Simple Synthesis of NdFeO3 Nanoparticles By the Co-Precipitation Method Based on a Study of Thermal Behaviors of Fe (III) and Nd (III) Hydroxides
Abstract
1. Introduction
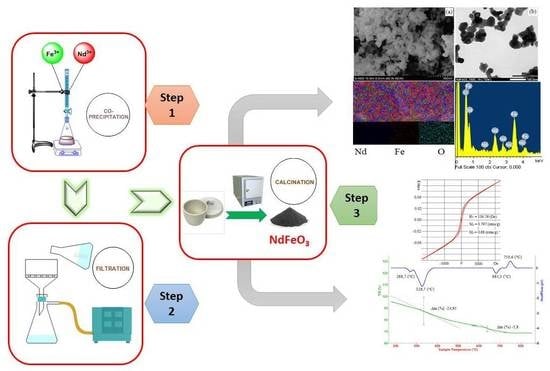
2. Experimental
2.1. Synthesis of Fe2O3, Nd2O3 and NdFeO3 Nanoscale Particles
2.2. Characteristics
3. Results and Discussion
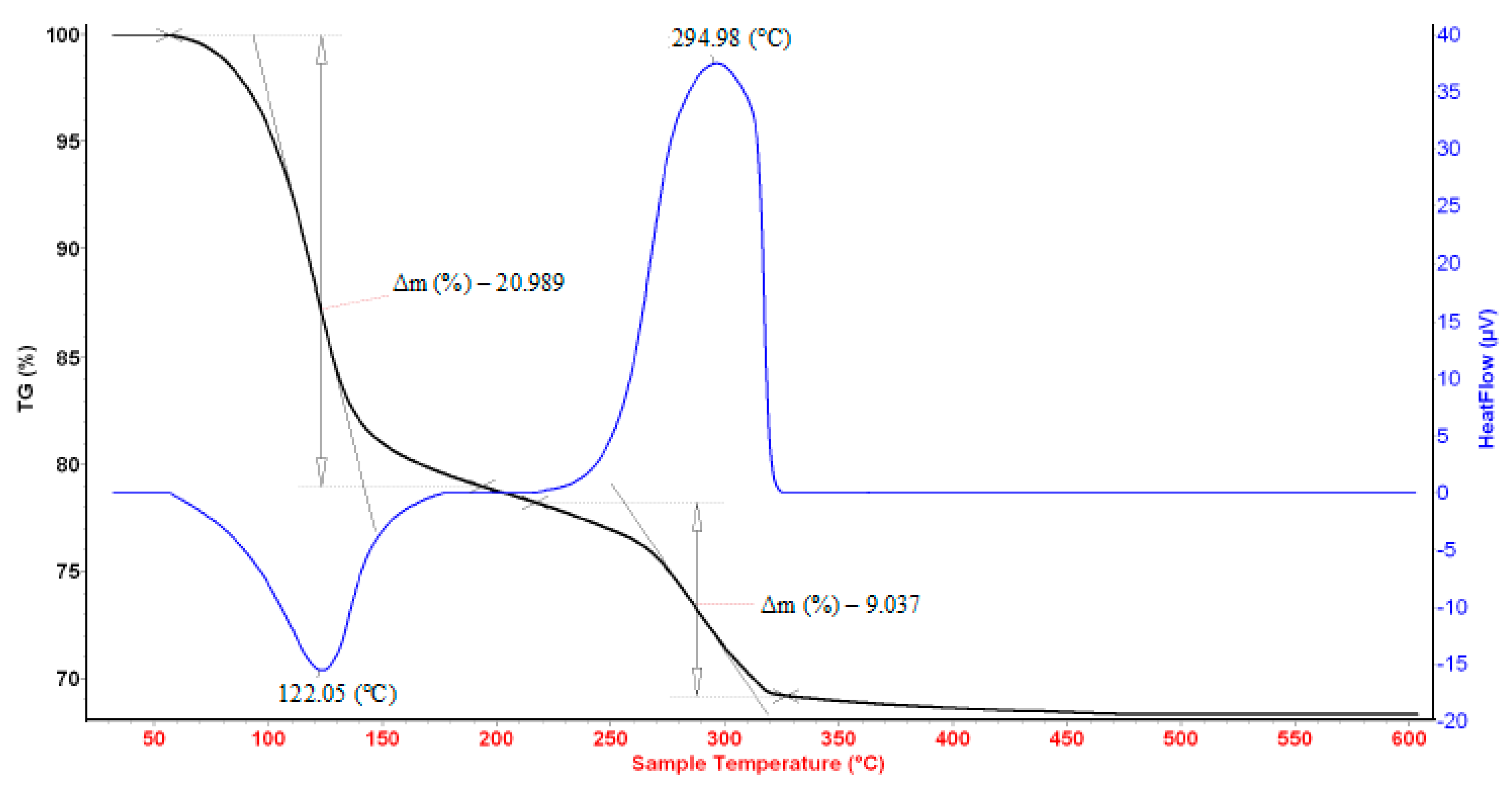
3.1. Thermal Behaviors of the Precipitates Containing Fe (III) or/and Nd (III)
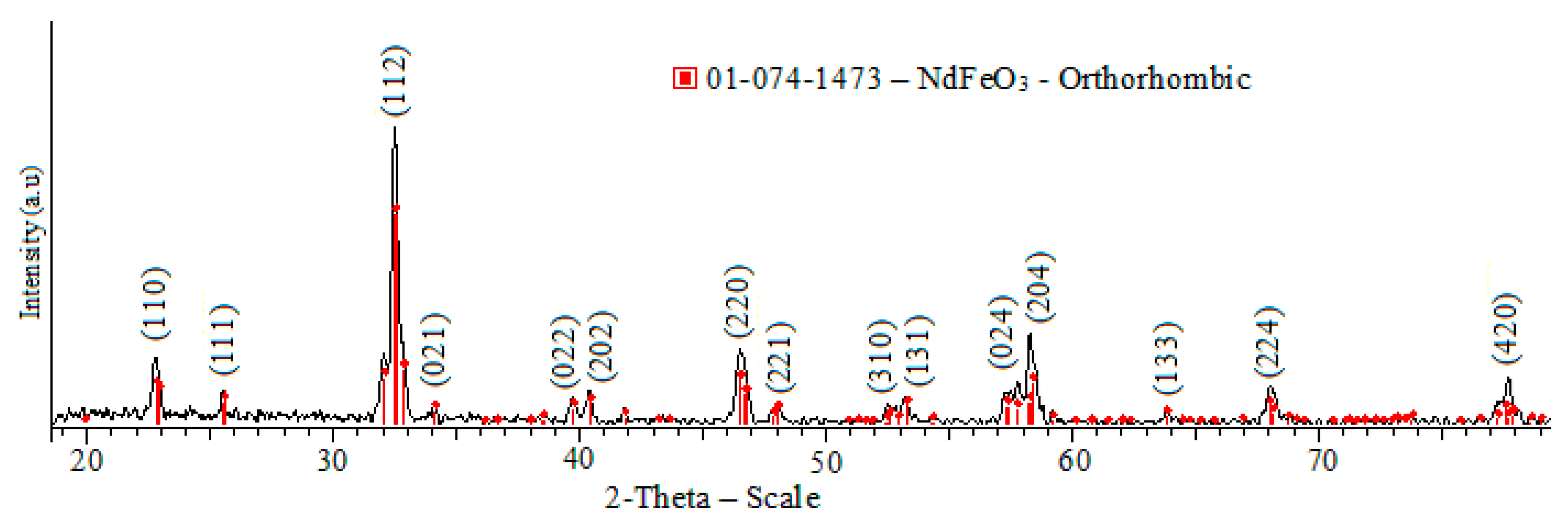
3.2. XRD Results
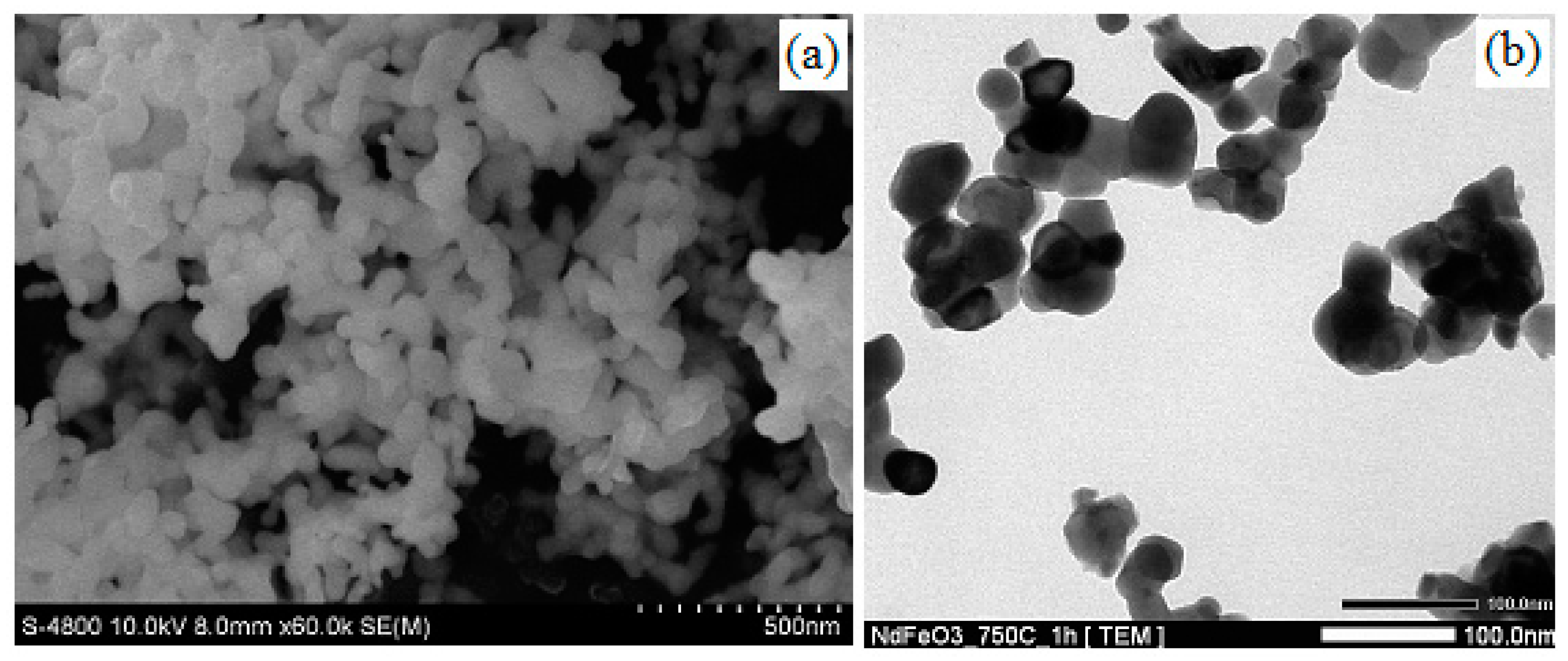
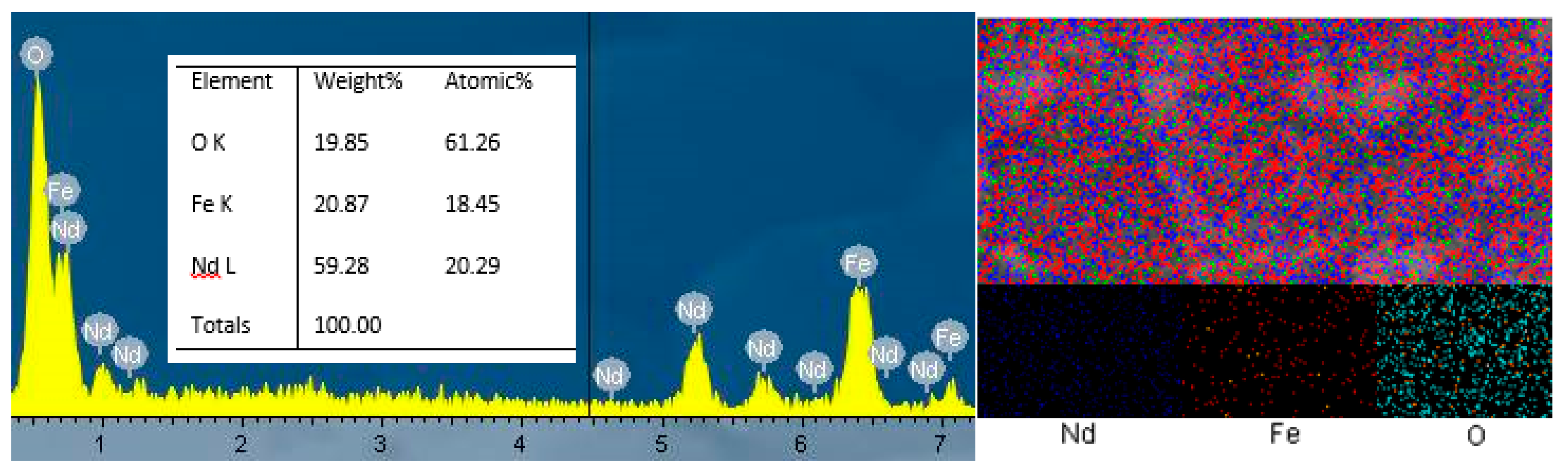
3.3. SEM, TEM, and EDX Results
3.4. VSM Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rempel, A.A. Nanotechnologies. Properties and applications of nanostructured materials. Russ. Chem. Rev. 2007, 76, 435–461. [Google Scholar] [CrossRef]
- Park, J.-H.; Shin, S.-H.; Kim, S.-H.; Park, J.-K.; Lee, J.-W.; Shin, J.-H.; Park, J.-H.; Kim, S.-W.; Choi, H.-J.; Lee, K.-S.; et al. Effect of synthesis time and composition on magnetic properties of FeCo nanoparticles by polyol method. J. Nanosci. Nanotechnol. 2018, 18, 7115–7119. [Google Scholar] [CrossRef]
- Bashir, A.; Ikram, M.; Kumar, R.; Lisboa-Filho, P.N. Structural, electronic structure and magnetic studies of GdFe1−xNixO3 (x ≤ 0.5). J. Alloys Compd. 2012, 251, 183–188. [Google Scholar] [CrossRef]
- Haron, W.; Thaweechai, T.; Wattanathana, W.; Laobuthee, A.; Manaspiya, H.; Veranitisagul, C.; Koonsaeng, N. Structural characteristics and dielectric properties of La1−xCoxFeO3 and LaFe1−xCoxO3 synthesized via metal organic complexes. Energy Procedia 2013, 34, 791–800. [Google Scholar] [CrossRef]
- Mastin, J.; Einarsrud, M.-A.; Grande, T. Crystal structure and thermal properties of La1−xCaxCoO3−δ (0 ≤ x ≤ 0.4). Chem. Mater. 2006, 18, 1680–1687. [Google Scholar] [CrossRef]
- Nada, F.A.; Ahmed, G.; Ekram, H.E.-A. Perovskite nanomaterials—Synthesis, characterization, and applications. In Perovskite Materials—Synthesis, Characterisation, Properties, and Applications, 1st ed.; Likun, P., Guang, Z., Eds.; InTechOpen: London, UK, 2016; Chapter 4; pp. 107–151. [Google Scholar]
- Shin, N.; Isao, A.; Yasuhiko, I.; Takeo, H. Preparation of Y(Mn1−xFex)O3 and electrical properties of the sintered bodies. Open J. Inorg. Chem. 2015, 5, 7–11. [Google Scholar]
- Luu, M.D.; Dao, N.N.; Nguyen, D.V.; Pham, N.C.; Vu, T.N.; Doan, T.D. A new perovskite-type NdFeO3 adsorbent: Synthesis, characterization, and As(V) adsorption. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 15–25. [Google Scholar] [CrossRef]
- Feng, C.; Ruan, S.; Li, J.; Zou, B.; Luo, J.; Chen, W.; Dong, W.; Wu, F. Ethanol sensing properties of LaCoxFe1−xO3 nanoparticles: Effects of calcinations temperature, Co-doping and carbon nanotub-treatment. Sens. Actuators B: Chem. 2011, 155, 232–238. [Google Scholar] [CrossRef]
- Jeffrey, W.F. Perovskite oxides for semiconductor-based gas sensors. Sens. Actuators B 2007, 123, 1169–1179. [Google Scholar]
- Oemar, U.; Ang, P.S.; Hidajat, K.; Kawi, S. Promotional effect of Fe on perovskite LaNixFe1−xO3 catalyst for hydrogen production via steam reforming of toluene. Int. J. Hydrog. Energy 2013, 38, 5525–5534. [Google Scholar] [CrossRef]
- Serna, P.V.; Campos, C.G.; Jesus, F.S.D.; Miro, A.M.B.; Loran, J.A.J.; Longwell, J. Mechanosynthesis, crystal structure and magnetic characterization of neodymium orthoferrite. Mater. Res. 2016, 19, 389–393. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.J.; Siddique, M.; Iqbal, M.; Hasan, M.M. Origin of anomalous octahedral distortions and collapse of magnetic ordering on Nd1−xSrxFeO3 (0 ≤ x ≤ 0.5). Ceram. Int. 2013, 39, 8901–8909. [Google Scholar] [CrossRef]
- Babu, P.R.; Babu, R. Starch assisted sol-gel synthesis and characterization of NdFeO3. Int. J. Chem. Tech. Res. 2016, 9, 364–369. [Google Scholar]
- Shanker, J.; Rao, G.N.; Venkataramana, K.; Babu, D.S. Investigation of structural and electrical properties of NdFeO3 perovskite nanocrystalline. Phys. Lett. A 2018, 382, 2974–2977. [Google Scholar] [CrossRef]
- Zharvan, V.; Kamaruddin, Y.N.; Samnur, S.; Sujiono, E.H. The effect of molar ratio on crystal structure and morphology of Nd1+xFeO3 (x = 0.1, 0.2 and 0.3) oxide alloy material synthesized by solid state reaction method. In Proceedings of the 4th International Conference on Advanced Materials Science and Technology, IOP Conf. Series: Materials Science and Engineerieng, Universitas Negeri Malang, Malang, Indonesia, 27–28 September 2017; Volume 202, p. 012072. [Google Scholar]
- Shanker, J.; Buchi Suresh, M.; Suresh Babu, D. Synthesis, chacracterization and impendance spectroscopy studies of NdFeO3 perovskite ceramics. Int. J. Sci. Eng. Res. (Ijser) 2015, 3, 194–197. [Google Scholar]
- Tugova, E.; Yastrebov, S.; Karpov, O.; Smith, R. NdFeO3 nanocrystals under glycine nitrate combustion formation. J. Cryst. Growth 2017, 467, 88–92. [Google Scholar] [CrossRef]
- Mostafa, Y.; Samaneh, S.Z.; Mozhgan, K.-M. Synthesis and characterization of nano-structured perovskite type neodymium orthoferrite NdFeO3. Curr. Chem. Lett. 2017, 6, 23–30. [Google Scholar]
- Mozhgan, K.-M.; Noroozifar, M.; Yousefi, M.; Jahani, S. Chemical synthesis and characterization of perovskite NdFeO3 nanocrystals via a co-precipitation method. Int. J. Nanosci. Nanotechnol. 2013, 9, 7–14. [Google Scholar]
- El Moussaoui, H.; Mounkachi, O.; Masrour, R.; Hamedoun, M.; Hlil, E.K.; Benyoussef, A. Synthesis and super-paramagnetic properties of neodymium ferrites nanorods. J. Alloys Compd. 2013, 581, 776–781. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Mittova, I.Y.; Almjasheva, O.V.; Kirillova, S.A.; Gusarov, V.V. Influence of the preparation conditions on the size and morphology of nanocrystalline lanthanum orthoferrite. Glass Phys. Chem. 2008, 34, 756–761. [Google Scholar]
- Nguyen, A.T.; Almjasheva, O.V.; Mittova, I.Y.; Stognei, O.V.; Soldatenko, S.A. Synthesis and magnetic properties of YFeO3 nanocrystals. Inorg. Mater. 2009, 45, 1304–1308. [Google Scholar]
- Nguyen, A.T.; Mittova, I.Y.; Solodukhin, D.O.; Almjasheva, O.V.; Mittova, V.O.; Demidova, S.Y. Sol-gel formation and properties of nanocrystals of solid solution Y1−xCaxFeO3. Russ. J. Inorg. Chem. 2014, 59, 40–45. [Google Scholar]
- Nguyen, A.T.; Chau, H.D.; Nguyen, T.T.L.; Mittova, V.O.; Do, T.H.; Mittova, I.Y. Structural and magnetic properties of YFe1−xCoxO3 (0.1 ≤ x ≤ 0.5) perovskite nanomaterials synthesized by co-precipitation method. Nanosyst.: Phys. Chem. Math. 2018, 9, 424–429. [Google Scholar]
- Berezhnaya, M.V.; Perov, N.S.; Almjasheva, O.V.; Mittova, V.O.; Nguyen, A.T.; Mittova, I.Y.; Druzhinina, L.V.; Alekhina, Y.A. Synthesis and magnetic properties of barium-doped nanocrystal lanthanum orthoferrie. Russ. J. Gen. Chem. 2019, 89, 480–485. [Google Scholar] [CrossRef]
- Berezhnaya, M.V.; Mittova, I.Y.; Perov, N.S.; Al’myasheva, O.V.; Nguyen, A.T.; Mittova, V.O.; Bessalova, V.V.; Viryutina, E.L. Production of zinc-doped yttrium ferrite nanopwders by the sol-gel method. Russ. J. Inorg. Chem. 2018, 63, 742–746. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 2nd ed.; Pearson, Prentice Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Kozo, N.; Wakita, H.; Mochizuki, A. The synthesis of crystalline rate earth carbonates. Bull. Chem. Soc. Jpn. 1973, 46, 152–156. [Google Scholar]
- Adachi, G.; Imanaka, N.; Kang, Z.C. Binary rate earth oxides; Kluwer Academic Publishers: New York, NY, USA; Bostin, MA, USA; Dordrecht, The Netherlands; Moscow, Russia, 2005; ISBN 1-4020-2569-6. [Google Scholar]
- Caro, P.; Lemaitre, M.; Blassé, M. Hydroxycarbonates deterres rates Ln2(CO3)x(OH)2(3−x)·nH2O. C. R. Seances Acad. Sci., Ser. C 1969, 269, 687–690. [Google Scholar]
- Yuan, S.J.; Wang, Y.; Shao, M.; Chang, F.; Kang, B.; Isikawa, Y.; Cao, S. Spin switching and magnetization reversal in single-crystal NdFeO3. Phys. Rev. B 2013, 87, 118405. [Google Scholar] [CrossRef]


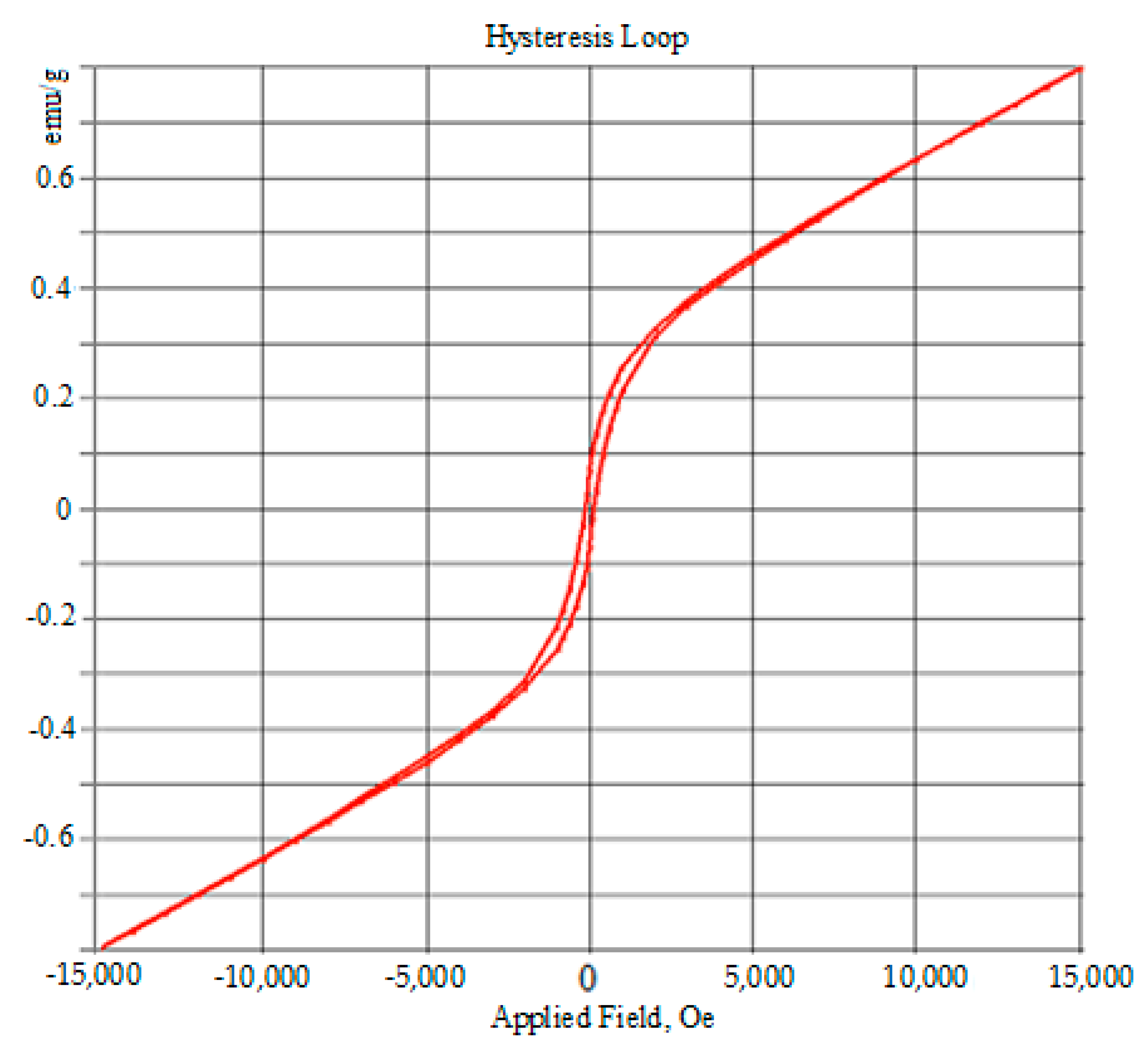
| D (nm) | a (Å) | b (Å) | c (Å) | V (Å3) | Ms (emu/g) | Hc (Oe) | Mr (emu/g) |
|---|---|---|---|---|---|---|---|
| 28.41 | 5.4990 | 5.5910 | 7.7592 | 238.5559 | 0.797 | 136.76 | 0.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.A.; Pham, V.; Pham, T.L.; Nguyen, L.T.T.; Mittova, I.Y.; Mittova, V.O.; Vo, L.N.; Nguyen, B.T.T.; Bui, V.X.; Viryutina, E.L. Simple Synthesis of NdFeO3 Nanoparticles By the Co-Precipitation Method Based on a Study of Thermal Behaviors of Fe (III) and Nd (III) Hydroxides. Crystals 2020, 10, 219. https://doi.org/10.3390/cryst10030219
Nguyen TA, Pham V, Pham TL, Nguyen LTT, Mittova IY, Mittova VO, Vo LN, Nguyen BTT, Bui VX, Viryutina EL. Simple Synthesis of NdFeO3 Nanoparticles By the Co-Precipitation Method Based on a Study of Thermal Behaviors of Fe (III) and Nd (III) Hydroxides. Crystals. 2020; 10(3):219. https://doi.org/10.3390/cryst10030219
Chicago/Turabian StyleNguyen, Tien A., V. Pham, Thanh L. Pham, Linh T. Tr. Nguyen, I. Ya. Mittova, V. O. Mittova, Lan N. Vo, Bich Tram T. Nguyen, Vuong X. Bui, and E. L. Viryutina. 2020. "Simple Synthesis of NdFeO3 Nanoparticles By the Co-Precipitation Method Based on a Study of Thermal Behaviors of Fe (III) and Nd (III) Hydroxides" Crystals 10, no. 3: 219. https://doi.org/10.3390/cryst10030219
APA StyleNguyen, T. A., Pham, V., Pham, T. L., Nguyen, L. T. T., Mittova, I. Y., Mittova, V. O., Vo, L. N., Nguyen, B. T. T., Bui, V. X., & Viryutina, E. L. (2020). Simple Synthesis of NdFeO3 Nanoparticles By the Co-Precipitation Method Based on a Study of Thermal Behaviors of Fe (III) and Nd (III) Hydroxides. Crystals, 10(3), 219. https://doi.org/10.3390/cryst10030219