovar Typhi. J. Photochem. Photobiol. B Biol. 2016, 161, 25–33. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Qu, C.; Su, Y.; Yu, S.; Zheng, W.; Liu, Y. Photovoltaic properties of graphene oxide sheets beaded with ZnO nanoparticles. J. Solid State Chem. 2011, 184, 881–887. [Google Scholar] [CrossRef]
- Kwon, B.J.; Kim, J.Y.; Choi, S.M.; An, S.J. Highly transparent and conducting graphene-embedded ZnO films with enhanced photoluminescence fabricated by aerosol synthesis. Nanotechnology 2014, 25, 85701–85707. [Google Scholar] [CrossRef]
- Anand, K.; Singh, O.; Singh, M.P.; Kaur, J.; Singh, R.C. Hydrogen sensor based on graphene/ZnO nanocomposite. Sens. Actuators B Chem. 2014, 195, 409–415. [Google Scholar] [CrossRef]
- Peng, Y.; Ji, J.; Chen, D. Ultrasound assisted synthesis of ZnO/reduced graphene oxide composites with enhanced photocatalytic activity and anti-photocorrosion. Appl. Surf. Sci. 2015, 356, 762–768. [Google Scholar] [CrossRef]
- Saranya, M.; Ramachandran, R.; Wang, F. Graphene-zinc oxide (G-ZnO) nanocomposite for electrochemical supercapacitor applications. J. Sci. Adv. Mater. Devices 2016, 1, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Q.; Xu, Z.; Lei, Z.; Dong, H.; Yu, H.; Qi, L.; Chen, Y. Enhanced nonlinear optical and optical limiting properties of graphene/ZnO hybrid organic glasses. Carbon 2014, 67, 214–220. [Google Scholar] [CrossRef]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Hussain, S.Z.; Ismail, A.F.; Khan, I.U. Graphene and its derivatives: Synthesis, modifications, and applications in wastewater treatment. Environ. Chem. Lett. 2018, 16, 1301–1323. [Google Scholar] [CrossRef]
- Norhayati, H.; Zuhailimuna, M.; Mohd Zobir, H.; Illyas, M.I.; Azmi, M.; Azlan, K.; AdilaMohamad, J. A Brief Review On Recent Graphene Oxide-Based Material Nanoco Mposites: Synthesis And Applications. J. Mater. Environ. Sci. 2016, 7, 3225–3243. [Google Scholar]
- Holder, E.; Tessler, N.; Rogach, A.L. Hybrid nanocomposite materials with organic and inorganic components for opto-electronic devices. J. Mater. Chem. 2008, 18, 1064–1078. [Google Scholar] [CrossRef]
- Liu, X.; Pan, L.; Lv, T.; Lu, T.; Zhu, G.; Sun, Z.; Sun, C. Microwave-assisted synthesis of ZnO–graphene composite for photocatalytic reduction of Cr (VI). Catal. Sci. Technol. 2011, 1, 1189–1193. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, T.; Zhou, H. Hydrothermal preparation of ZnO-reduced graphene oxide hybrid with high performance in photocatalytic degradation. Appl. Surf. Sci. 2012, 258, 6204–6211. [Google Scholar] [CrossRef]
- Saranya, M.; Garg, S.; Singh, I.; Ramachandran, R.; Santhosh, C.; Harish, C.; Grace, A.N. Solvothermal preparation of ZnO/graphene nanocomposites and its photocatalytic properties. Nanosci. Nanotechnol. Lett. 2013, 5, 349–354. [Google Scholar] [CrossRef]
- Alam, K.; Sim, Y.; Yu, J.-H.; Gnanaprakasam, J.; Choi, H.; Chae, Y.; Sim, U.; Cho, H. In-situ Deposition of Graphene Oxide Catalyst for Efficient Photoelectrochemical Hydrogen Evolution Reaction Using Atmospheric Plasma. Materials 2020, 13, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodwihok, C.; Wongratanaphisan, D.; Thi Ngo, Y.L.; Khandelwal, M.; Hur, S.H.; Chung, J.S. Effect of GO Additive in ZnO/rGO Nanocomposites with Enhanced Photosensitivity and Photocatalytic Activity. Nanomaterials 2019, 9, 1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ergin, B.; Ketenci, E.; Atay, F. Characterization of ZnO films obtained by ultrasonic spray pyrolysis technique. Int. J. Hydrogen Energy 2009, 34, 5249–5254. [Google Scholar] [CrossRef]
- Bilgin, V.; Kose, S.; Atay, F.; Akyuz, I. The effect of Zn concentration on some physical properties of tin oxide films obtained by ultrasonic spray pyrolysis. Mater. Lett. 2004, 58, 3686–3693. [Google Scholar] [CrossRef]
- Akhavan, O. The effect of heat treatment on formation of graphene thin films from graphene oxide nanosheets. Carbon 2010, 48, 509–519. [Google Scholar] [CrossRef]
- Qu, W.W.; Jin, W.; Xu, J.Y.; Yang, S.; Peng, J.H. A Theoretical Investigation of the Thermal Decomposition of Zinc Acetate and Zn-Oxo Complex Based on the Pyrolysis Characteristics of the Zinc-Containing Spent Catalyst. Adv. Mater. Res. 2012, 538, 2438–2443. [Google Scholar] [CrossRef]
- Mihaiu, S.; Szilágyi, I.M.; Atkinson, I.; Mocioiu, O.C.; Hunyadi, D.; Pandele-Cusu, J.; Toader, A.; Munteanu, C.; Boyadjiev, S.; Pokol, G.; et al. Thermal study on the synthesis of the doped ZnO to be used in TCO films. J. Therm. Anal. Calorim. 2016, 124, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.; Deng, W.; Wang, W.; Liu, Z. Facile fabrication of nanoporous graphene powder for high-rate lithium–sulfur batteries. RSC Adv. 2017, 7, 5177–5182. [Google Scholar] [CrossRef] [Green Version]
- Ciszewski, M.; Mianowski, A.; Szatkowski, P.; Nawrat, G.; Adamek, J. Reduced graphene oxide–bismuth oxide composite as electrode material for supercapacitors. Ionics 2015, 21, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.Y.; Chang, K.H.; Tien, H.W.; Lee, Y.F.; Li, S.M.; Wang, Y.S.; Wang, J.Y.; MA, C.C.M.; Hu, C.C. Design and tailoring of a hierarchical graphene-carbon nanotube architecture for supercapacitors. J. Mater. Chem. 2011, 21, 2374–2380. [Google Scholar] [CrossRef]
- Siburian, R.; Sihotang, H.; Raja, S.L.; Supeno, M.; Simanjuntak, C. New route to synthesize of graphene nano sheets. Orient. J. Chem. 2018, 34, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Jabeen, M.; Ishaq, M.; Song, W.; Xu, L.; Maqsood, I.; Deng, Q. UV-assisted photocatalytic synthesis of ZnO-reduced graphene oxide nanocomposites with enhanced photocatalytic performance in degradation of methylene blue. ECS J. Solid State Sci. Technol. 2017, 6, M36. [Google Scholar] [CrossRef]
- Chen, Y.L.; Hu, Z.A.; Chang, Y.Q.; Wang, H.W.; Zhang, Z.Y.; Yang, Y.Y.; Wu, H.Y. Zinc oxide/reduced graphene oxide composites and electrochemical capacitance enhanced by homogeneous incorporation of reduced graphene oxide sheets in zinc oxide matrix. J. Phys. Chem. C 2011, 115, 2563–2571. [Google Scholar] [CrossRef]
- Gong, Y.; Zou, C.; Yao, Y.; Fu, W.; Wang, M.; Yin, G.; Chen, X. A facile approach to synthesize rose-like ZnO/reduced graphene oxide composite: Fluorescence and photocatalytic properties. J. Mater. Sci. 2014, 49, 5658–5666. [Google Scholar] [CrossRef]
- Flores-Carrasco, G.; Muñoz-Fernández, L.; Alcántara-Iniesta, S.; Soto-Cruz, B.S.; Milosevic, O.; Rabanal, M.E. Structural and functional properties of ZnO thin films grown on Si substrates by air assisted USP method from non-aqueous solutions at low-temperature. Adv. Powder Technol. 2017, 28, 93–100. [Google Scholar] [CrossRef]
- Haldorai, Y.; Voit, W.; Shim, J.J. Nano ZnO@ reduced graphene oxide composite for high performance supercapacitor: Green synthesis in supercritical fluid. Electrochim. Acta 2014, 120, 65–72. [Google Scholar] [CrossRef]
- Longo, A.; Verucchi, R.; Aversa, L.; Tatti, R.; Ambrosio, A.; Orabona, E.; Maddalena, P. Graphene oxide prepared by graphene nanoplatelets and reduced by laser treatment. Nanotechnology 2017, 28, 224002. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, A.K.; Patel, H.S.; Gupta, B.K.; Varma, G.D. Facile synthesis of ZnO–reduced graphene oxide nanocomposites for NO2 gas sensing applications. Eur. J. Inorg. Chem. 2015, 11, 1912–1923. [Google Scholar] [CrossRef]
- Posa, V.R.; Annavaram, V.; Koduru, J.R.; Ammireddy, V.R.; Somala, A.R. Graphene-ZnO nanocomposite for highly efficient photocatalytic degradation of methyl orange dye* under solar light irradiation. Korean J. Chem. Eng. 2016, 33, 456–464. [Google Scholar] [CrossRef]
- Sengupta, I.; Chakraborty, S.; Talukdar, M.; Pal, S.K.; Chakraborty, S. Thermal reduction of graphene oxide: How temperature influences purity. J. Mater. Res. 2018, 33, 4113–4122. [Google Scholar] [CrossRef]
- Samuel, M.; Koshy, J.; Chandran, A.; George, K.C. Optical phonon confinement in ZnO nanorods and nanotubes. Indian J. Pure Appl. Phys. 2010, 48, 703–708. [Google Scholar]
- Radoń, A.; Włodarczyk, P.; Łukowiec, D. Structure, temperature and frequency dependent electrical conductivity of oxidized and reduced electrochemically exfoliated graphite. Phys. E Lowdimens. Syst. Nanostruct. 2018, 99, 82–90. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, V.; Saxena, V.; Singh, P.; Singh, V.K. Two-step synthesis of reduced graphene oxide with columnar-shaped ZnO composites and their photocatalytic performance with natural dye. J. Aust. Ceram. Soc. 2019, 1, 1–12. [Google Scholar] [CrossRef]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of ZnO decorated graphene nanocomposite for enhanced photocatalytic properties. J. Appl. Phys. 2014, 115, 173504. [Google Scholar] [CrossRef]
- Boukhoubza, I.; Khenfouch, M.; Achehboune, M.; Mothudi, B.M.; Zorkani, I.; Jorio, A. Graphene oxide/ZnO nanorods/graphene oxide sandwich structure: The origins and mechanisms of photoluminescence. J. Alloys Compd. 2019, 797, 1320–1326. [Google Scholar] [CrossRef]
- Shuai, W.; Hu, Y.; Chen, Y.; Hu, K.; Zhang, X.; Zhu, W.; Lao, Z. Composite structure of ZnO films coated with reduced graphene oxide: Structural, electrical and electrochemical properties. J. Semicond. 2018, 39, 23001–23006. [Google Scholar] [CrossRef]
- Jilani, S.M.; Gamot, T.D.; Banerji, P. Thin-film transistors with a graphene oxide nanocomposite channel. Langmuir 2012, 28, 16485–16489. [Google Scholar] [CrossRef]
- Qu, G.; Fan, G.; Zhou, M.; Rong, X.; Li, T.; Zhang, R.; Chen, D. Graphene-Modified ZnO Nanostructures for Low-Temperature NO2 Sensing. ACS Omega 2019, 4, 4221–4232. [Google Scholar] [CrossRef] [Green Version]
- Al-Fandi, M.; Oweis, R.; Albiss, B.A.; AlZoubi, T.; Al-Akhras, M.A.; Qutaish, H.; Al-Shawwa, E. A prototype ultraviolet light sensor based on ZnO nanoparticles/graphene oxide nanocomposite using low temperature hydrothermal method. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Irbid, Jordan, 2015; Volume 92, p. 012009. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Sun, B.; Ni, L.; Wei, X.; Guo, T.; Shi, Z.; Duan, L. The mechanism of photocurrent enhancement of ZnO ultraviolet photodetector by reduced graphene oxide. Curr. Appl. Phys. 2018, 18, 859–863. [Google Scholar] [CrossRef]

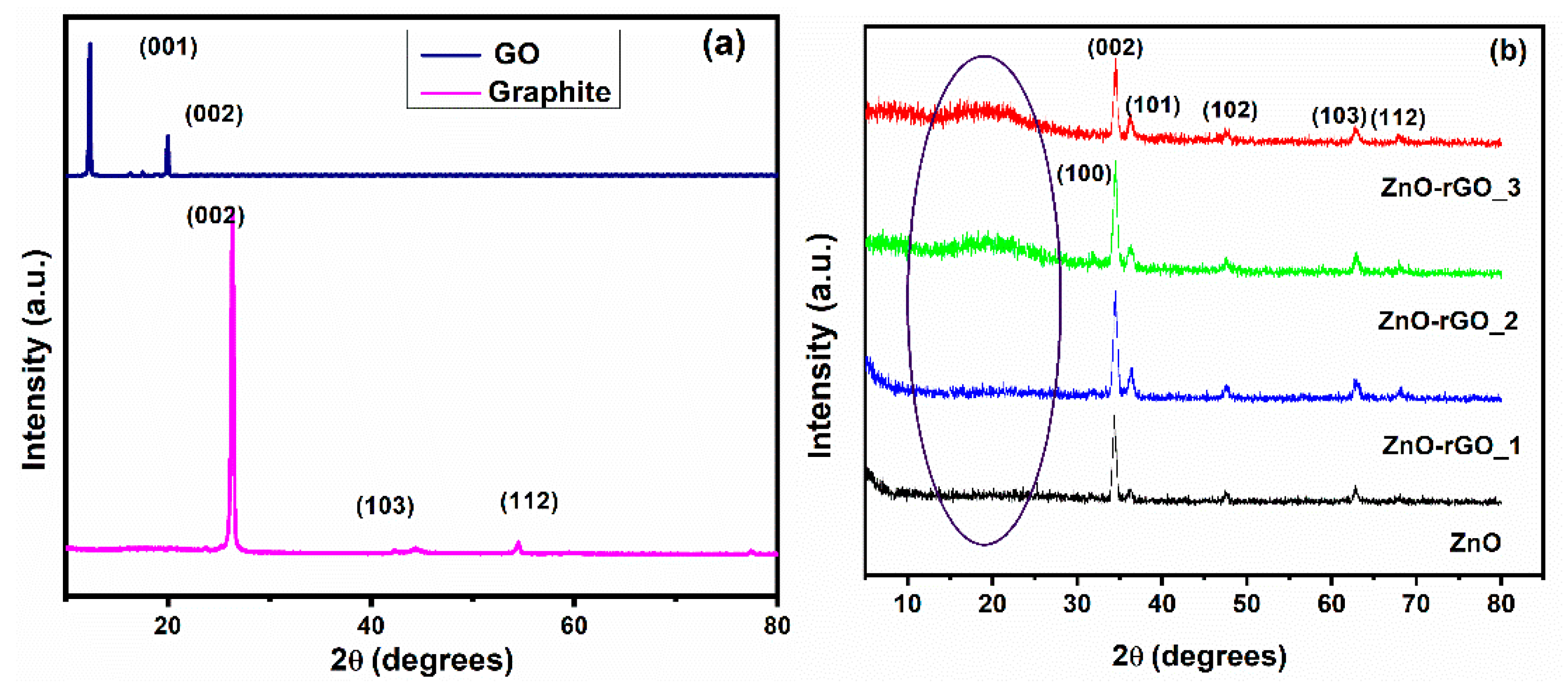
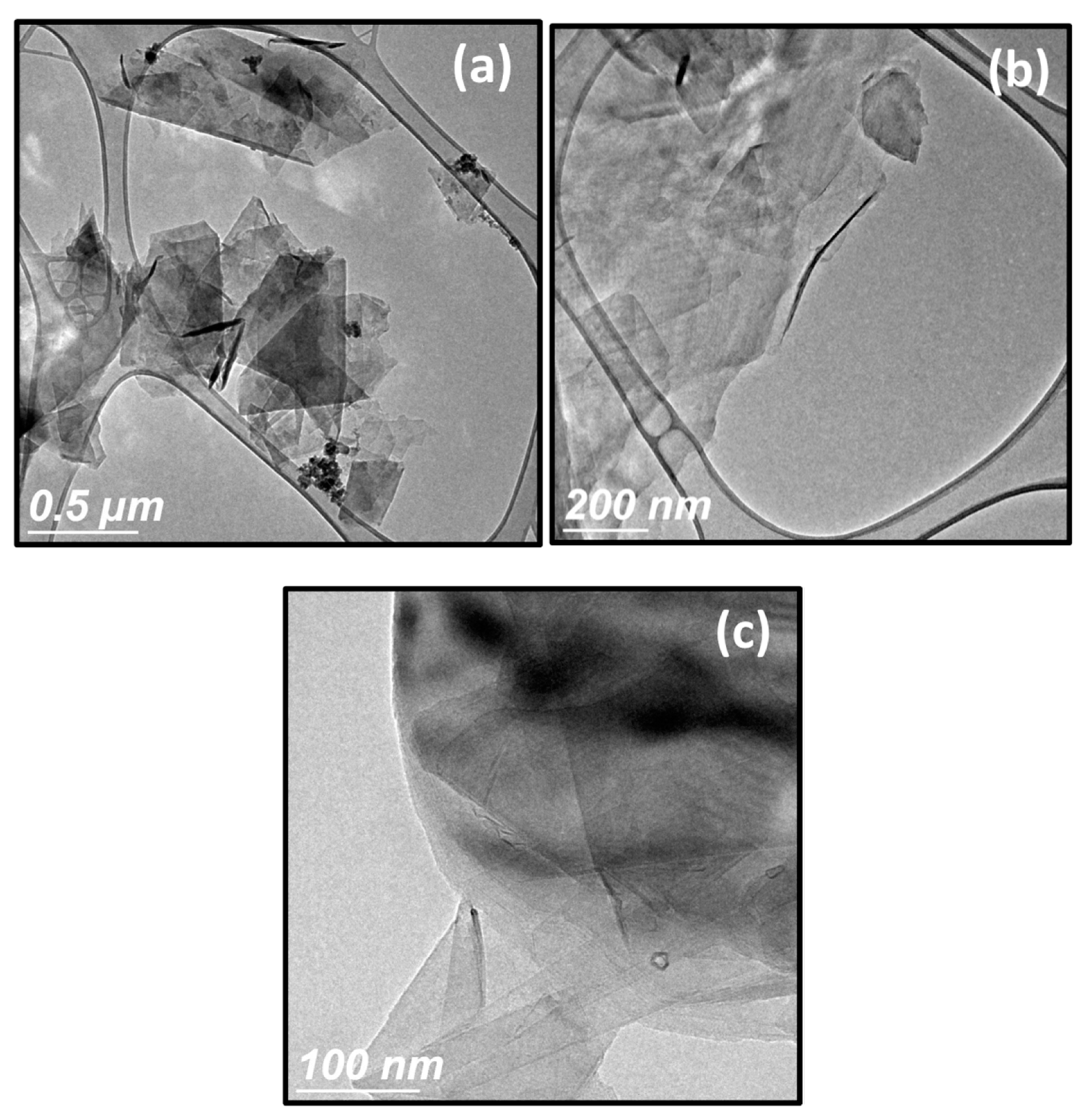


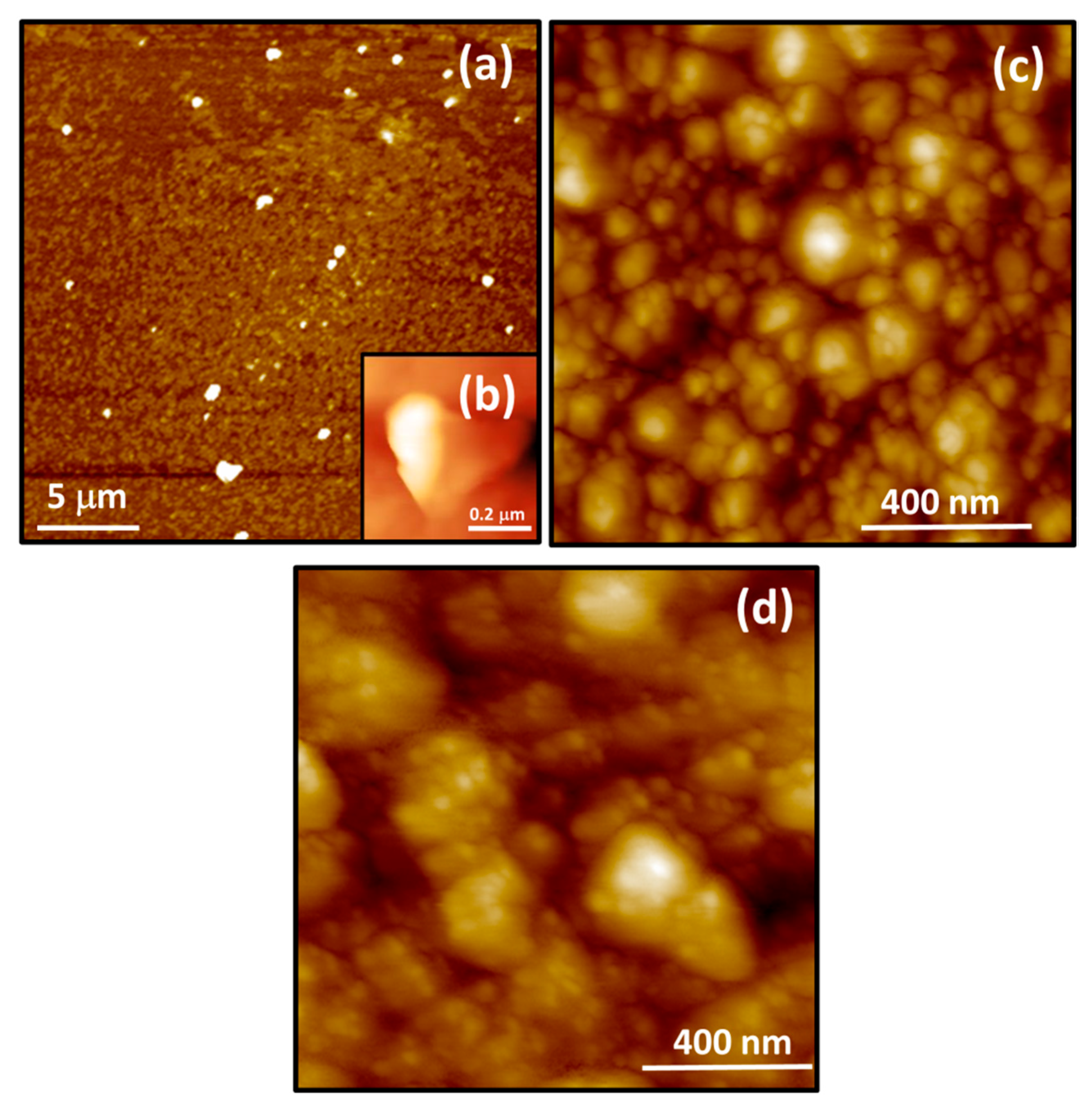
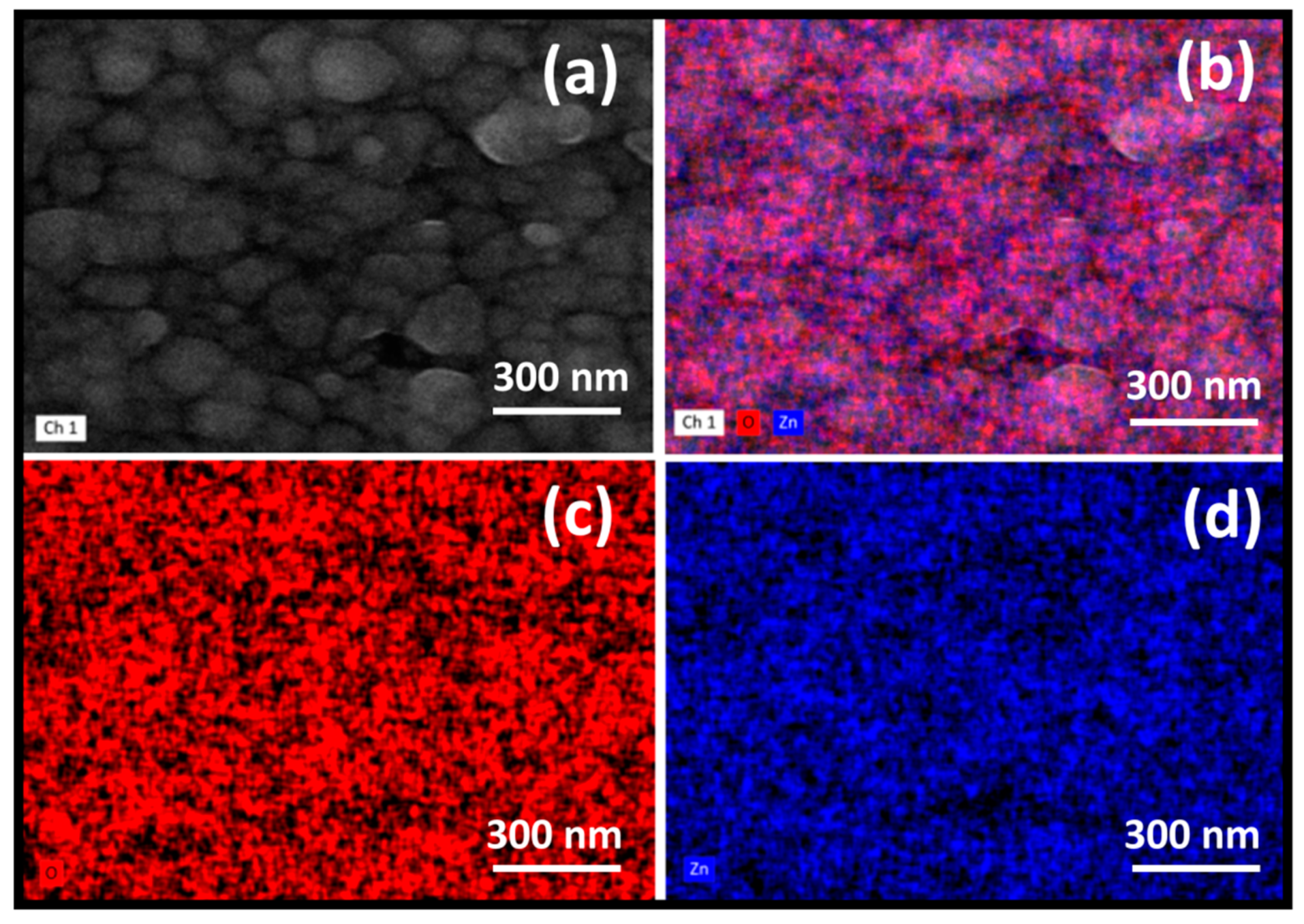
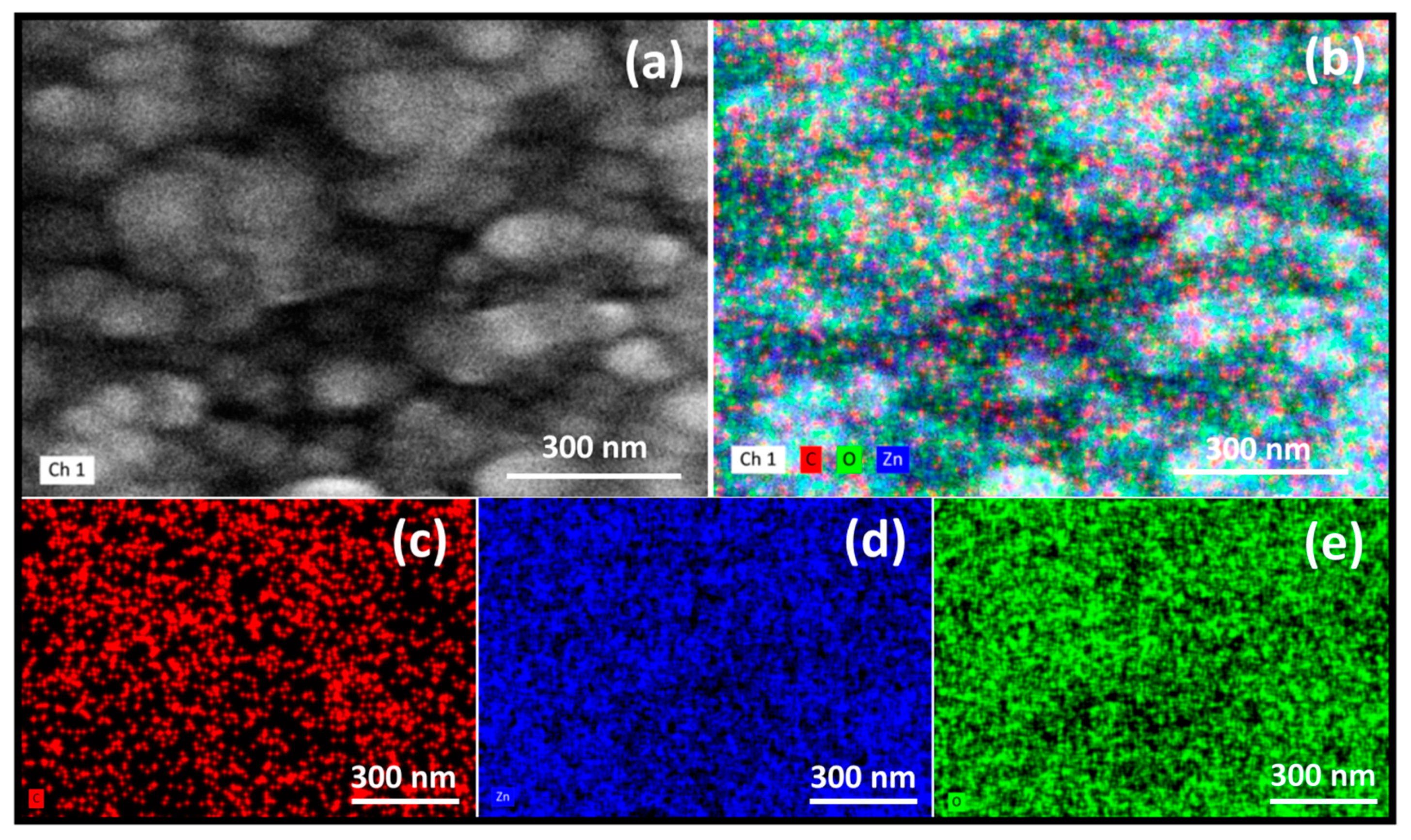

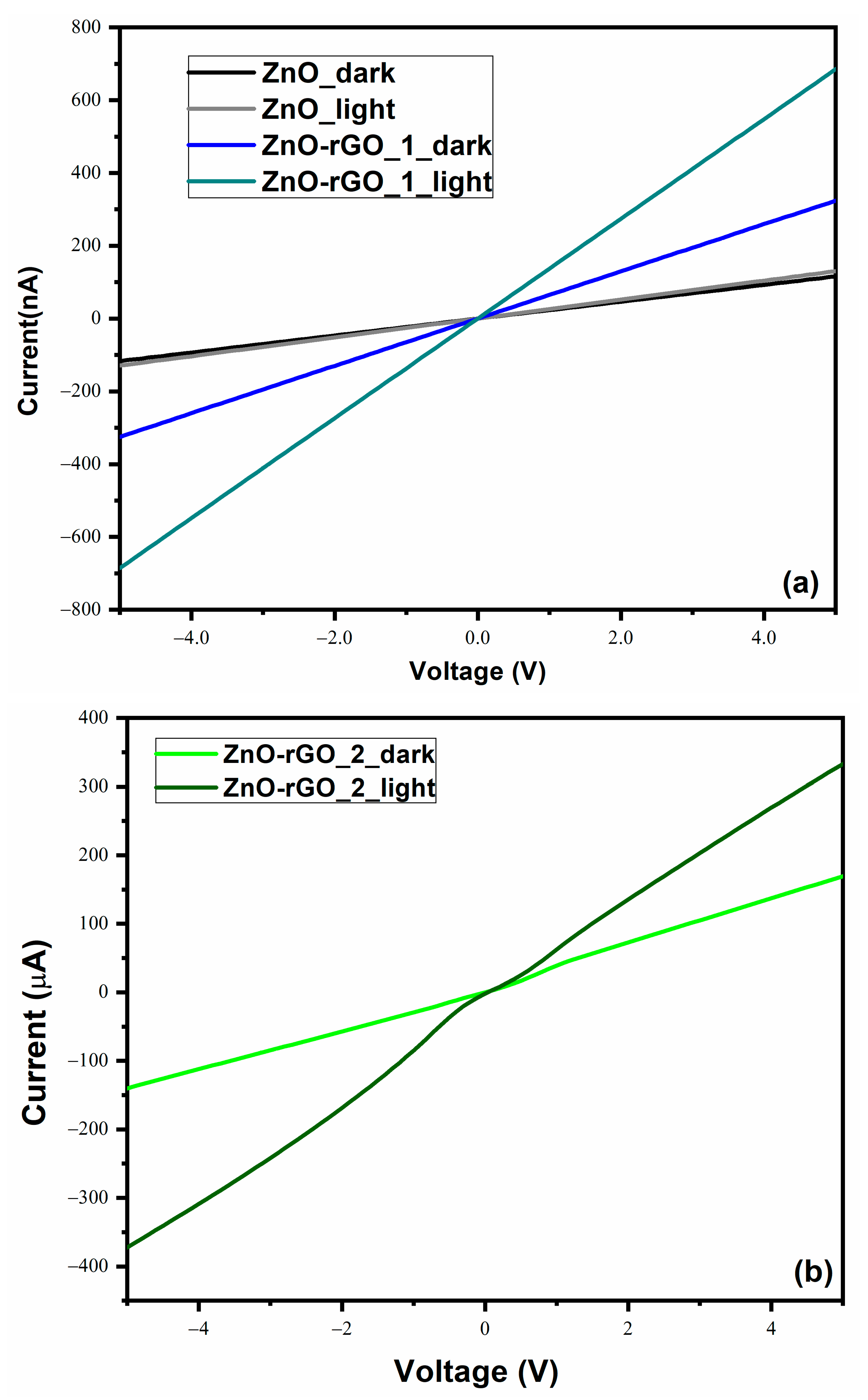
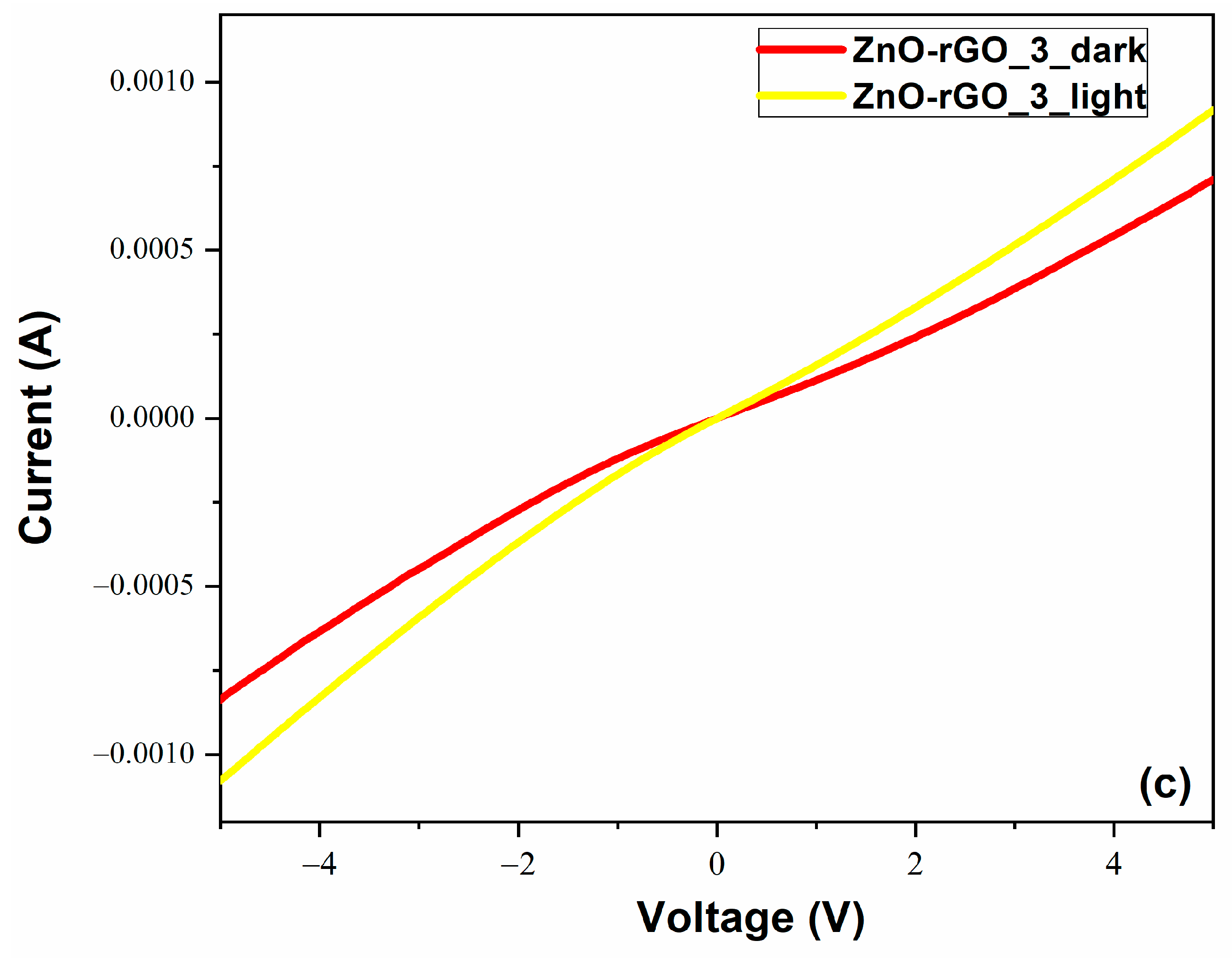
| Sample | Td (min) | Thicknesses (nm) | Position of (002) Peak (°) | B (°) (Broading or Half Width) | Crystallite Size (nm) | R(%) |
|---|---|---|---|---|---|---|
| ZnO | 2 | 130 | 34.60 | 0.506 | 16.0(1) | 8.96 |
| ZnO-rGO_1 | 2 | 150 | 34.39 | 0.577 | 16.3(1) | 9.76 |
| ZnO-rGO_2 | 3.5 | 250 | 34.31 | 0.550 | 17.2(2) | 9.29 |
| ZnO-rGO_3 | 5 | 370 | 34.44 | 0.545 | 17.5(1) | 9.91 |
| Sample | Concentration Atom (%) | |||
|---|---|---|---|---|
| Si | C | O | Zn | |
| ZnO | 5.10 | 0 | 49.81 | 45.00 |
| ZnO-rGO_1 | 0 | 2.96 | 43.45 | 52.77 |
| ZnO-rGO_2 | 0 | 8.57 | 31.42 | 60.01 |
| ZnO-rGO_3 | 0 | 13.79 | 27.58 | 58.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Amador, R.; Alvarado, J.; Flores-Carrasco, G.; Morales-de la Garza, L.; Alcántara-Iniesta, S.; Luna-Flores, A.; Bernal, Y.P.; Méndez Rojas, M.Á.; Gervacio-Arciniega, J.J.; Martínez Hernández, H.P.; et al. The Influence of Deposition Time on the Structural, Morphological, Optical and Electrical Properties of ZnO-rGO Nanocomposite Thin Films Grown in a Single Step by USP. Crystals 2020, 10, 73. https://doi.org/10.3390/cryst10020073
Ramírez-Amador R, Alvarado J, Flores-Carrasco G, Morales-de la Garza L, Alcántara-Iniesta S, Luna-Flores A, Bernal YP, Méndez Rojas MÁ, Gervacio-Arciniega JJ, Martínez Hernández HP, et al. The Influence of Deposition Time on the Structural, Morphological, Optical and Electrical Properties of ZnO-rGO Nanocomposite Thin Films Grown in a Single Step by USP. Crystals. 2020; 10(2):73. https://doi.org/10.3390/cryst10020073
Chicago/Turabian StyleRamírez-Amador, R., J. Alvarado, G. Flores-Carrasco, L. Morales-de la Garza, S. Alcántara-Iniesta, A. Luna-Flores, Y. P. Bernal, Miguel Ángel Méndez Rojas, J. J. Gervacio-Arciniega, H. P. Martínez Hernández, and et al. 2020. "The Influence of Deposition Time on the Structural, Morphological, Optical and Electrical Properties of ZnO-rGO Nanocomposite Thin Films Grown in a Single Step by USP" Crystals 10, no. 2: 73. https://doi.org/10.3390/cryst10020073






