Influence of Oxidation on Temperature-Dependent Photoluminescence Properties of Hydrogen-Terminated Silicon Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of ncSi-H
2.3. Characterization
3. Results
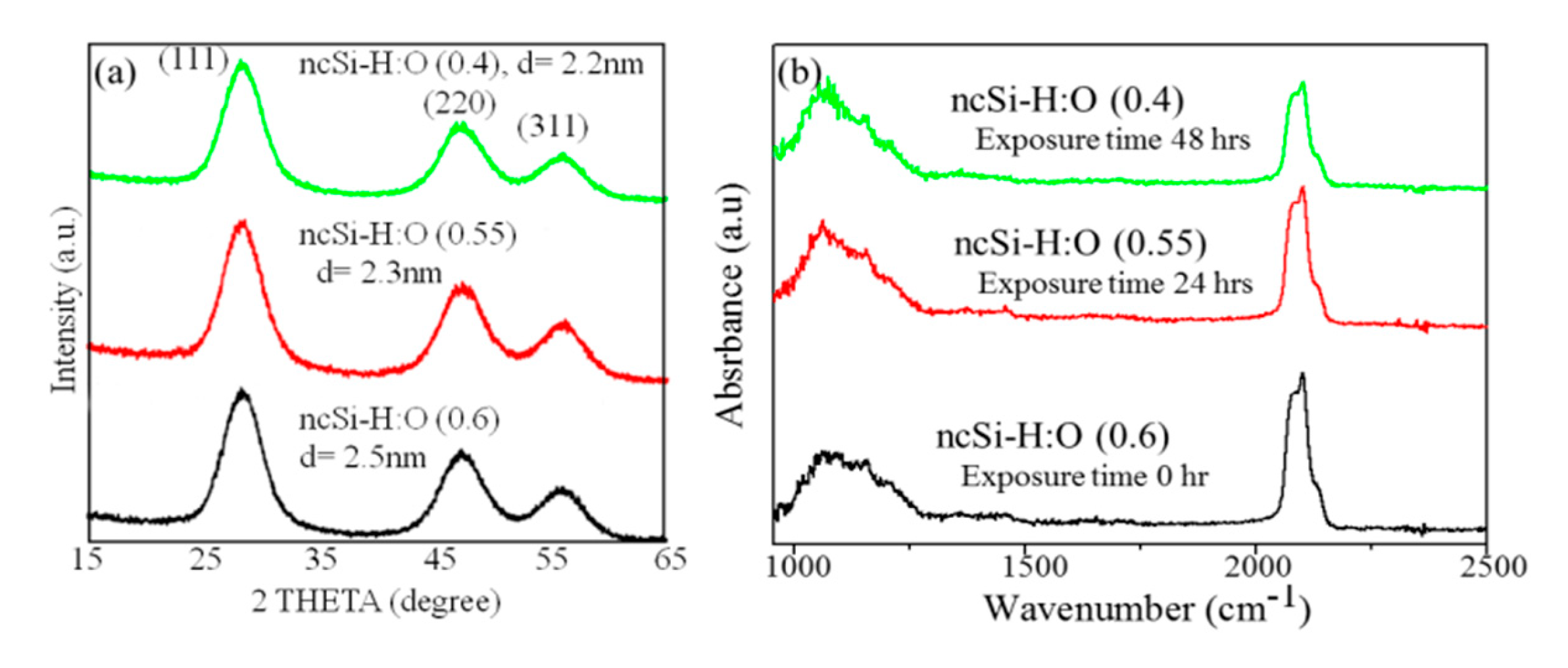
3.1. Analysis of the Surface Configuration
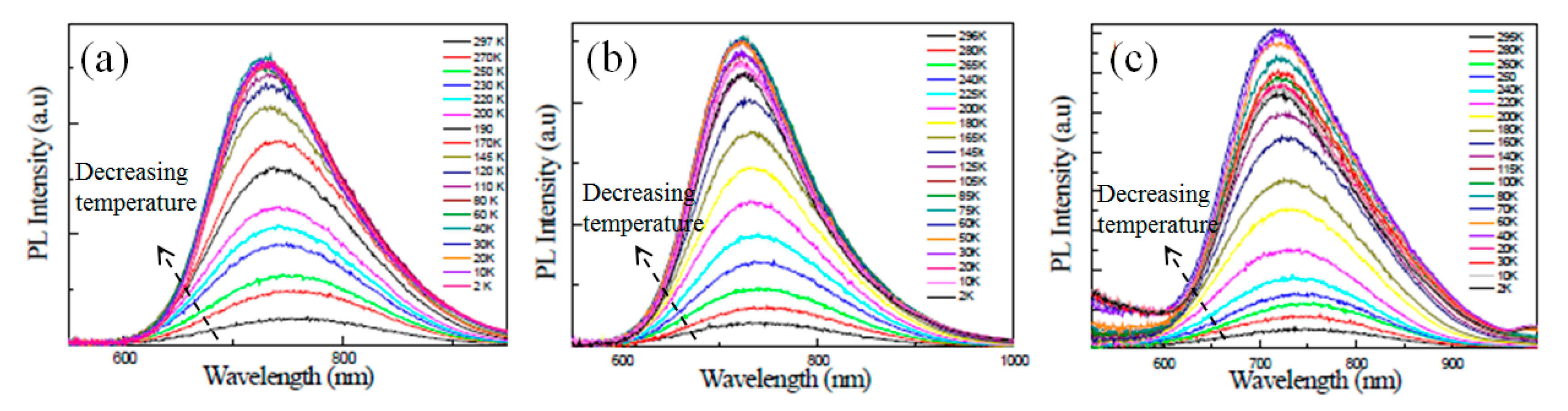
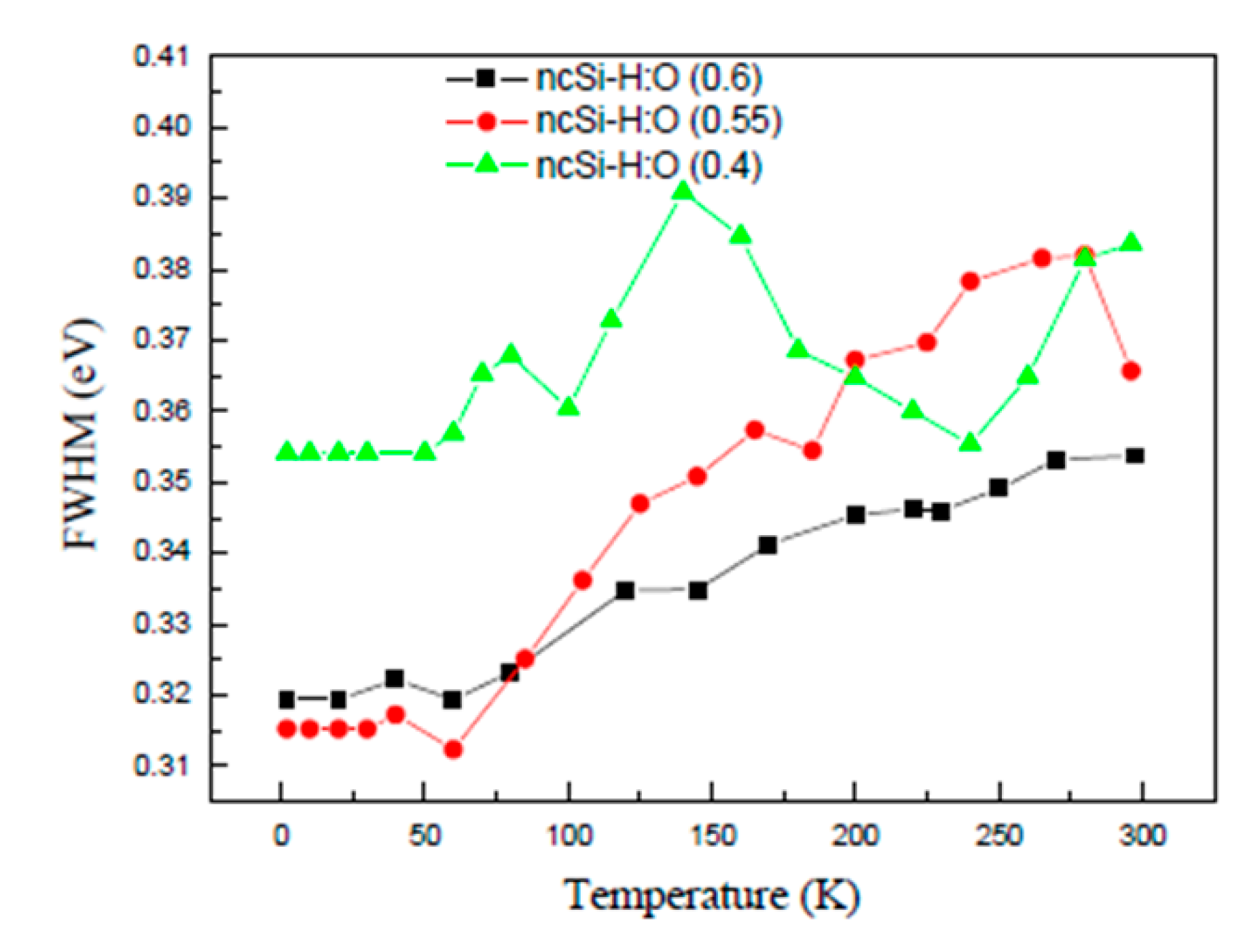
3.2. Temperature Dependence of the PL Intensity
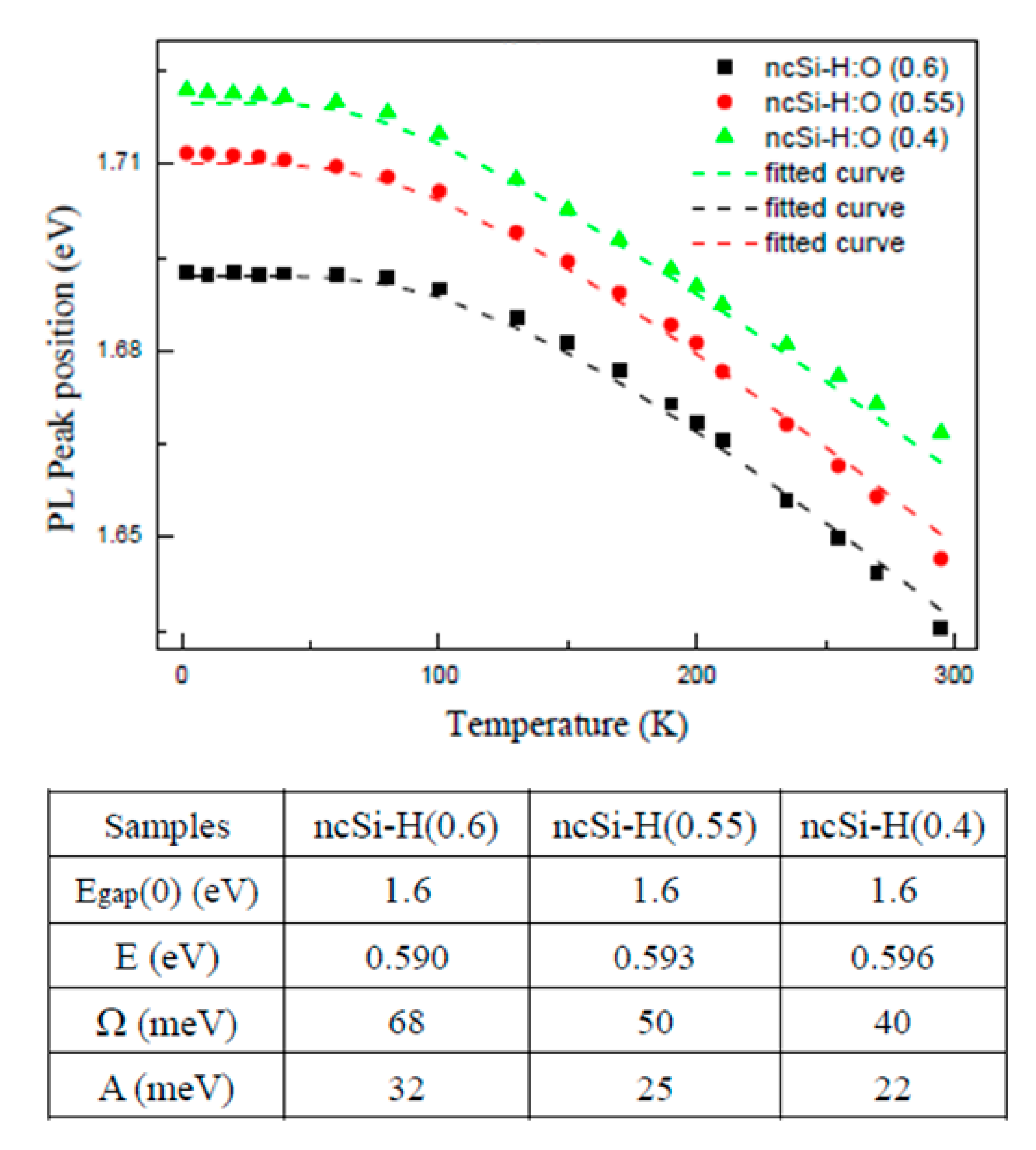
3.3. Temperature Dependence of the PL Energy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chandra, S.; Ghosh, B.; Beaune, G.; Nagarajan, U.; Yasui, T.; Nakamura, J.; Tsuruoka, T.; Baba, Y.; Shirahata, N.; Winnik, F.M. Functional Double-Shelled Silicon Nanocrystals for Two-Photon Fluorescence Cell Imaging: Spectral Evolution and Tuning. Nanoscale 2016, 8, 9009. [Google Scholar] [CrossRef] [PubMed]
- Maier-Flaig, F.; Rinck, J.; Stephan, M.; Bocksrocker, T.; Bruns, M.; Kübel, C.; Powell, A.K.; Ozin, G.A.; Lemmer, U. Multicolor Silicon Light-Emitting Diodes (SiLEDs). Nano Lett. 2013, 13, 475. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Masuda, Y.; Wakayama, Y.; Imanaka, Y.; Inoue, J.I.; Hashi, K.; Deguchi, K.; Yamada, H.; Sakka, Y.; Ohki, S. Hybrid White Light Emitting Diode Based on Silicon Nanocrystals. Adv. Funct. Mater. 2014, 24, 7151. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S.; Gu, W.; Zhang, Y.; Qiao, X.; Ni, Z.; Pi, X.; Yang, D. Light-Emitting Diodes Based on Colloidal Silicon Quantum Dots with Octyl and Phenylpropyl Ligands. ACS Appl. Mater. Interfaces 2018, 10, 5959. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-Y.; Anthony, R.; Kortshagen, U.R.; Holmes, R.J. High-Efficiency Silicon Nanocrystal Light-Emitting Devices. Nano Lett. 2011, 11, 1952. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, D.P.; Henderson, E.J.; Helander, M.G.; Wang, Z.; Ozin, G.A.; Lu, Z. Visible Colloidal Nanocrystal Silicon Light-Emitting Diode. Nano Lett. 2011, 11, 1585. [Google Scholar] [CrossRef]
- Ghosh, B.; Yamada, H.; Chinnathambi, S.; Özbilgin, İ.N.G.; Shirahata, N. Inverted Device Architecture for Enhanced Performance of Flexible Silicon Quantum Dot Light-Emitting Diode. J. Phys. Chem. Lett. 2018, 9, 5400–5407. [Google Scholar] [CrossRef]
- Erogbogbo, F.; Chang, C.-W.; May, J.L.; Liu, L.; Kumar, R.; Law, W.-C.; Ding, H.; Yong, K.T.; Roy, I.; Sheshadri, M. Bioconjugation of luminescent silicon quantum dots to gadolinium ions for bioimaging applications. Nanoscale 2012, 4, 5483–5489. [Google Scholar] [CrossRef]
- Montalti, M.; Cantelli, A.; Battistelli, G. Nanodiamonds and silicon quantum dots: Ultrastable and biocompatible luminescent nanoprobes for long-term bioimaging. Chem. Soc. Rev. 2015, 44, 4853–4921. [Google Scholar] [CrossRef]
- Ghosh, B.; Hamaoka, T.; Nemoto, Y.; Takeguchi, M.; Shirahata, N. Impact of Anchoring Monolayers on the Enhancement of Radiative Recombination in Light-Emitting Diodes Based on Silicon Nanocrystals. J. Phys. Chem. C 2018, 122, 6422. [Google Scholar] [CrossRef]
- Hessel, C.M.; Reid, D.; Panthani, M.G.; Rasch, M.R.; Goodfellow, B.W.; Wei, J.; Fujii, H.; Akhavan, V.; Korgel, B.A. Synthesis of Ligand-Stabilized Silicon Nanocrystals with Size-Dependent Photoluminescence Spanning Visible to Near-Infrared Wavelengths. Chem. Mater. 2012, 24, 393. [Google Scholar] [CrossRef]
- Jurbergs, D.; Rogojina, E.; Mangolini, L.; Kortshagen, U. Silicon nanocrystals with ensemble quantum yields exceeding 60%. Appl. Phys. Lett. 2006, 88, 233116. [Google Scholar] [CrossRef]
- Henderson, E.J.; Kelly, J.A.; Veinot, J.G.C. Influence of HSiO1.5 Sol–Gel Polymer Structure and Composition on the Size and Luminescent Properties of Silicon Nanocrystals. Chem. Mater. 2009, 21, 5426–5434. [Google Scholar]
- Veinot, J.G.C. Synthesis, surface functionalization, and properties of freestanding silicon nanocrystals. Chem. Commun. 2006, 4160–4168. [Google Scholar] [CrossRef]
- Pi, X.D.; Mangolini, L.; Campbell, S.A.; Kortshagen, U. Room-temperature atmospheric oxidation of Si nanocrystals after HF etching. Phys. Rev. B 2007, 75, 85423. [Google Scholar] [CrossRef]
- Mastronardi, M.L.; Henderson, E.J.; Puzzo, D.P.; Chang, Y.; Wang, Z.B.; Helander, M.G.; Jeong, J.; Kherani, N.P.; Lu, Z.; Ozin, G.A. Silicon Nanocrystal Oleds: Effect of Organic Capping Group on Performance. Small 2012, 8, 3647. [Google Scholar] [CrossRef]
- Puzder, A.; Williamson, A.J.; Grossman, J.C.; Galli, G. Surface Chemistry of Silicon Nanoclusters. Phys. Rev. Lett. 2002, 88, 97401. [Google Scholar] [CrossRef] [Green Version]
- Takeoka, S.; Fujii, M.; Hayashi, S. Size-dependent photoluminescence from surface-oxidized Si nanocrystals in a weak confinement regime. Phys. Rev. B 2000, 62, 16820–16825. [Google Scholar] [CrossRef]
- Shirahata, N.; Hirakawa, D.; Sakka, Y. Interfacial-related color tuning of colloidal Si nanocrystals. Green Chem. 2010, 12, 2139–2141. [Google Scholar] [CrossRef]
- Mitra, S.; Švrček, V.; Macias-Montero, M.; Velusamy, T.; Mariotti, D. Temperature-dependent photoluminescence of surface-engineered silicon nanocrystals. Sci. Rep. 2016, 6, 27727. [Google Scholar] [CrossRef] [Green Version]
- Švrček, V.; Pelant, I.; Rehspringer, J.-L.; Gilliot, P.; Ohlmann, D.; Crégut, O.; Hönerlage, B.; Chvojka, T.; Valenta, J.; Dian, J. Photoluminescence properties of sol–gel derived SiO2 layers doped with porous silicon. Mater. Sci. Eng. C 2002, 19, 233–236. [Google Scholar] [CrossRef]
- Bürkle, M.; Lozac’h, M.; McDonald, C.; Mariotti, D.; Matsubara, K.; Švrček, V. Bandgap Engineering in OH-Functionalized Silicon Nanocrystals: Interplay between Surface Functionalization and Quantum Confinement. Adv. Funct. Mater. 2017, 27, 1701898. [Google Scholar] [CrossRef] [Green Version]
- Valenta, J.; Greben, M.; Dyakov, S.A.; Gippius, N.A.; Hiller, D.; Gutsch, S.; Zacharias, M. Nearly perfect near-infrared luminescence efficiency of Si nanocrystals: A comprehensive quantum yield study employing the Purcell effect. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guerra, R.; Degoli, E.; Ossicini, S. Size, oxidation, and strain in small Si/SiO 2 nanocrystals. Phys. Rev. B 2009, 80, 155332. [Google Scholar] [CrossRef] [Green Version]
- Svrcek, V.; Dohnalova, K.; Mariotti, D.; Trinh, M.T.; Limpens, R.; Mitra, S.; Gregorkiewicz, T.; Matsubara, K.; Kondo, M. Dramatic Enhancement of Photoluminescence Quantum Yields for Surface-Engineered Si Nanocrystals within the Solar Spectrum. Adv. Funct. Mater. 2013, 23, 6051–6058. [Google Scholar] [CrossRef]
- Wen, X.; Van Dao, L.; Hannaford, P. Temperature dependence of photoluminescence in silicon quantum dots. J. Phys. D Appl. Phys. 2007, 40, 3573. [Google Scholar] [CrossRef]
- Suemoto, T.; Tanaka, K.; Nakajima, A. Interpretation of the temperature dependence of the luminescence intensity, lifetime, and decay profiles in porous Si. Phys. Rev. B 1994, 49, 11005–11009. [Google Scholar] [CrossRef]
- Rinnert, H.; Jambois, O.; Vergnat, M. Photoluminescence properties of size-controlled silicon nanocrystals at low temperatures. J. Appl. Phys. 2009, 106, 23501. [Google Scholar] [CrossRef]
- Van Sickle, A.R.; Miller, J.B.; Moore, C.; Anthony, R.J.; Kortshagen, U.R.; Hobbie, E.K. Temperature dependent photoluminescence of size-purified silicon nanocrystals. ACS Appl. Mater. Interfaces 2013, 5, 4233–4238. [Google Scholar] [CrossRef]
- Alex, V.; Finkbeiner, S.; Weber, J. Temperature dependence of the indirect energy gap in crystalline silicon. J. Appl. Phys. 1996, 79, 6943–6946. [Google Scholar] [CrossRef]
- Gupta, A.; Wiggers, H. Freestanding silicon quantum dots: Origin of red and blue luminescence. Nanotechnology 2010, 22, 55707. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Takeguchi, M.; Nakamura, J.; Nemoto, Y.; Hamaoka, T.; Chandra, S.; Shirahata, N. Origin of the photoluminescence quantum yields enhanced by alkane-termination of freestanding silicon nanocrystals: Temperature-dependence of optical properties. Sci. Rep. 2016, 6, 36951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartel, A.M.; Gutsch, S.; Hiller, D.; Zacharias, M. Fundamental temperature-dependent properties of the Si nanocrystal band gap. Phys. Rev. B 2012, 85, 165306. [Google Scholar] [CrossRef]
- Ghosh, B.; Shirahata, N. Colloidal silicon quantum dots: Synthesis and luminescence tuning from the near-UV to the near-IR range. Sci. Technol. Adv. Mater. 2014, 15, 014207. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.I.; Syväjärvi, M.; Bergman, J.P.; Kordina, O.; Henry, A.; Monemar, B.; Janzén, E. Time-resolved decay of the blue emission in porous silicon. Appl. Phys. Lett. 1994, 65, 2451. [Google Scholar] [CrossRef]
- Kovalev, D.; Heckler, H.; Polisski, G.; Koch, F. Optical properties of Si nanocrystals. Phys. Stat. Sol. 1999, 215, 871–932. [Google Scholar] [CrossRef]
- De Boer, W.; Timmerman, D.; Gregorkiewicz, T.; Zhang, H.; Buma, W.J.; Poddubny, A.N.; Prokofiev, A.A.; Yassievich, I.N. Self-trapped exciton state in Si nanocrystals revealed by induced absorption. Phys. Rev. B 2012, 85, 161409. [Google Scholar] [CrossRef] [Green Version]
- Prezhdo, O.V. Multiple excitons and the electron–phonon bottleneck in semiconductor quantum dots: An ab initio perspective. Chem. Phys. Lett. 2008, 460, 1–9. [Google Scholar] [CrossRef]
- Varshni, Y.P. Temperature dependence of the energy gap in semiconductors. Physica 1967, 34, 149–154. [Google Scholar] [CrossRef]
- Viña, L.; Logothetidis, S.; Cardona, M. Temperature dependence of the dielectric function of germanium. Phys. Rev. B 1984, 30, 1979–1991. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, B.; Shirahata, N. Influence of Oxidation on Temperature-Dependent Photoluminescence Properties of Hydrogen-Terminated Silicon Nanocrystals. Crystals 2020, 10, 143. https://doi.org/10.3390/cryst10030143
Ghosh B, Shirahata N. Influence of Oxidation on Temperature-Dependent Photoluminescence Properties of Hydrogen-Terminated Silicon Nanocrystals. Crystals. 2020; 10(3):143. https://doi.org/10.3390/cryst10030143
Chicago/Turabian StyleGhosh, Batu, and Naoto Shirahata. 2020. "Influence of Oxidation on Temperature-Dependent Photoluminescence Properties of Hydrogen-Terminated Silicon Nanocrystals" Crystals 10, no. 3: 143. https://doi.org/10.3390/cryst10030143





