Effect of Zn Doping in CuO Octahedral Crystals towards Structural, Optical, and Gas Sensing Properties
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Chemicals
2.2. Synthesis Procedure
2.3. Sample Characterizations
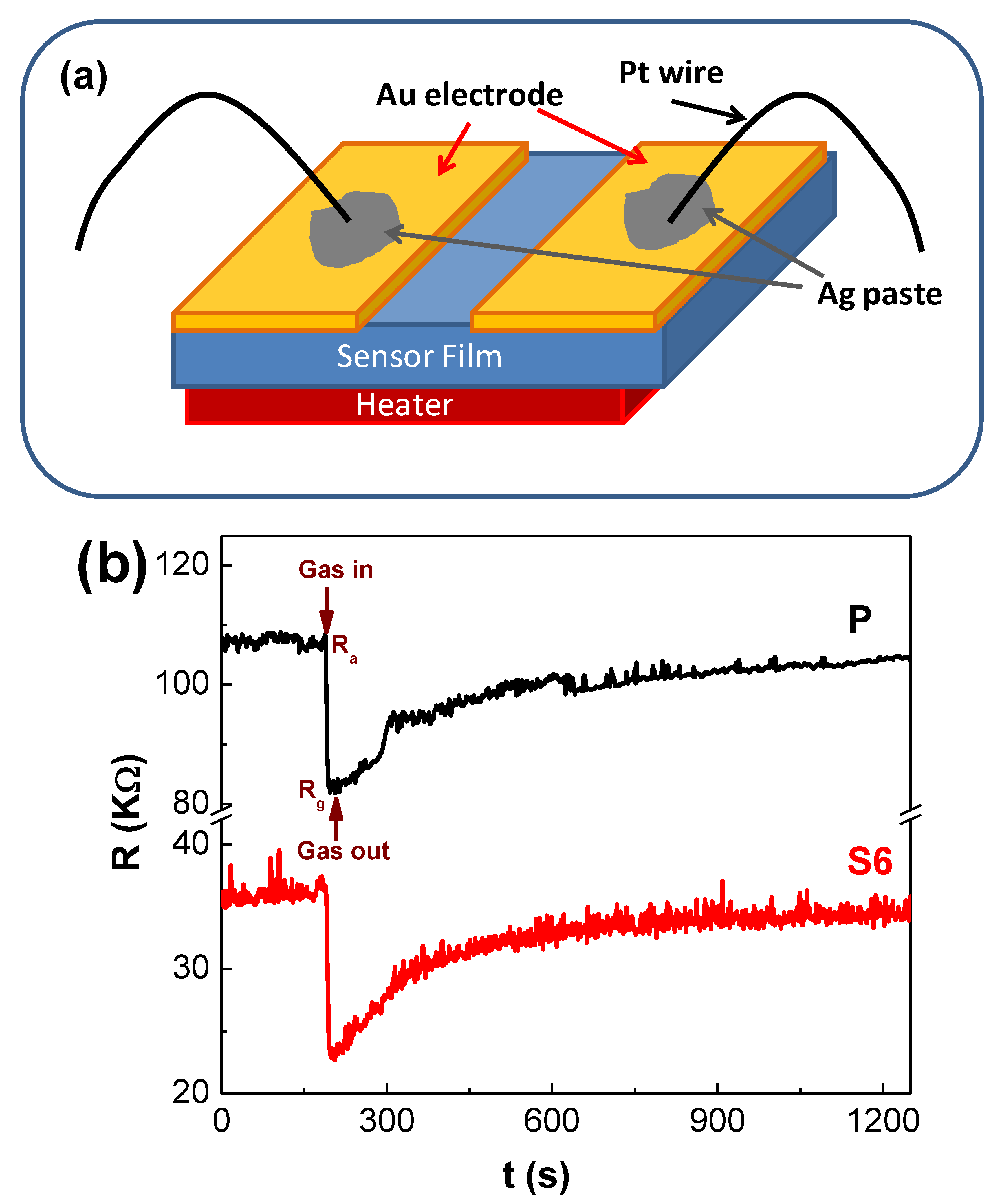
2.4. Gas Sensing Measurements
2.5. Work Function Measurements
3. Results and Discussion
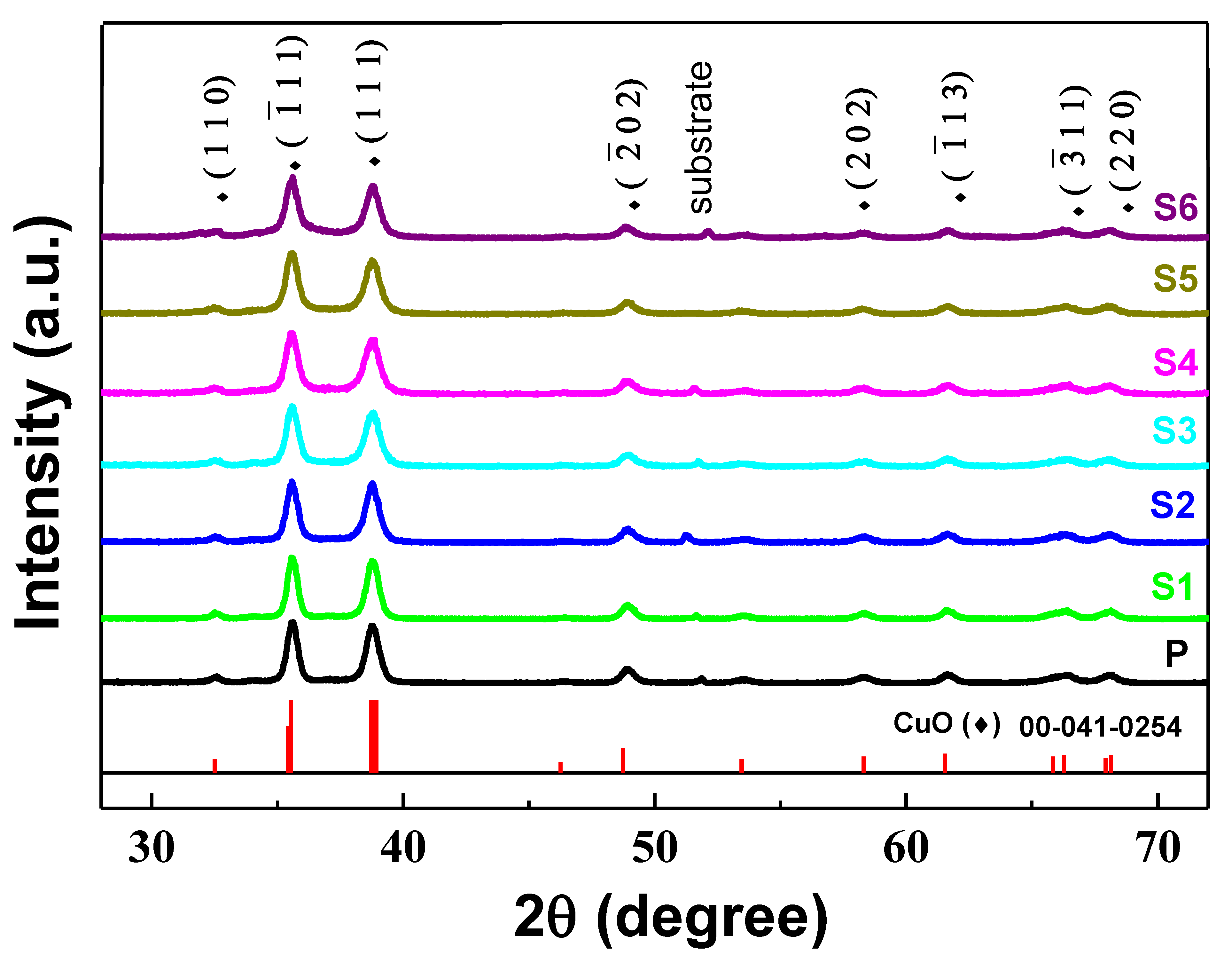
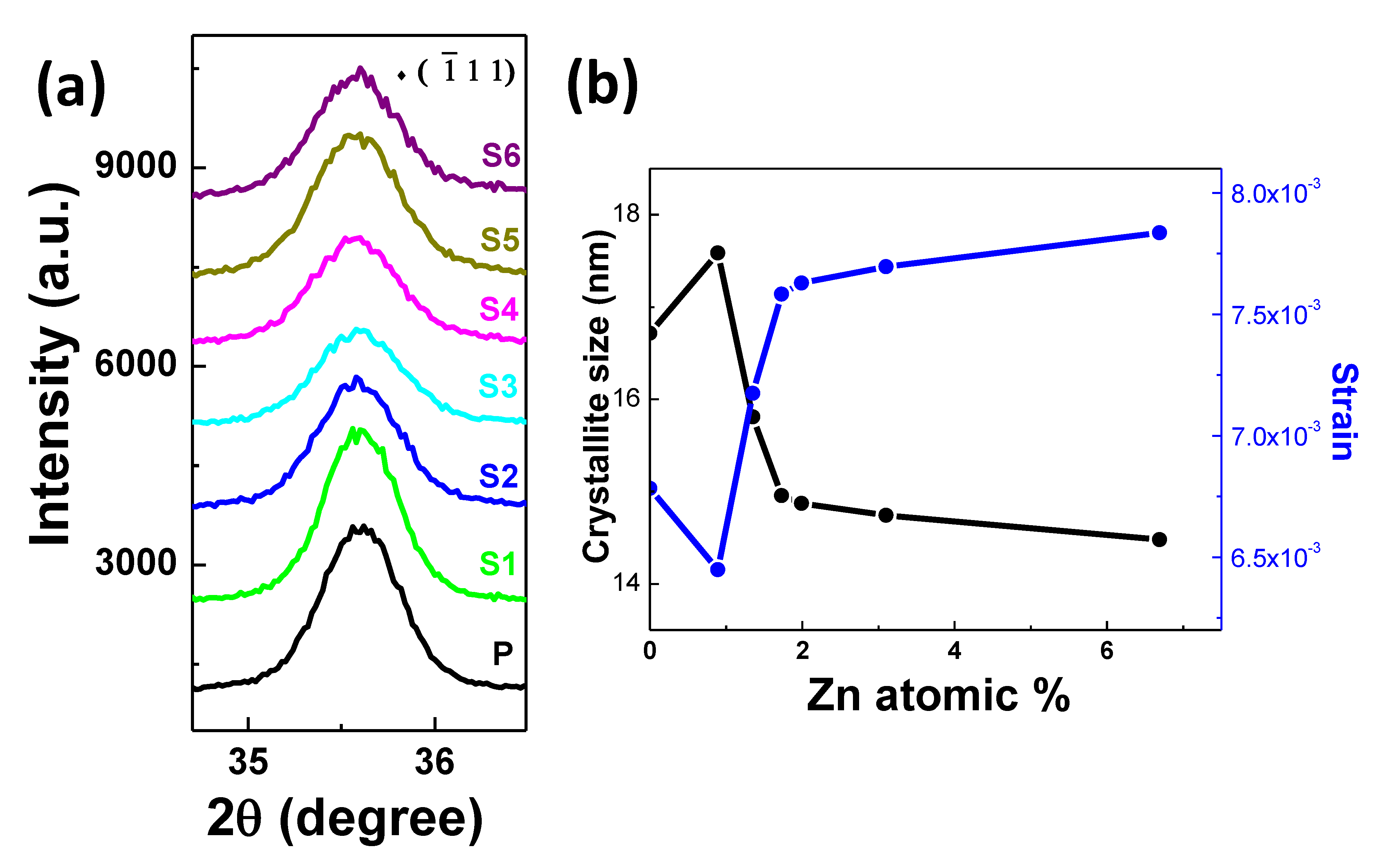
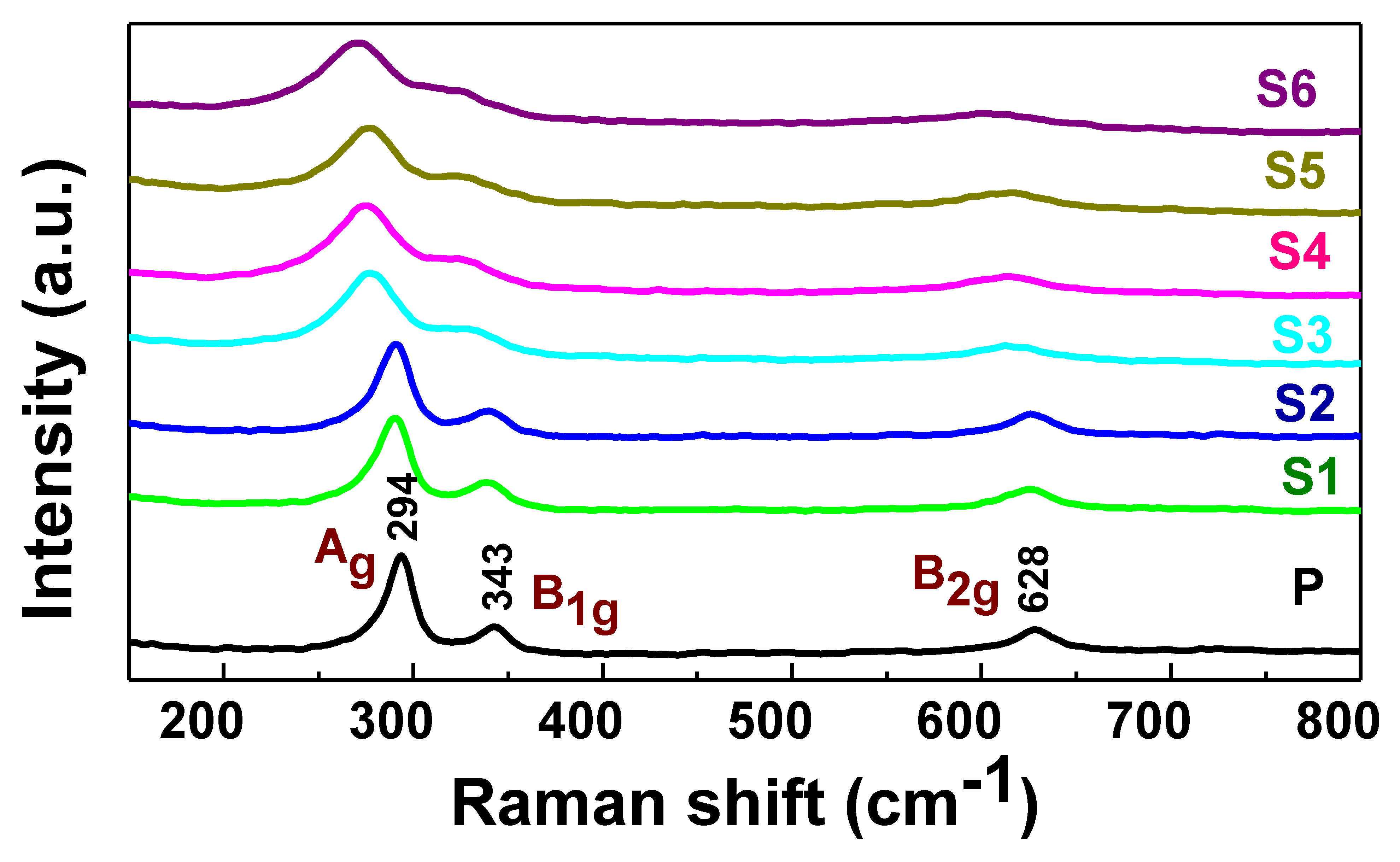
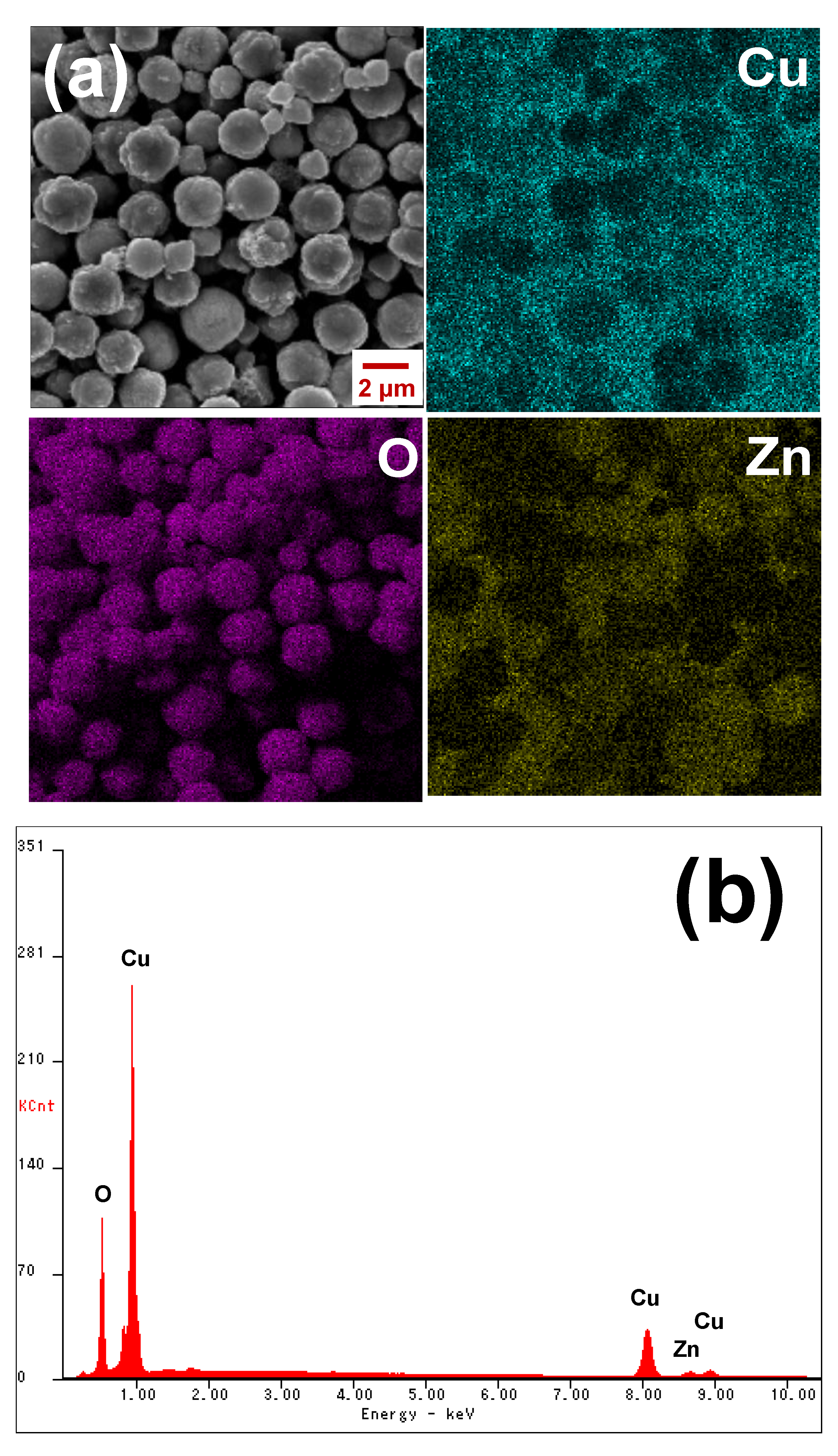
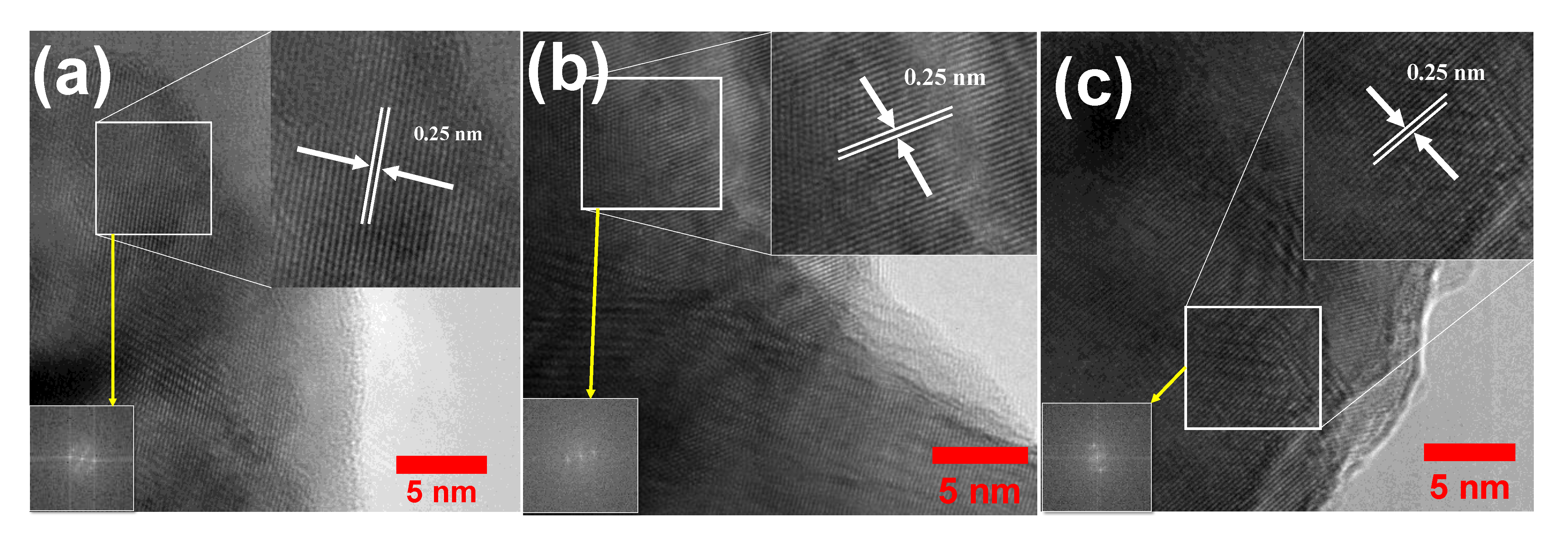
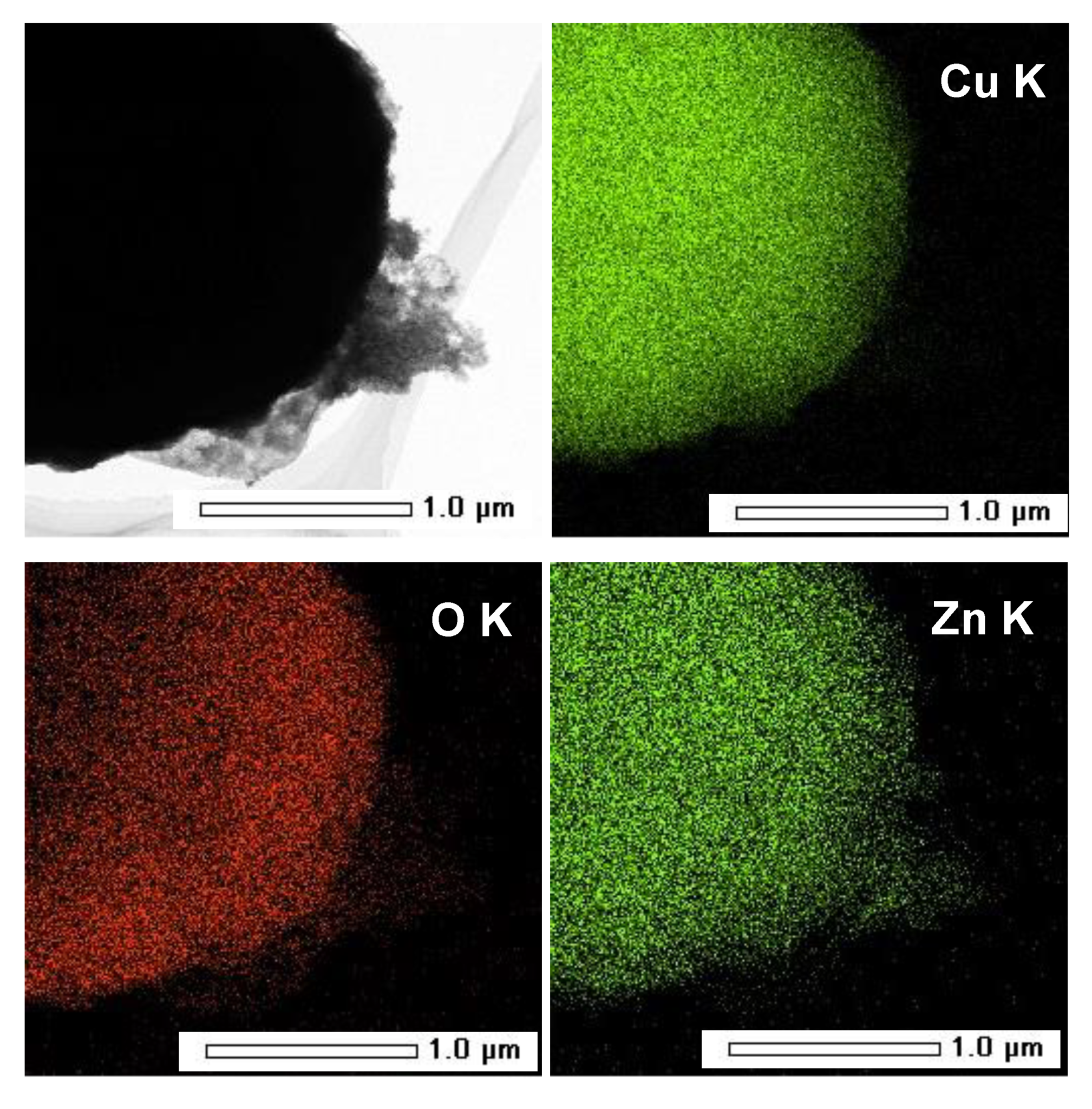
3.1. Structural and Morphological Characterization
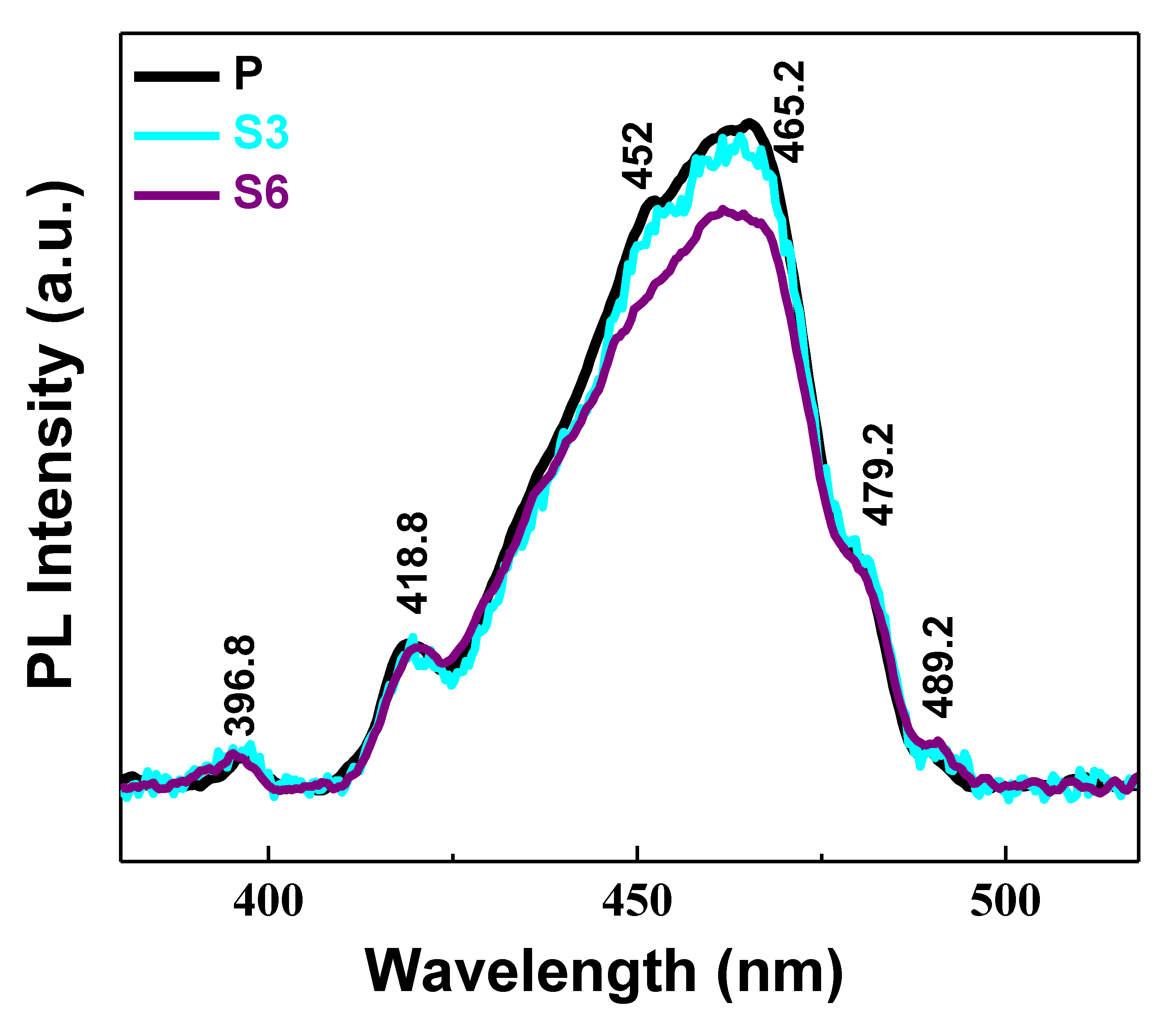
3.2. Optical Studies
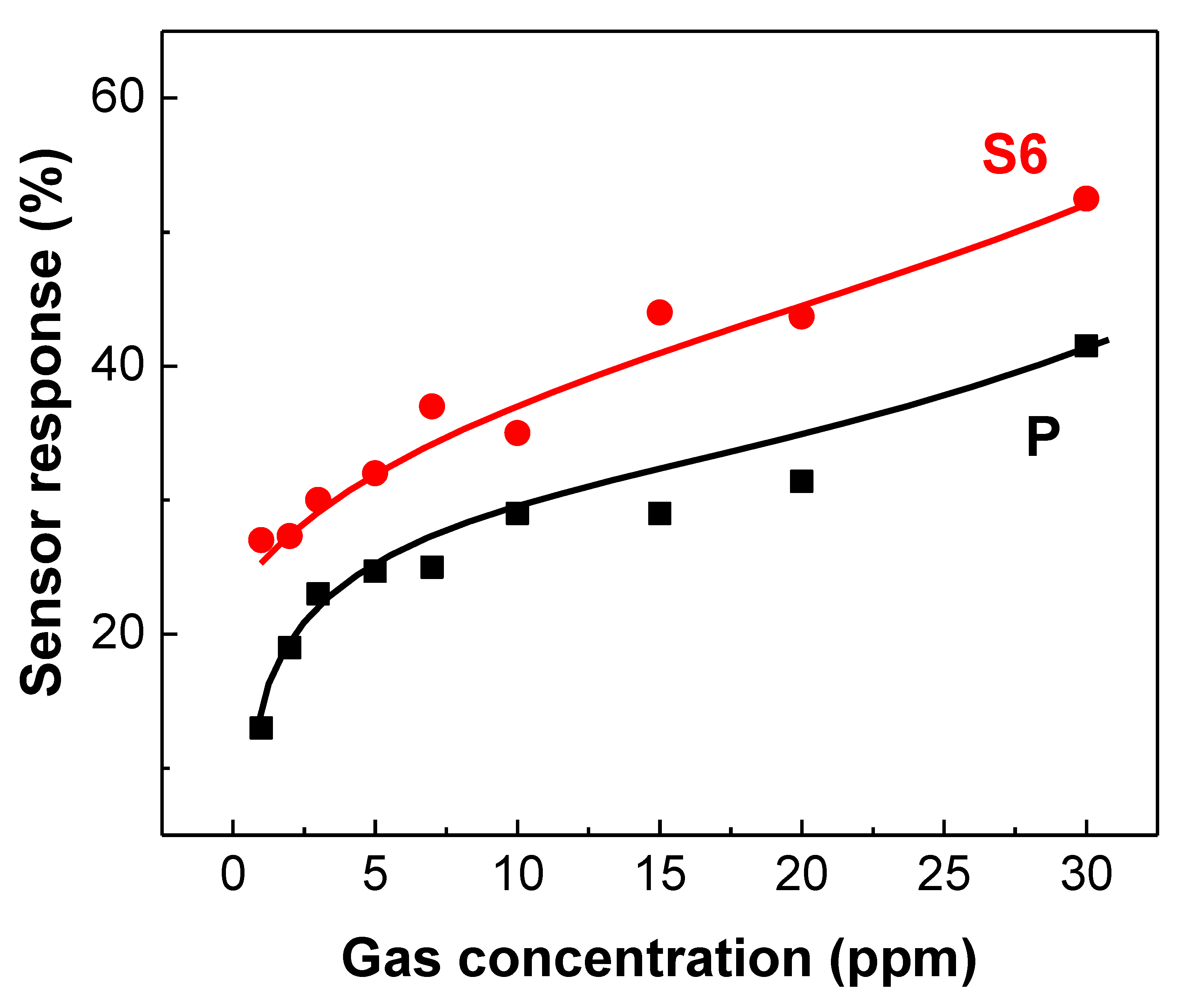
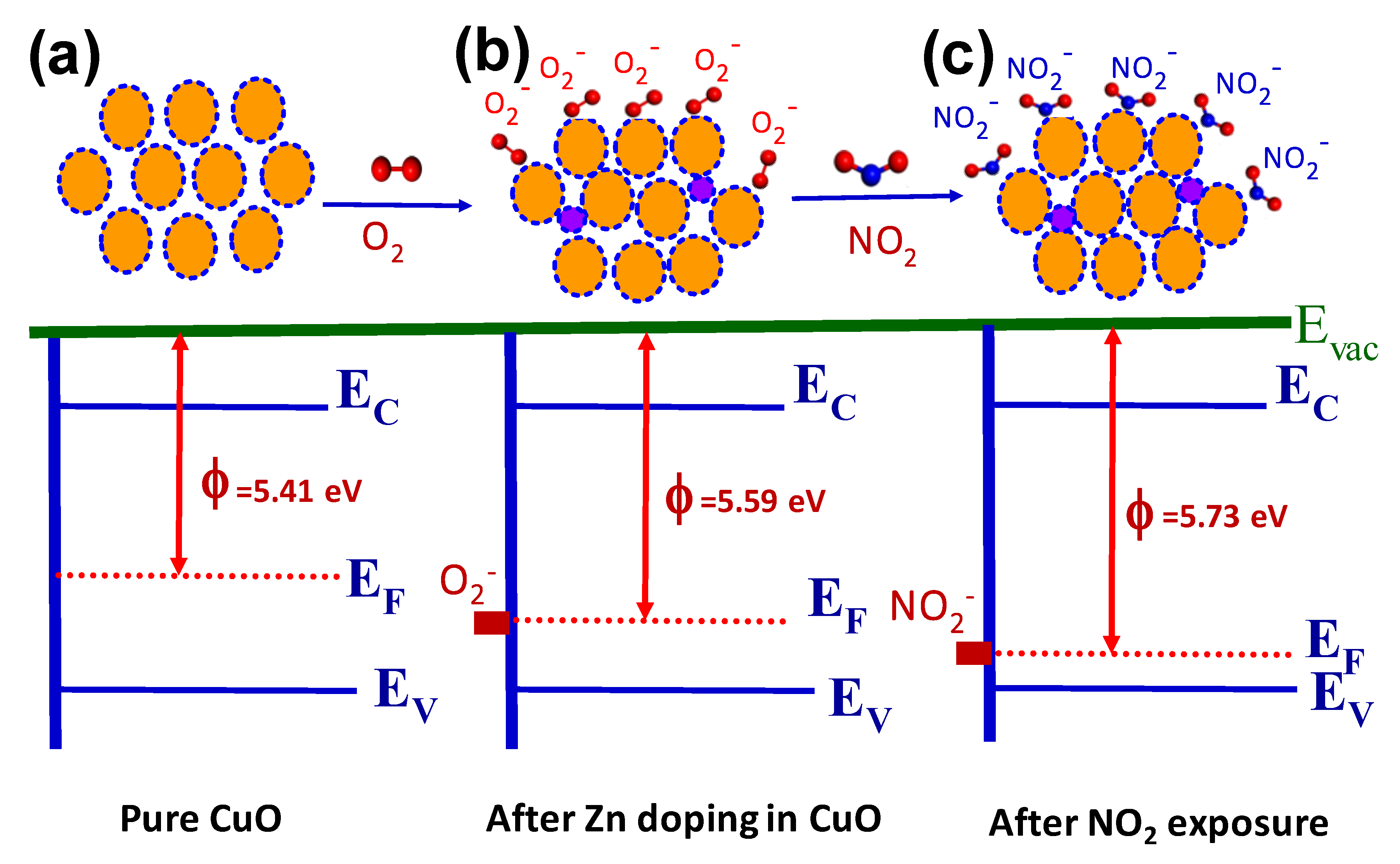
3.3. Gas Sensing Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burdett, J.K.; Sevov, S. Stability of the Oxidation States of Copper. J. Am. Chem. Soc. 1995, 117, 12788–12792. [Google Scholar] [CrossRef]
- Scuderi, V.; Amiard, G.; Boninelli, S.; Scalese, S.; Miritello, M.; Sberna, P.M.; Impellizzeri, G.; Privitera, V. Photocatalytic activity of CuO and Cu2O nanowires. Mater. Sci. Semicond. Process. 2016, 42, 89–93. [Google Scholar] [CrossRef]
- Murali, D.S.; Kumar, S.; Choudhary, R.J.; Wadikar, A.D.; Jain, M.K.; Subrahmanyam, A. Synthesis of Cu2O from CuO thin films: Optical and electrical properties. AIP Adv. 2015, 5, 047143. [Google Scholar] [CrossRef]
- Wong, T.K.S.; Zhuk, S.; Masudy-Panah, S.; Dalapati, G.K. Current status and future prospects of copper oxide heterojunction solar cells. Materials 2016, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.; Biscu, E.; Holban, A.; Gestal, M.; Grumezescu, A. Methods of Synthesis, Properties and Biomedical Applications of CuO Nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Ramgir, N.S.; Ganapathi, S.K.; Kaur, M.; Datta, N.; Muthe, K.P.; Aswal, D.K.; Gupta, S.K.; Yakhmi, J.V. Sub-ppm H2S sensing at room temperature using CuO thin films. Sens. Actuators B Chem. 2010, 151, 90–96. [Google Scholar] [CrossRef]
- Yang, C.; Su, X.; Xiao, F.; Jian, J.; Wnag, J. Gas sensing properties of CuO nanorods synthesized by a microwave-assisted hydrothermal method. Sens. Actuators B Chem. 2011, 158, 299–303. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Yuan, Z.; Sun, Y.; Chang, F.; Deng, H.; Xiea, L.; Lic, H. A highly sensitive gas sensor based on CuO nanoparticles synthetized via a sol–gel method. RSC Adv. 2016, 6, 79343–79349. [Google Scholar] [CrossRef]
- Jayathilaka, C.; Kapaklis, V.; Siripala, W.; Jayanetti, S. Improved efficiency of electrodeposited p-CuO/n-Cu2O heterojunction solar cell. Appl. Phys. Express 2015, 8, 065503. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, G.; Pei, S.; Wang, J. Investigation of optical bandgap variation and photoluminescence behavior in nanocrystalline CuO thin films. Optik (Stuttg) 2018, 158, 382–390. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, V.T. Copper Oxide Nanomaterials Prepared by Solution Methods, Some Properties, and Potential Applications: A Brief Review. Int. Sch. Res. Not. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Xuan, Y.; Zhang, H.; Deng, X.; Bai, M.; Wang, L. A facile coordination precipitation route to prepare porous CuO microspheres with excellent photo-Fenton catalytic activity and electrochemical performance. Cryst. Eng. Commun. 2019, 21, 648–655. [Google Scholar] [CrossRef]
- Nesa, M.; Momin, M.A.; Sharmin, M.; Bhuiyan, A.H. Structural, optical and electronic properties of CuO and Zn-doped CuO: DFT based First-principles calculations. Chem. Phys. 2020, 528, 110536. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Y.; Meng, D.; Wang, D. One-step hydrothermal synthesis and enhanced photocatalytic performance of pine-needle-like Zn-doped CuO nanostructures. J. Mater. Sci. Mater. Electron. 2016, 27, 12884–12890. [Google Scholar] [CrossRef]
- Lu, J.; Wang, J.; Zou, Q.; Zhao, Y.; Fang, J.; He, S.; He, D.; Luo, Y. Catalytic performance of transition metals (Co, Ni, Zn, Mo) doped CuO-Ce0.8Zr0.2O2 based catalysts for CO preferential oxidation in H2-rich streams. J. Alloys Compd. 2019, 784, 1248–1260. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, Z.; Mao, J.; Zhou, R. Doping effect of transition metals (Zr, Mn, Ti and Ni) on well-shaped CuO/CeO2 (rods): Nano/micro structure and catalytic performance for selective oxidation of CO in excess H2. Phys. Chem. Chem. Phys. 2018, 20, 25983–25994. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Jin, S.; Chen, H.; Lee, W.; Liu, M.; Chen, Y. Anionic defect engineering of transition metal oxides for oxygen reduction and evolution reactions. J. Mater. Chem. A 2019, 7, 5875–5897. [Google Scholar] [CrossRef]
- Sonia, S.; Jose Annsi, I.; Suresh Kumar, P.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Hydrothermal synthesis of novel Zn-doped CuO nanoflowers as an efficient photodegradation material for textile dyes. Mater. Lett. 2015, 144, 127–130. [Google Scholar] [CrossRef]
- Yathisha, R.O.; Arthoba Nayaka, Y. Structural, optical and electrical properties of zinc incorporated copper oxide nanoparticles: Doping effect of Zn. J. Mater. Sci. 2018, 53, 678–691. [Google Scholar] [CrossRef]
- Iqbal, J.; Jan, T.; Ul-Hassan, S.; Ahmed, I.; Mansoor, Q.; Umair Ali, M.; Abbas, F.; Ismail, M. Facile synthesis of Zn-doped CuO hierarchical nanostructures: Structural, optical and antibacterial properties. AIP Adv. 2015, 5, 127112. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zou, C.; Su, Y.; Li, M.; Han, Y.; Kong, E.S.W.; Yang, Z.; Zhang, Y. An ultrasensitive NO2 gas sensor based on a hierarchical Cu2O/CuO mesocrystal nanoflower. J. Mater. Chem. A 2018, 6, 17120–17131. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Yin, J.; Shang, Y.; Du, J.; Kang, Z.; Wang, R.; Chen, Y.; Sun, D.; Jiang, J. An ultrafast responsive NO2 gas sensor based on a hydrogen-bonded organic framework material. Chem. Commun. 2020, 56, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shao, C.; Lu, D.; Lu, G.; Li, X.; Liu, Y. Octahedral-Like CuO/In2O3Mesocages with Double-Shell Architectures: Rational Preparation and Application in Hydrogen Sulfide Detection. ACS Appl. Mater. Interfaces 2017, 9, 44632–44640. [Google Scholar] [CrossRef] [PubMed]
- Bhangare, B.; Ramgir, N.S.; Jagtap, S.; Debnath, A.K.; Muthe, K.P.; Terashima, C.; Aswal, D.K.; Gosavi, S.W.; Fujishima, A. XPS and Kelvin probe studies of SnO2/RGO nanohybrids based NO2 sensors. Appl. Surf. Sci. 2019, 487, 918–929. [Google Scholar] [CrossRef]
- Ramgir, N.; Datta, R.; Avhad, K.; Bhusari, R.; Vedpathak, M.M.; Meera, M.; Katke, V.V.; Ravisankar, E.; Debnath, A.K.; Saha, T.K.; et al. A Table Top Static Gas Sensing Unit: Model Tpd-barc-ch. BARC Newslett. 2017, 14–18. [Google Scholar]
- Ramgir, N.S.; Goyal, C.P.; Sharma, P.K.; Goutam, U.K.; Bhattacharya, S.; Datta, N.; Kaur, M.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Selective H2S sensing characteristics of CuO modified WO3 thin films. Sens. Actuators B Chem. 2013, 188, 525–532. [Google Scholar] [CrossRef]
- Chidambaram, D.; Vattikondala, G.; Marappan, G.; Sivalingam, Y. Indium content dependent VOCs interactions in monolithic InGaN/GaN multi quantum well structures grown by MOCVD. Mater. Sci. Semicond. Process. 2019, 104, 104694. [Google Scholar] [CrossRef]
- Samarasekara, P.; Karunarathna, P.G.D.C.K.; Weeramuni, H.P.; Fernando, C.A.N. Electrical properties of spin coated Zn-doped CuO films. Mater. Res. Express 2018, 5, 066418. [Google Scholar] [CrossRef]
- Cretu, V.; Postica, V.; Mishra, A.K.; Hoppe, M.; Tiginyanu, I.; Mishra, Y.K.; Chow, L.; De Leeuw, N.H.; Adelung, R.; Lupan, O. Synthesis, characterization and DFT studies of zinc-doped copper oxide nanocrystals for gas sensing applications. J. Mater. Chem. A 2016, 4, 6527–6539. [Google Scholar] [CrossRef] [Green Version]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles- a comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Xu, J.; Ji, W.; Shen, Z.; Li, W.; Tang, S.; Ye, X.; Jia, D.; Xin, X. Raman spectra of CuO nanocrystals. J. Raman Spectrosc. 1999, 30, 413–415. [Google Scholar] [CrossRef]
- Sriyutha Murthy, P.; Venugopalan, V.P.; Arunya, D.D.; Dhara, S.; Pandiyan, R.; Tyagi, A.K. Antibiofilm activity of nano sized CuO. In Proceedings of the International Conference on Nanoscience, Engineering and Technology, ICONSET 2011, Chennai, Tamilnadu, 28–30 November 2011; pp. 580–583. [Google Scholar]
- Jan, T.; Iqbal, J.; Farooq, U.; Gul, A.; Abbasi, R.; Ahmad, I.; Malik, M. Structural, Raman and optical characteristics of Sn doped CuO nanostructures: A novel anticancer agent. Ceram. Int. 2015, 41, 13074–13079. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, W.; Wang, G.; Yu, Y.; Qin, S.; Fang, J.; Wang, F.; Zhang, X.A. The Raman redshift of graphene impacted by gold nanoparticles. AIP Adv. 2015, 5, 057133. [Google Scholar] [CrossRef]
- Jacquemin, M.; Genet, M.J.; Gaigneaux, E.M.; Debecker, D.P. Calibration of the X-ray photoelectron spectroscopy binding energy scale for the characterization of heterogeneous catalysts: Is everything really under control? ChemPhysChem 2013, 14, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.F.; Ji, W.; Shen, Z.X.; Tang, S.H.; Ye, X.R.; Jia, D.Z.; Xin, X.Q. Preparation and Characterization of CuO Nanocrystals. J. Solid State Chem. 1999, 147, 516–519. [Google Scholar] [CrossRef]
- Li, K.; Xue, D. Estimation of Electronegativity Values of Elements in Different Valence States. J. Phys. Chem. A 2006, 110, 11332–11337. [Google Scholar] [CrossRef]
- Qiu, X.; Burda, C. Chemically synthesized nitrogen-doped metal oxide nanoparticles. Chem. Phys. 2007, 339, 1–10. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Liu, Z.; Xia, P.; Xie, Y.; Xiong, D. Synthesis of the 0D/3D CuO/ZnO Heterojunction with Enhanced Photocatalytic Activity. J. Phys. Chem. C 2018, 122, 9531–9539. [Google Scholar] [CrossRef]
- Dupin, J.C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Ciftyürek, E.; Šmíd, B.; Li, Z.; Matolín, V.; Schierbaum, K. Spectroscopic understanding of Sno2 and Wo3 metal oxide surfaces with advanced synchrotron based; XPS-UPS and near ambient pressure (NAP) XPS surface sensitive techniques for gas sensor applications under operational conditions. Sensors 2019, 19, 4737. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, S.P.; Ley, L.; McFeely, F.R.; Pollak, R.A.; Shirley, D.A. Relative effect of extra-atomic relaxation on Auger and binding-energy shifts in transition metals and salts. Phys. Rev. B 1974, 9, 381–391. [Google Scholar] [CrossRef]
- Alexander, V.N.; Anna, K.V.; Stephen, W.G.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database. Available online: http://srdata.nist.gov/xps/Default.aspx (accessed on 14 February 2020).
- Bhuvaneshwari, S.; Gopalakrishnan, N. Hydrothermally synthesized Copper Oxide (CuO) superstructures for ammonia sensing. J. Colloid Interface Sci. 2016, 480, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, C.; Pavithra, G.; Bououdina, M.; Dhanapandian, S.; Dhamodharan, P. Characterization and study of antibacterial activity of spray pyrolysed ZnO:Al thin films. Appl. Nanosci. 2016, 6, 815–825. [Google Scholar] [CrossRef] [Green Version]
- Vuong, N.M.; Chinh, N.D.; Huy, B.T.; Lee, Y.I. CuO-decorated ZnO hierarchical nanostructures as efficient and established sensing materials for H2S Gas Sensors. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhuri, A.; Gupta, V.; Sreenivas, K. Enhanced catalytic activity of ultrathin CuO islands on SnO2 films for fast response H2S gas sensors. IEEE Sens. J. 2003, 3, 680–686. [Google Scholar] [CrossRef]
- Navazani, S.; Shokuhfar, A.; Hassanisadi, M.; Di Carlo, A.; Yaghoobi Nia, N.; Agresti, A. A PdPt decorated SnO2-rGO nanohybrid for high-performance resistive sensing of methane. J. Taiwan Inst. Chem. Eng. 2019, 95, 438–451. [Google Scholar] [CrossRef]
- Kooti, M.; Keshtkar, S.; Askarieh, M.; Rashidi, A. Progress toward a novel methane gas sensor based on SnO2 nanorods-nanoporous graphene hybrid. Sens. Actuators B Chem. 2019, 281, 96–106. [Google Scholar] [CrossRef]
- Jaaniso, R.; Tan, O.K. Semiconductor Gas Sensors; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780857092366. [Google Scholar]





| Sample | Composition (atomic %) | ||
|---|---|---|---|
| Cu | O | Zn | |
| P | 68.64 | 31.36 | - |
| S1 | 66.68 | 32.43 | 0.89 |
| S2 | 64.21 | 34.44 | 1.35 |
| S3 | 68.10 | 30.17 | 1.73 |
| S4 | 68.06 | 29.95 | 1.99 |
| S5 | 65.17 | 31.73 | 3.10 |
| S6 | 52.12 | 41.19 | 6.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, C.P.; Goyal, D.; K. Rajan, S.; Ramgir, N.S.; Shimura, Y.; Navaneethan, M.; Hayakawa, Y.; Muthamizhchelvan, C.; Ikeda, H.; Ponnusamy, S. Effect of Zn Doping in CuO Octahedral Crystals towards Structural, Optical, and Gas Sensing Properties. Crystals 2020, 10, 188. https://doi.org/10.3390/cryst10030188
Goyal CP, Goyal D, K. Rajan S, Ramgir NS, Shimura Y, Navaneethan M, Hayakawa Y, Muthamizhchelvan C, Ikeda H, Ponnusamy S. Effect of Zn Doping in CuO Octahedral Crystals towards Structural, Optical, and Gas Sensing Properties. Crystals. 2020; 10(3):188. https://doi.org/10.3390/cryst10030188
Chicago/Turabian StyleGoyal, Chandra Prakash, Deepak Goyal, Sinjumol K. Rajan, Niranjan S. Ramgir, Yosuke Shimura, Mani Navaneethan, Yasuhiro Hayakawa, C. Muthamizhchelvan, Hiroya Ikeda, and S. Ponnusamy. 2020. "Effect of Zn Doping in CuO Octahedral Crystals towards Structural, Optical, and Gas Sensing Properties" Crystals 10, no. 3: 188. https://doi.org/10.3390/cryst10030188





