Experimental Study on Zn-Doped Al-Rich Alloys for Fast on-Board Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Characterization
2.3. Measurement of Hydrogen Production
3. Results
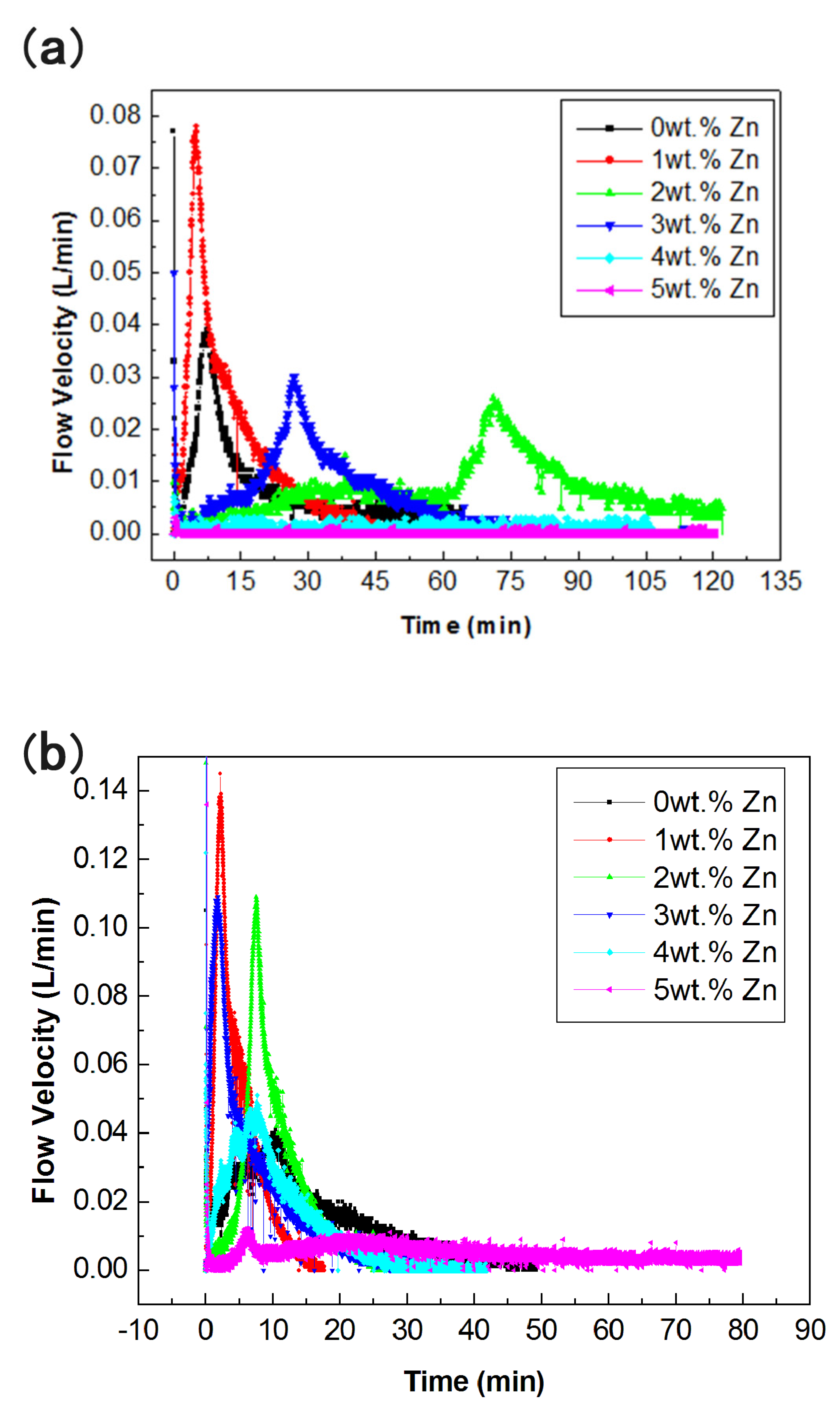
3.1. Hydrogen Generation Performance
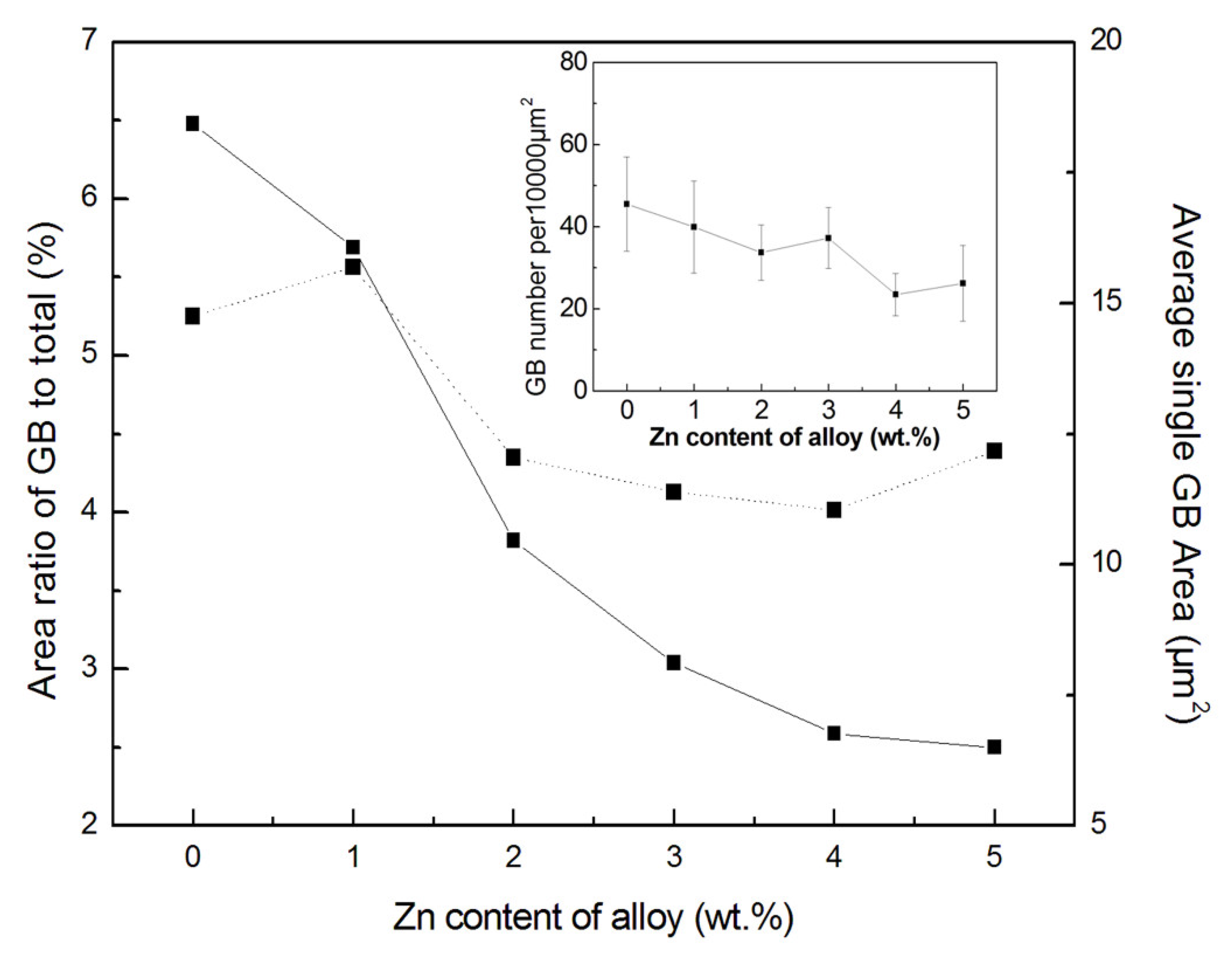
3.2. Characterization of Al Alloys
3.2.1. XRD Analysis
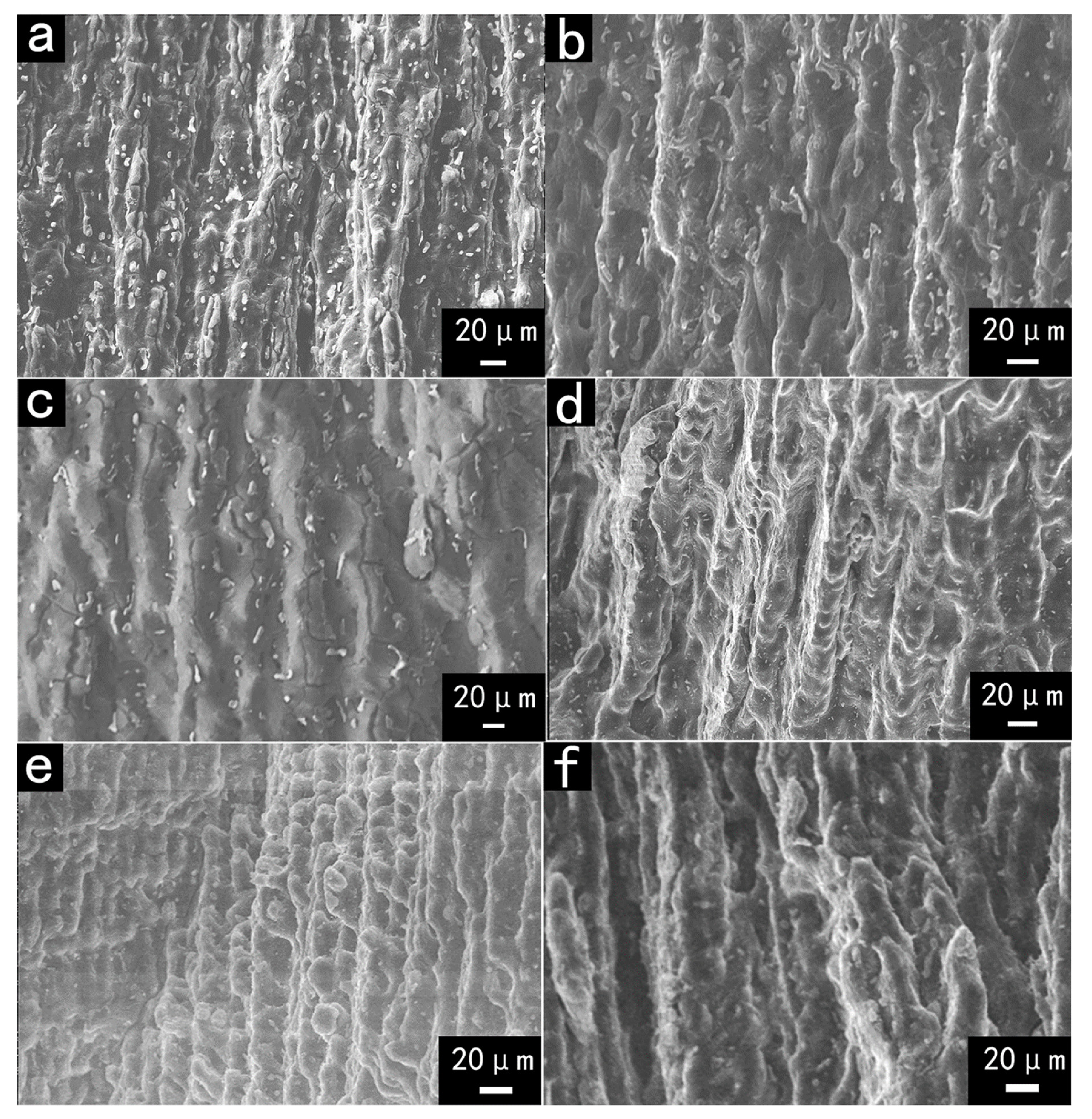
3.2.2. SEM Observation
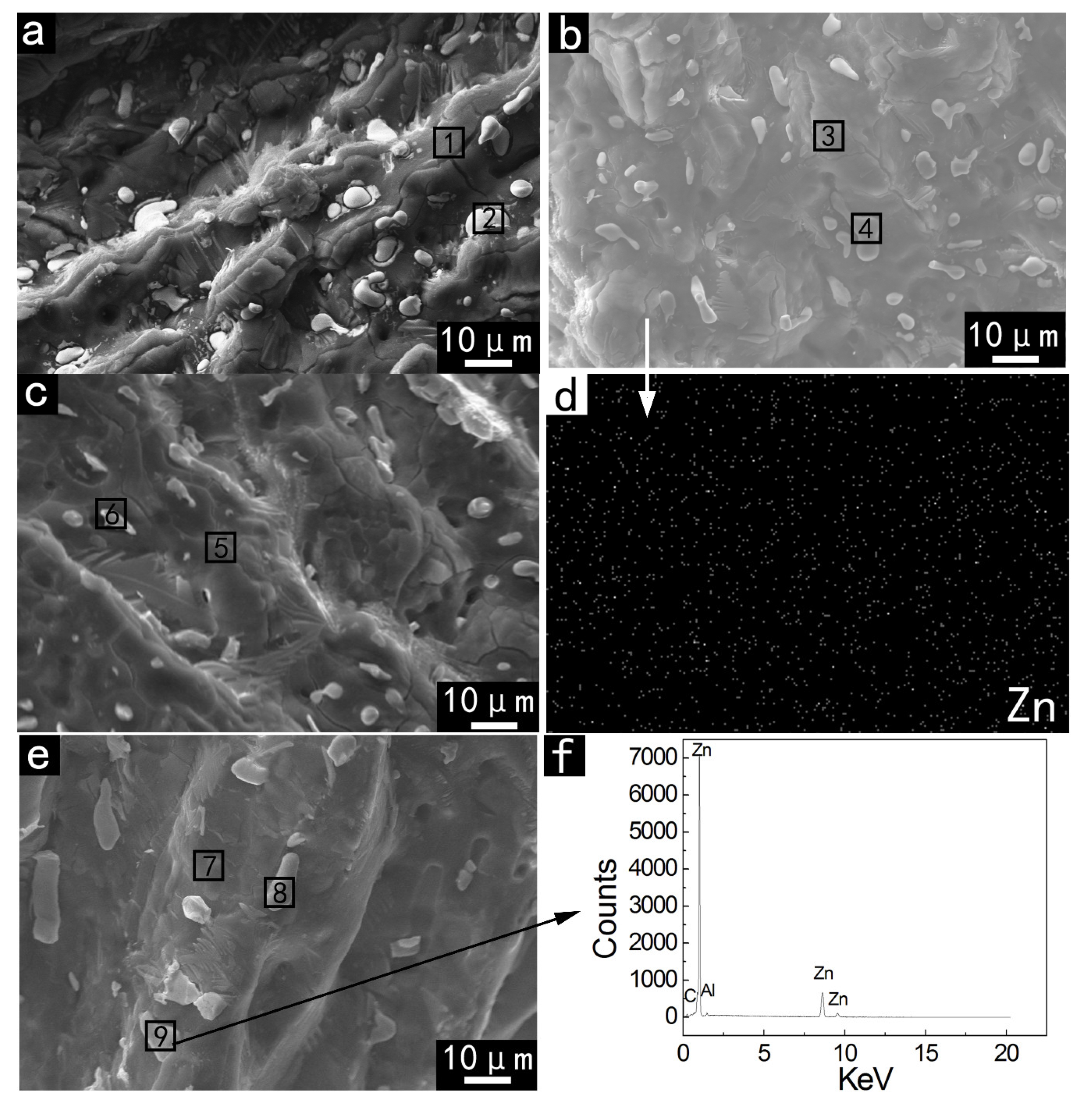
3.2.3. EDX Analysis
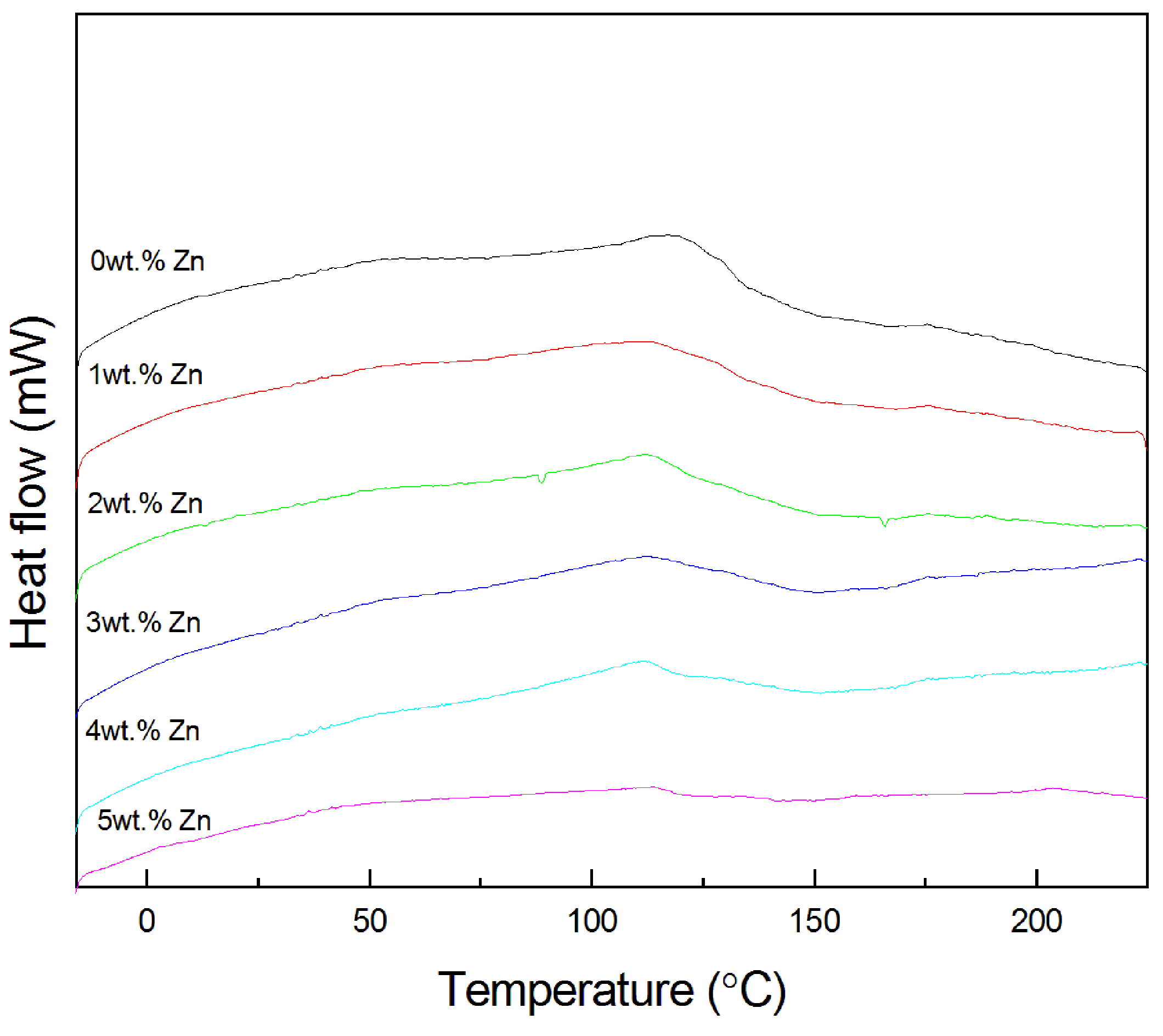
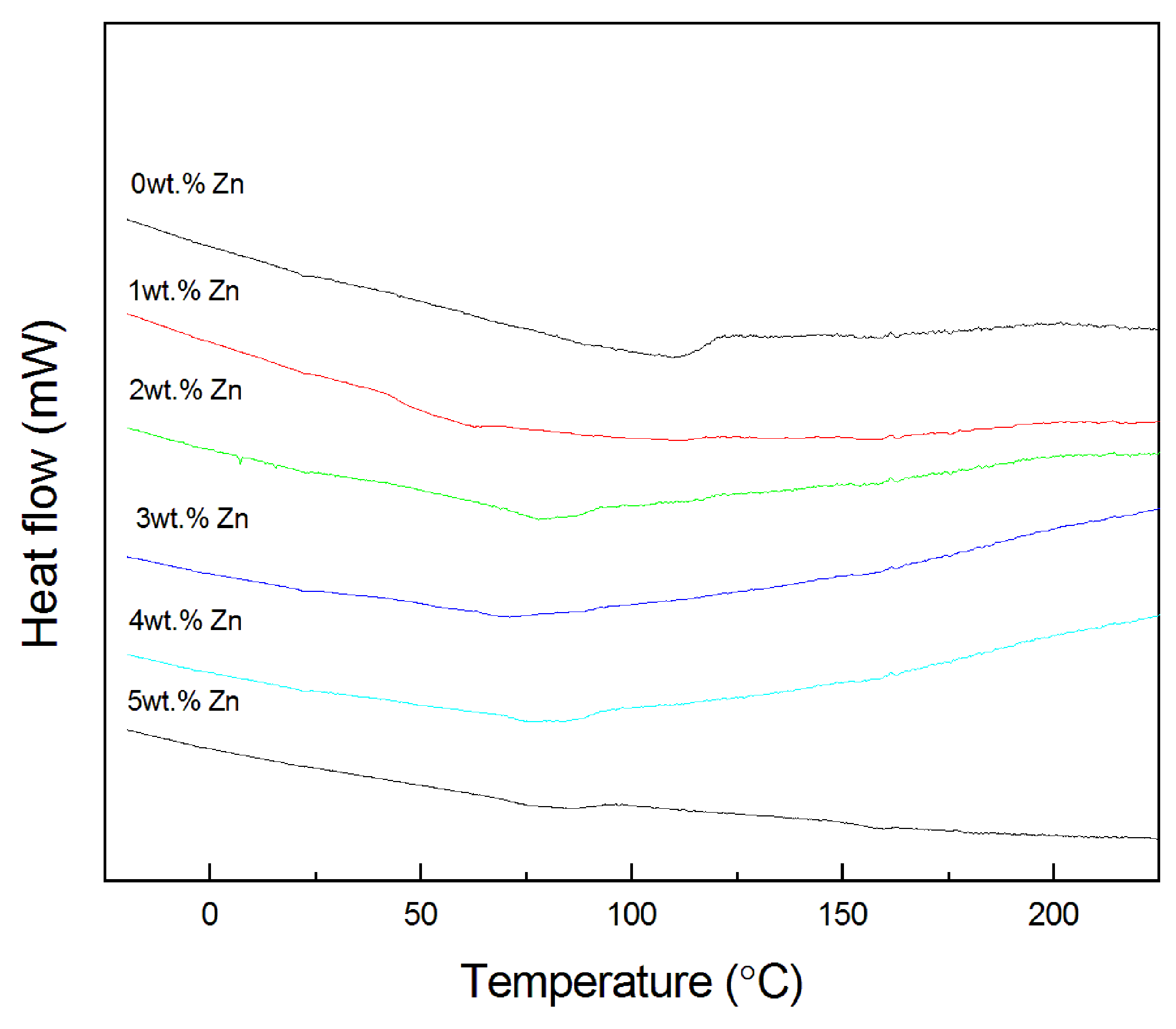
3.2.4. DSC Curves
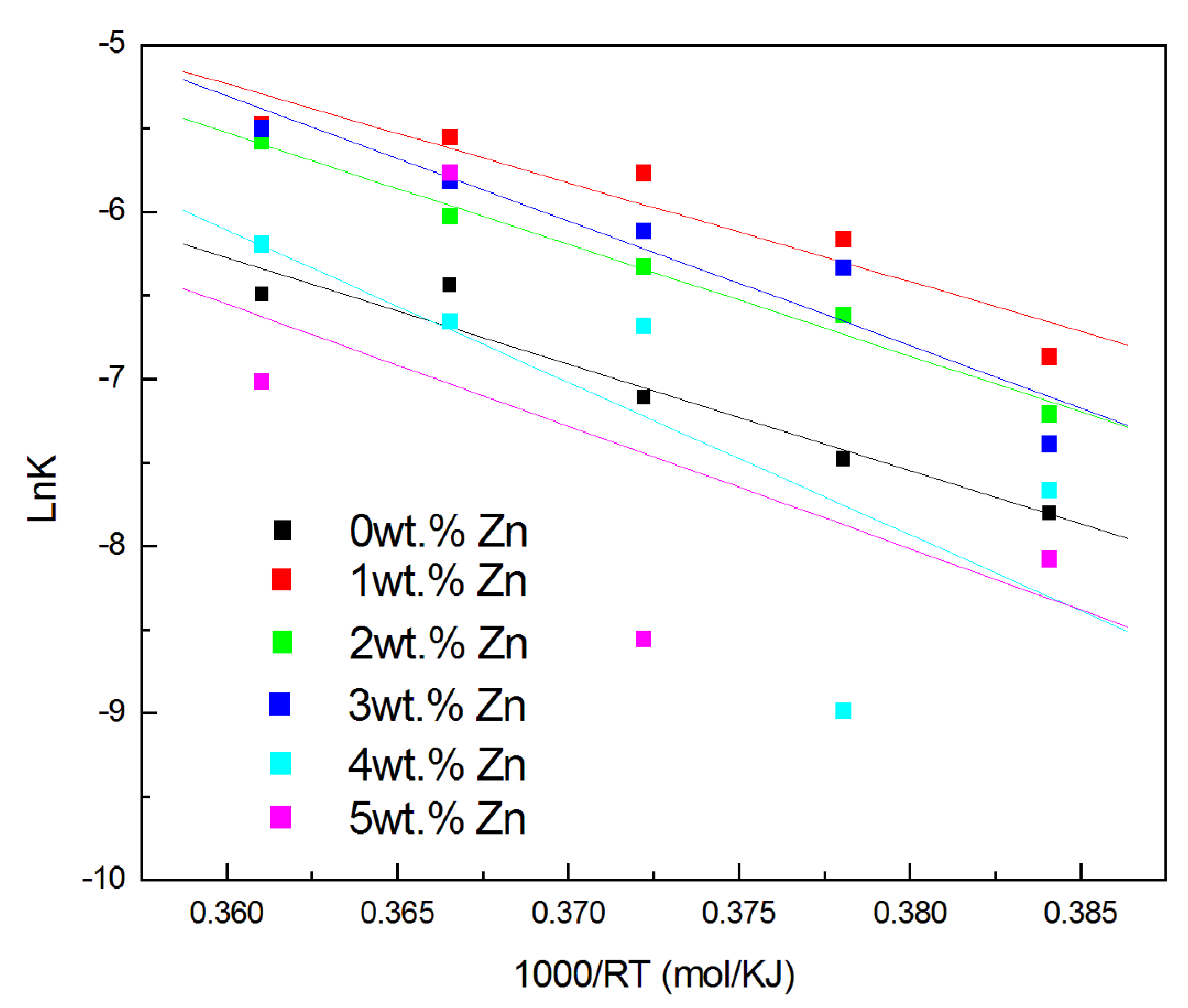
3.3. Kinetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ajanovic, A.; Haas, R. Economic prospects and policy framework for hydrogen as fuel in the transport sector. Energy Policy 2018, 123, 280–288. [Google Scholar] [CrossRef]
- Lincoln, S.F. Fossil fuels in the 21st century. Ambio 2005, 34, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Veziroglu, T.N. 21st century's energy: Hydrogen energy system. In Assessment of Hydrogen Energy for Sustainable Development; Sheffield, J.W., Sheffield, C., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 9–31. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H.M. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Secer, A.; Kucet, N.; Faki, E.; Hasanoglu, A. Comparison of co-gasification efficiencies of coal, lignocellulosic biomass and biomass hydrolysate for high yield hydrogen production. Int. J. Hydrogen Energy 2018, 43, 21269–21278. [Google Scholar] [CrossRef]
- Demirbas, A. Comparison of thermochemical conversion processes of biomass to hydrogen-rich gas mixtures. Energy Sources A Recovery Util. Environ. Eff. 2016, 38, 2971–2976. [Google Scholar] [CrossRef]
- Yuan, B.; Tan, S.; Liu, J. Dynamic hydrogen generation phenomenon of aluminum fed liquid phase Ga–In alloy inside NaOH electrolyte. Int. J. Hydrogen Energy 2016, 41, 1453–1459. [Google Scholar] [CrossRef]
- Xu, S.; Liu, J. Metal-based direct hydrogen generation as unconventional high density energy. Front. Energy 2018, 13, 27–53. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, J.; Jiang, T.; Gou, Y.; Hou, X.; Pan, B. Effect of heat treatment on Al Mg Ga In Sn alloy for hydrogen generation through hydrolysis reaction. Int. J. Hydrogen Energy 2017, 42, 24393–24403. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Gai, W.-Z.; Zhang, X.-Y.; Pan, H.-Y.; Cheng, Z.; Xu, P.; Deng, Z.-Y. Effect of storage environment on hydrogen generation by the reaction of Al with water. RSC Adv. 2017, 7, 2103–2109. [Google Scholar] [CrossRef] [Green Version]
- Tekade, S.P.; Pednekar, A.S.; Jadhav, G.R.; Kalekar, S.E.; Shende, D.Z.; Wasewar, K.L. Hydrogen generation through water splitting reaction using waste aluminum in presence of gallium. Int. J. Hydrogen Energy 2019. [Google Scholar] [CrossRef]
- Guan, X.; Zhou, Z.; Luo, P.; Wu, F.; Dong, S. Effects of preparation method on the hydrolytic hydrogen production performance of Al-rich alloys. J. Alloys Compd. 2019, 796, 210–220. [Google Scholar] [CrossRef]
- Guan, X.; Zhou, Z.; Luo, P.; Wu, F.; Dong, S. Hydrogen generation from the reaction of Al-based composites activated by low-melting-point metals/oxides/salts with water. Energy 2019, 188. [Google Scholar] [CrossRef]
- Yu, M.; Kim, M.; Yoon, B.; Oh, S.; Nam, D.-H.; Kwon, H. Carbon nanotubes/aluminum composite as a hydrogen source for PEMFC. Int. J. Hydrogen Energy 2014, 39, 19416–19423. [Google Scholar] [CrossRef]
- Liu, Y.; An, J.C.; Jin, W.Z.; Cui, J.; Zhang, W. Insight into the effect of In addition on hydrogen generation behavior and hydrolysis mechanism of Al-based ternary alloys in distilled water—A new active Al-Ga-In hydrolysis hydrogen generation alloy. Int. J. Energy Res. 2019, 43, 8973–8984. [Google Scholar] [CrossRef]
- Godart, P.; Fischman, J.; Seto, K.; Hart, D. Hydrogen production from aluminum-water reactions subject to varied pressures and temperatures. Int. J. Hydrogen Energy 2019, 44, 11448–11458. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, X.; Liu, J. Liquid metal activated aluminum-water reaction for direct hydrogen generation at room temperature. Renew. Sustain. Energy Rev. 2018, 92, 17–37. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, T.; Zhou, J.; Shi, W.; Liu, J.; Cen, K. Experimental study on the effect of low melting point metal additives on hydrogen production in the aluminum–water reaction. Energy 2015, 88, 537–543. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X.M.; Chen, D.M.; Yang, K. Insight into the reactivity of Al–Ga–In–Sn alloy with water. Int. J. Hydrogen Energy 2012, 37, 2187–2194. [Google Scholar] [CrossRef]
- He, T.; Xiong, Y.; Du, S.; Yuan, Z.; Liang, X.; Huttula, M.; Cao, W. Impact of Li Addition in Al-Rich Alloys on Hydrogen Production in Water. J. Mater. Eng. Perform. 2019, 28, 2459–2464. [Google Scholar] [CrossRef]
- Qiao, D.; Lu, Y.; Tang, Z.; Fan, X.; Wang, T.; Li, T.; Liaw, P.K. The superior hydrogen-generation performance of multi-component Al alloys by the hydrolysis reaction. Int. J. Hydrogen Energy 2019, 44, 3527–3537. [Google Scholar] [CrossRef]
- Wang, F.-Q.; Wang, H.-H.; Wang, J.; Lu, J.; Luo, P.; Chang, Y.; Ma, X.-G.; Dong, S.-J. Effects of low melting point metals (Ga, In, Sn) on hydrolysis properties of aluminum alloys. Trans. Nonferrous Met. Soc. China 2016, 26, 152–159. [Google Scholar] [CrossRef]
- An, Q.; Hu, H.; Li, N.; Liu, D.; Xu, S.; Liu, Z.; Wei, C.; Luo, F.; Xia, M.; Gao, Q. Effects of Bi composition on microstructure and Al-water reactivity of Al-rich alloys with low-In. Int. J. Hydrogen Energy 2018, 43, 10887–10895. [Google Scholar] [CrossRef]
- An, Q.; Hu, H.; Li, N.; Wei, J.; Wei, C.; Wang, H.; Woodall, J.M.; Gao, Q. Strategies for improving flow rate control of hydrogen generated by Al-rich alloys for on-board applications. Int. J. Hydrogen Energy 2019, 44, 27695–27703. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Z.; Chen, C.; Xu, Z.; Dong, S. Ti promotes the reaction of Al alloy with water to rapidly generate hydrogen. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Z.; Wei, J.; Liu, D.; Xu, S.; An, Q.; Xu, S.; Wang, H.; Gao, Q. Effect of alumina on the microstructure and hydrogen production of Al-riched bulk alloys. Chem. Phys. Lett. 2020, 738. [Google Scholar] [CrossRef]
- Xie, Z.; Dong, S.; Luo, P.; Wang, H. Enhanced Hydrogen Generation Properties of Al-Ga-In-Sn Alloy in Reaction with Water by Trace Amount of AlTi5B Additives. Mater. Trans. 2017, 58, 724–727. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Chen, W.; Wang, W.; Ren, F.; Stock, H.-R. Effect of different Cu contents on the microstructure and hydrogen production of Al–Cu-Ga-In-Sn alloys for dissolvable materials. J. Alloys Compd. 2020, 821. [Google Scholar] [CrossRef]
- Huang, T.; Gao, Q.; Liu, D.; Xu, S.; Guo, C.; Zou, J.; Wei, C. Preparation of Al-Ga-In-Sn-Bi quinary alloy and its hydrogen production via water splitting. Int. J. Hydrogen Energy 2015, 40, 2354–2362. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.M.; Yang, K. Investigation on microstructure and hydrogen generation performance of Al-rich alloys. Int. J. Hydrogen Energy 2010, 35, 12011–12019. [Google Scholar] [CrossRef]
- He, T.T.; Wang, W.; Chen, W.; Chen, D.M.; Yang, K. Influence of In and Sn compositions on the reactivity of Al–Ga–In–Sn alloys with water. Int. J. Hydrogen Energy 2017, 42, 5627–5637. [Google Scholar] [CrossRef]
- Du, B.D.; Wang, W.; Chen, W.; Chen, D.M.; Yang, K. Grain refinement and Al-water reactivity of Al Ga In Sn alloys. Int. J. Hydrogen Energy 2017, 42, 21586–21596. [Google Scholar] [CrossRef]
- Kirchheim, R. Grain coarsening inhibited by solute segregation. Acta Mater. 2002, 50, 413–419. [Google Scholar] [CrossRef]
- Dillon, S.J.; Tang, M.; Carter, W.C.; Harmer, M.P. Complexion: A new concept for kinetic engineering in materials science. Acta Mater. 2007, 55, 6208–6218. [Google Scholar] [CrossRef]
- Ahadi, A.; Kalidindi, A.R.; Sakurai, J.; Matsushita, Y.; Tsuchiya, K.; Schuh, C.A. The role of W on the thermal stability of nanocrystalline NiTiWx thin films. Acta Mater. 2018, 142, 181–192. [Google Scholar] [CrossRef]
- Ziebarth, J.T.; Woodall, J.M.; Kramer, R.A.; Choi, G. Liquid phase-enabled reaction of Al–Ga and Al–Ga–In–Sn alloys with water. Int. J. Hydrogen Energy 2011, 36, 5271–5279. [Google Scholar] [CrossRef]
- Wei, C.; Liu, D.; Xu, S.; Cui, T.; An, Q.; Liu, Z.; Gao, Q. Effects of Cu additives on the hydrogen generation performance of Al-rich alloys. J. Alloys Compd. 2018, 738, 105–110. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Yan, Y.; Zhu, H.; Fang, H.; Luo, X.; Dai, Y.; Yu, K. Effects of alloying elements on the electrochemical behaviors of Al-Mg-Ga-In based anode alloys. Int. J. Hydrogen Energy 2019, 44, 12073–12084. [Google Scholar] [CrossRef]






| No. | Al | Ga | In | Sn | Zn |
|---|---|---|---|---|---|
| 1 | 90 | 2.5 | 5.58 | 1.92 | 0 |
| 2 | 90 | 2.5 | 4.83 | 1.67 | 1 |
| 3 | 90 | 2.5 | 4.09 | 1.41 | 2 |
| 4 | 90 | 2.5 | 3.35 | 1.15 | 3 |
| 5 | 90 | 2.5 | 2.60 | 0.90 | 4 |
| 6 | 90 | 2.5 | 1.86 | 0.64 | 5 |
| Zone | Phase | Element (at.%) | |||||
|---|---|---|---|---|---|---|---|
| Al | Ga | In | Sn | Zn | O | ||
| 1 | G | 47.44(98.18) | 0.72(1.49) | 0.08(0.17) | 0.08(0.17) | - | 51.68 |
| 2 | GB | 12.81(32.65) | 0.82(2.09) | 19.22(48.98) | 6.39(16.28) | - | 60.76 |
| 3 | G | 38.90(97.71) | 0.55(1.38) | 0.08(0.20) | 0.11(0.28) | 0.16(0.40) | 60.19 |
| 4 | GB | 11.82(22.38) | 1.27(2.40) | 30.02(56.83) | 9.50(17.99) | 0.22(0.42) | 47.18 |
| 5 | G | 47.40(97.15) | 0.57(1.17) | 0.28(0.57) | 0.06(0.12) | 0.47(0.96) | 51.21 |
| 6 | GB | 13.96(20.03) | 1.67(2.40) | 43.72(62.74) | 9.42(13.52) | 0.91(1.31) | 30.32 |
| 7 | G | 68.46(95.99) | 1.13(1.58) | 0.17(0.24) | 0.02(0.03) | 1.54(2.16) | 28.68 |
| 8 | GB | 3.09(3.58) | 3.14(3.64) | 61.03(70.78) | 18.24(21.15) | 0.73(0.85) | 13.77 |
| 9 | High-Zn region | 4.71(4.85) | 0.58(0.60) | - | 0.20(0.21) | 91.70(94.37) | 2.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Gao, Q.; An, Q.; Wang, H.; Wei, J.; Wei, C. Experimental Study on Zn-Doped Al-Rich Alloys for Fast on-Board Hydrogen Production. Crystals 2020, 10, 167. https://doi.org/10.3390/cryst10030167
Liu D, Gao Q, An Q, Wang H, Wei J, Wei C. Experimental Study on Zn-Doped Al-Rich Alloys for Fast on-Board Hydrogen Production. Crystals. 2020; 10(3):167. https://doi.org/10.3390/cryst10030167
Chicago/Turabian StyleLiu, Dan, Qian Gao, Qi An, Hongchao Wang, Jilun Wei, and Cundi Wei. 2020. "Experimental Study on Zn-Doped Al-Rich Alloys for Fast on-Board Hydrogen Production" Crystals 10, no. 3: 167. https://doi.org/10.3390/cryst10030167
APA StyleLiu, D., Gao, Q., An, Q., Wang, H., Wei, J., & Wei, C. (2020). Experimental Study on Zn-Doped Al-Rich Alloys for Fast on-Board Hydrogen Production. Crystals, 10(3), 167. https://doi.org/10.3390/cryst10030167




