Crystal Structure, Spectroscopy and Photocatalytic Properties of a Co(II) Complex Based on 5-(1,2,4-triazol-1-yl)pyridine-3-carboxylic Acid
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of [Co(tpa)2(H2O)4]·2H2O (1)
2.3. X-ray Crystallography
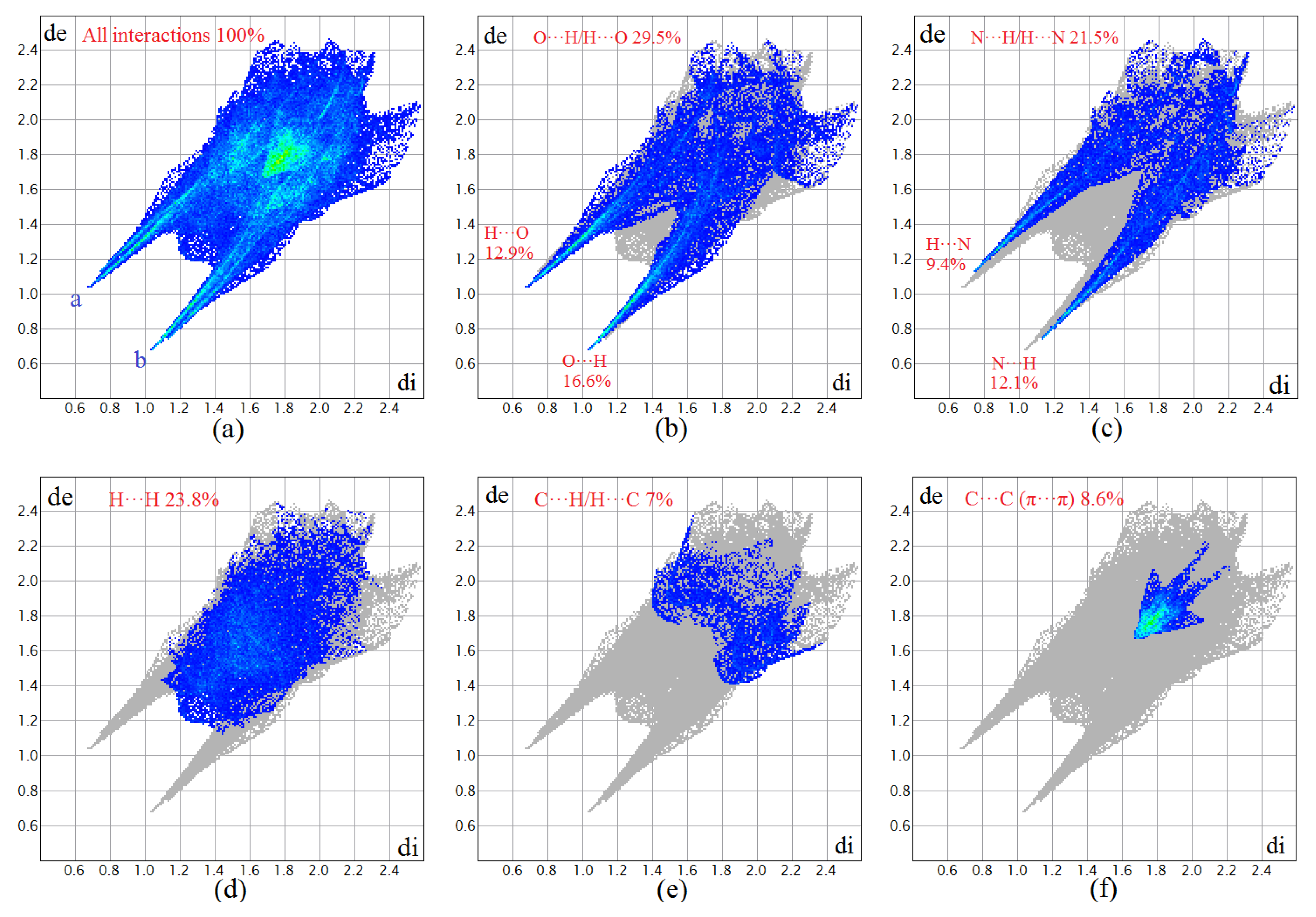
2.4. Hirshfeld Surface Analysis
2.5. Photocatalytic Experiments
3. Results
3.1. Crystal Structure of [Co(tpa)2(H2O)4]·2H2O
3.2. Hirshfeld Surface of [Co(tpa)2(H2O)4]·2H2O (1)
3.3. XRD and Thermal Stability
3.4. FT-IR and UV-Vis Absorption Spectra
3.5. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.L.; Fu, J.H.; Wei, J.J.; Xu, X.; Li, X.F.; Liu, Q.Y. Noncentrosymmetric organic solid and its zinc coordination polymer with diamonded network prepared from an ionothermal reaction: Syntheses, crystal structures, and second-order nonlinear optics properties. Cryst. Growth Des. 2012, 12, 4663–4668. [Google Scholar] [CrossRef]
- Luo, Y.S.; Chen, J.L.; Zeng, X.H.; Qiu, L.; He, L.H.; Liu, S.J.; Wen, H.R. A highly stable and luminescent mononuclear Cu(I) bis-{5-tert-butyl-3-(6-methyl-2-pyridyl)-1H-1,2,4-triazole} complex. Chin. Chem. Lett. 2017, 28, 1027–1030. [Google Scholar] [CrossRef]
- Yan, Y.T.; Zhang, S.S.; Yang, G.P.; Zhang, W.Y.; Zhang, F.; Cao, F.; Yang, R.F.; Wang, Y.Y. The influence of coordination modes and active sites of a 5-(triazol-1-yl) nicotinic ligand on the assembly of diverse MOFs. Dalton Trans. 2017, 46, 9784–9793. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.F.; Guo, M.; Li, X.L.; Zhao, L.; Sun, Q.F.; Layfield, R.A.; Tang, J.K. From double-shelled grids to supramolecular frameworks. Chem. Commun. 2018, 54, 12097–12100. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Wang, T.; Yan, T.; Du, L.; Zhao, Q.H. Crystal structures and spectroscopic characterizations of two Cd(II) complexes based on [1,2,4]-triazole derivatives. Chin. J. Inorg. Chem. 2017, 33, 1443–1449. [Google Scholar]
- Wang, D.W.; Wang, T.; Yan, T.; Du, L.; Zhao, Q.H. Crystal structure, spectroscopic and thermal properties of copper(II) and manganese(II) coordination polymers based on triazole-benzoic acid ligands. Transit. Met. Chem. 2018, 43, 1–8. [Google Scholar] [CrossRef]
- Harandi, Z.J.; Nasab, S.G.; Teimouri, A. Synthesis and characterisation of magnetic activated carbon/diopside nanocomposite for removal of reactive dyes from aqueous solutions: Experimental design and optimization. Int. J. Environ. Anal. Chem. 2019. [Google Scholar] [CrossRef]
- Cui, J.W.; Hou, S.X.; Li, Y.H.; Cui, G.H. A multifunctional Ni(II) coordination polymer: Synthesis, crystal structure and applications as a luminescent sensor, electrochemical probe, and photocatalyst. Dalton Trans. 2017, 46, 16911–16924. [Google Scholar] [CrossRef]
- Li, Z.R.; Mei, J.X.; Bai, L. Synthesis of C3N4‑decorated ZnO and Ag/ZnO nanoparticles via calcination of ZIF‑8 and melamine for photocatalytic removal of methyl orange. Chem. Pap. 2019, 73, 883–889. [Google Scholar] [CrossRef]
- Li, Z.C.; Ma, J.J.; Zhang, B.; Song, C.X.; Wang, D.B. Crystal phase- and morphology-controlled synthesis of MoO3 materials. CrystEngComm 2017, 19, 1479–1485. [Google Scholar] [CrossRef]
- Foura, G.; Chouchou, N.; Soualah, A.; Kouachi, K.; Guidotti, M.; Robert, D. Fe-doped TiO2 supported on HY zeolite for solar photocatalytic treatment of dye pollutants. Catalysts 2017, 7, 344. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Xu, K.C.; Wu, J.G.; Tan, C.F.; Ho, G.W.; Wei, A.; Hong, M.H. Ag-CuO-ZnO metal-semiconductor multiconcentric nanotubes for achieving superior and perdurable photodegradation. Nanoscale 2017, 9, 11574–11583. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; He, J.; Wang, S.Q.; Zou, L.K.; Wu, X.R. Syntheses, crystal structure, and photocatalytic property of two new complexes of an unsymmetrical Schiff base ligand. Inorg. Chim. Acta 2017, 458, 218–223. [Google Scholar] [CrossRef]
- Qin, L.; Xiao, S.L.; Ma, P.J.; Cui, G.H. Synthesis, crystal structures and catalytic properties of Ag(I) and Co(II) 1D coordination polymers constructed from bis(benzimidazolyl)butane. Transit. Met. Chem. 2013, 38, 627–633. [Google Scholar] [CrossRef]
- Li, J.X.; Li, Y.F.; Liu, L.W.; Cui, G.H. Luminescence, electrochemical and photocatalytic properties of sub-micron nickel(II) and cobalt(II) coordination polymers synthesized by sonochemical process. Ultrason. Sonochem. 2018, 41, 196–205. [Google Scholar] [CrossRef]
- Wang, D.W.; Yang, S.L.; Zhuang, C.F.; Wang, Y.; Shi, Z.J. Crystal structure, Hirshfeld surface analysis and photocatalytic activities of a cobalt(III) complex based on acid and alkaline mixed ligands. Transit. Met. Chem. 2019, 44, 455–461. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Zhang, C.G.; Li, Y.; Luo, Y.H.; Sun, B.W. Two novel salts of tris(hydroxymethyl)aminomethane (THAM): Synthesis, crystal structure, thermal and Hirshfeld surfaces analysis. J. Chem. Crystallogr. 2013, 43, 576–584. [Google Scholar] [CrossRef]
- Roy, S.; Harms, K.; Chattopadhyay, S. Synthesis, characterization and photocatalytic activity of a dinuclear thiocyanate bridged cadmium(II) Schiff base complex. Polyhedron 2017, 127, 471–477. [Google Scholar] [CrossRef]
- Luo, Y.H.; Liu, Q.L.; Yang, L.J.; Wang, W.; Ling, Y.; Sun, B.W. Quantitative comparisons between α, β, γ and δ pyrazinamide (PZA) polymorphs. Res. Chem. Intermed. 2015, 41, 7059–7072. [Google Scholar] [CrossRef]
- Seth, S.K.; Maity, G.C.; Kar, T. Structural elucidation, Hirshfeld surface analysis and quantum mechanical study of para-nitro benzylidene methyl arjunolate. J. Mol. Struct. 2011, 1000, 120–126. [Google Scholar] [CrossRef]
- Fang, D.L.; Mo, S.Y.; Wu, K.F.; Huang, Z.J. Synthesis, crystal structures, and properties of three coordination polymers of 5-(1H-imidazol-1-yl) isophthalic acid. Transit. Met. Chem. 2017, 42, 273–283. [Google Scholar] [CrossRef]
- Wang, D.W.; Wang, T.; Du, L.; Zhou, J.; Yan, T.; Zhao, Q.H. Four supramolecular transition metal(II) complexes based on triazole-benzoic acid derivatives: Crystal structure, Hirshfeld surface analysis, spectroscopic and thermal properties. Struct. Chem. 2018, 29, 1013–1023. [Google Scholar] [CrossRef]
- Azam, M.; Al-Resayes, S.; Wabaidur, S.M.; Trzesowska-Kruszynska, A.; Kruszynski, R.; Mohapatra, R.K.; Siddiqui, M.R. Cd(II) complex constructed from dipyridyl imine ligand: Design, synthesis and exploration of its photocatalytic degradation properties. Inorg. Chim. Acta 2018, 471, 698–704. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.L.; Xu, C.X.; Chen, S.F.; Fu, X.L. One-pot synthesis of ZnO2/ZnO composite with enhanced photocatalytic performance for organic dye removal. J. Nanosci. Nanotechnol. 2013, 13, 657–665. [Google Scholar] [CrossRef]
- Mansour, A.M.; Bakry, E.M.; Abdel-Ghani, N.T. Photocatalytic degradation of methylene blue with copper(II) oxide synthesized by thermal decomposition of flubendazole complexes. J. Photoch. Photobiol. A 2016, 327, 21–24. [Google Scholar] [CrossRef]
- Neto, J.O.M.; Bellato, C.R.; Souza, C.H.F.; Silva, R.C.; Rocha, P.A. Synthesis, characterization and enhanced photocatalytic activity of iron oxide/carbon nanotube/Ag-doped TiO2 nanocomposites. J. Braz. Chem. Soc. 2017, 28, 2301–2312. [Google Scholar]
- Zhang, X.T.; Fan, L.M.; Fan, W.L.; Li, B.; Liu, G.Z.; Liu, X.Z.; Zhao, X. Structural diversity, luminescence and photocatalytic properties of six coordination polymers based on designed bifunctional 2-(imidazol-1-yl)terephthalic acid. CrystEngComm 2016, 18, 6914–6925. [Google Scholar] [CrossRef]
- Wang, X.L.; Luan, J.; Lin, H.Y.; Lu, Q.L.; Le, M.; Liu, G.C.; Shao, J.Y. Metal(II)-organic coordination polymers modulated by two isomeric semirigid bis-pyridyl–bis-amide ligands: Structures, fluorescent sensing behavior, and selective photocatalysis. ChemPlusChem 2014, 79, 1691–1702. [Google Scholar] [CrossRef]
- Korala, L.; Germain, J.R.; Chen, E.; Pala, I.R.; Li, D.; Brock, S.L. CdS aerogels as efficient photocatalysts for degradation of organic dyes under visible light irradiation. Inorg. Chem. Front. 2017, 4, 1451–1457. [Google Scholar] [CrossRef]
- Lin, S.; Diercks, C.S.; Zhang, Y.B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.D.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1212. [Google Scholar] [CrossRef] [Green Version]




| Complex 1 | |
|---|---|
| Empirical formula | C16H22CoN8O10 |
| Formula weight | 545.34 |
| Temperature/K | 293(2) |
| Crystal system | triclinic |
| Space group | P ī |
| a/Å | 6.8212(9) |
| b/Å | 9.0225(12) |
| c/Å | 10.1018(13) |
| α/° | 63.8650(10) |
| β/° | 83.2140(10) |
| γ/° | 76.5600(10) |
| Volume/Å3 | 542.77(12) |
| Z | 1 |
| ρcalcg/cm3 | 1.668 |
| F(000) | 281.0 |
| Crystal size/mm3 | 0.34 × 0.23 × 0.14 |
| Radiation | MoKα (λ = 0.71073) |
| Independent reflections | 1880, Rint = 0.0149 |
| restraints/parameters | 0/165 |
| Goodness-of-fit on F2 | 1.087 |
| Final R indexes [I > = 2σ (I)] | R1 = 0.0296, wR2 = 0.0781 |
| Final R indexes [all data] | R1 = 0.0311, wR2 = 0.0788 |
| L. diff. peak/hole /e Å−3 | 0.36/−0.34 |
| Complex 1 | |||
|---|---|---|---|
| Co1−N1 | 2.1821(17) | O1W1−Co1−N11 | 89.62(6) |
| Co1−O1W | 2.0933(16) | O1W−Co1−N11 | 90.38(6) |
| Co1−O2W | 2.0757(15) | O2W−Co1−O1W | 89.98(7) |
| O1W−Co1−O1W1 | 180.0 | O2W1−Co1−O1W | 90.02(7) |
| D-H···A | d(D-H)/Å | d(H···A)/Å | d(D···A)/Å | <DHA/° | Symmetry Code 1 |
|---|---|---|---|---|---|
| O1W-H1WA···N3A | 0.881 | 1.970 | 2.818 | 161.31 | −x, −y, −z + 1 |
| O1W-H1WB···O1 | 0.880 | 1.953 | 2.778 | 155.63 | −x, −y, −z |
| O2W-H2WA···N2A | 0.858 | 2.200 | 2.892 | 137.61 | −x, −y, −z |
| O2W-H2WB···O3W | 0.858 | 1.905 | 2.651 | 144.42 | −x, −y + 1, −z |
| O3W-H3WA···O1 | 0.850 | 1.914 | 2.733 | 161.22 | −x + 1, −y, −z |
| O3W-H3WB···O2 | 0.850 | 1.846 | 2.691 | 172.85 | x, y, z + 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhao, N.; Wang, T.; Zhuang, C.; Wang, Y.; Yang, B. Crystal Structure, Spectroscopy and Photocatalytic Properties of a Co(II) Complex Based on 5-(1,2,4-triazol-1-yl)pyridine-3-carboxylic Acid. Crystals 2020, 10, 98. https://doi.org/10.3390/cryst10020098
Wang D, Zhao N, Wang T, Zhuang C, Wang Y, Yang B. Crystal Structure, Spectroscopy and Photocatalytic Properties of a Co(II) Complex Based on 5-(1,2,4-triazol-1-yl)pyridine-3-carboxylic Acid. Crystals. 2020; 10(2):98. https://doi.org/10.3390/cryst10020098
Chicago/Turabian StyleWang, Dawei, Ning Zhao, Tao Wang, Changfu Zhuang, Ying Wang, and Bin Yang. 2020. "Crystal Structure, Spectroscopy and Photocatalytic Properties of a Co(II) Complex Based on 5-(1,2,4-triazol-1-yl)pyridine-3-carboxylic Acid" Crystals 10, no. 2: 98. https://doi.org/10.3390/cryst10020098
APA StyleWang, D., Zhao, N., Wang, T., Zhuang, C., Wang, Y., & Yang, B. (2020). Crystal Structure, Spectroscopy and Photocatalytic Properties of a Co(II) Complex Based on 5-(1,2,4-triazol-1-yl)pyridine-3-carboxylic Acid. Crystals, 10(2), 98. https://doi.org/10.3390/cryst10020098





