Microstructural Stability and Softening Resistance of a Novel Hot-Work Die Steel
Abstract
1. Introduction
2. Materials and Experiments
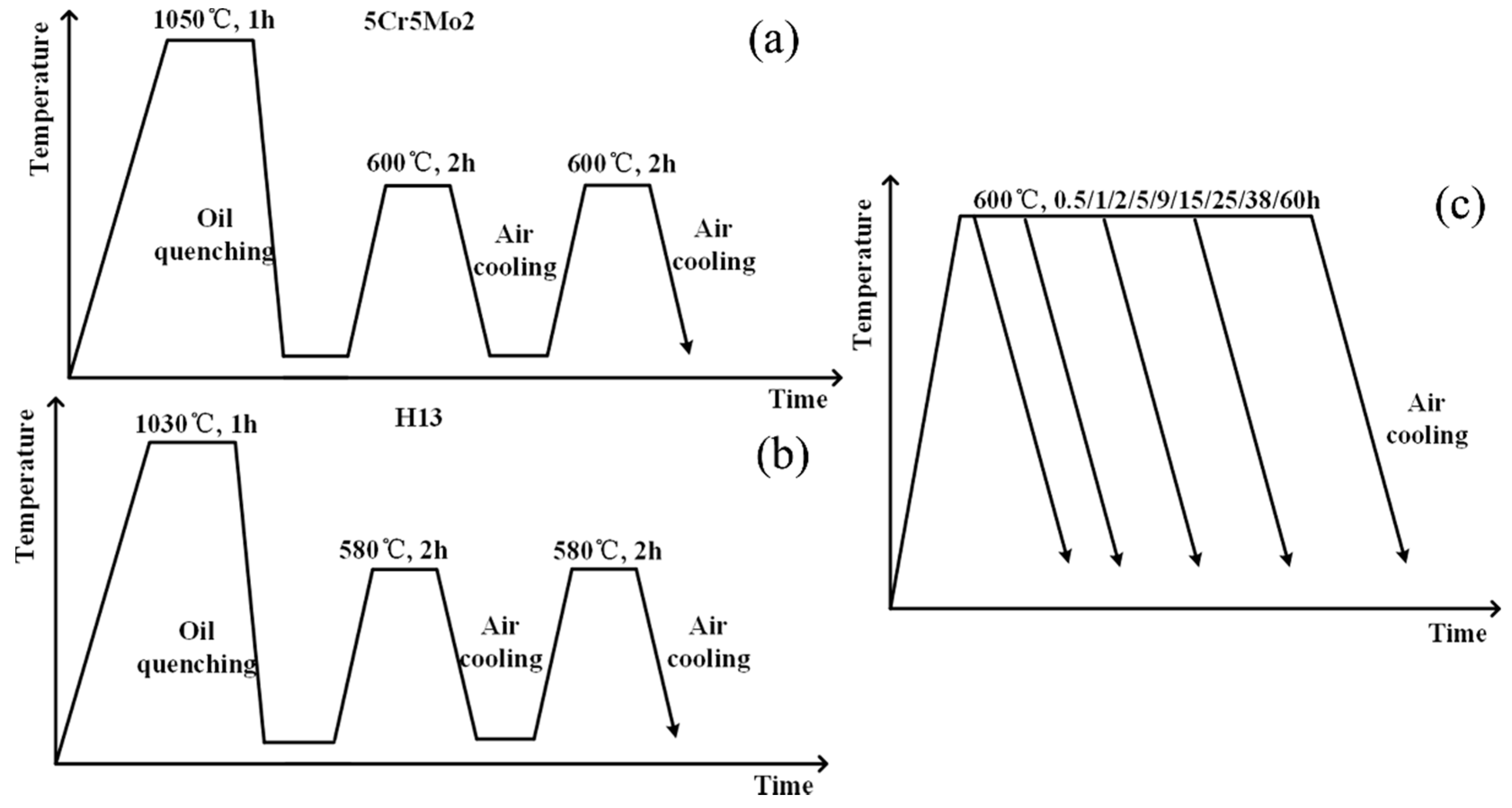
2.1. Materials and Heat Treatment
2.2. Mechanical Tests
2.3. Microstructure Observations
2.4. X-ray Diffraction Analysis
3. Results and Discussion
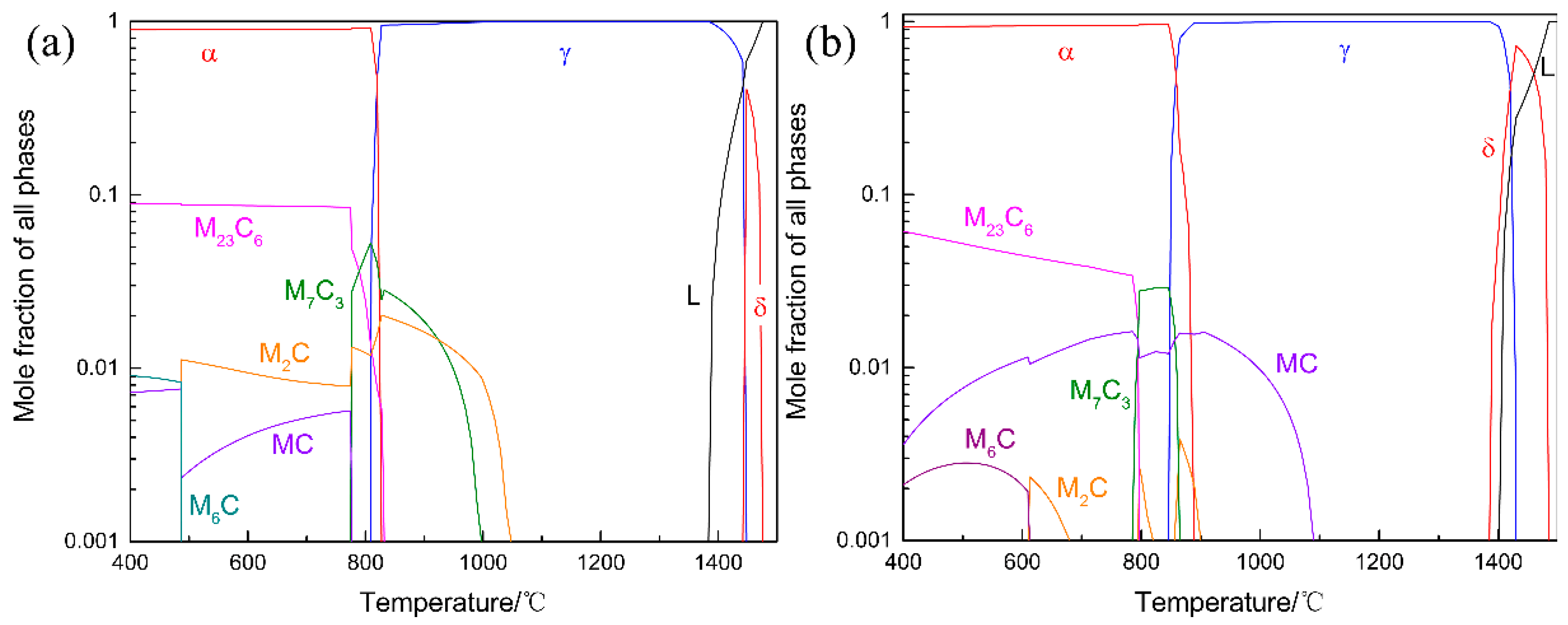
3.1. Alloy Design
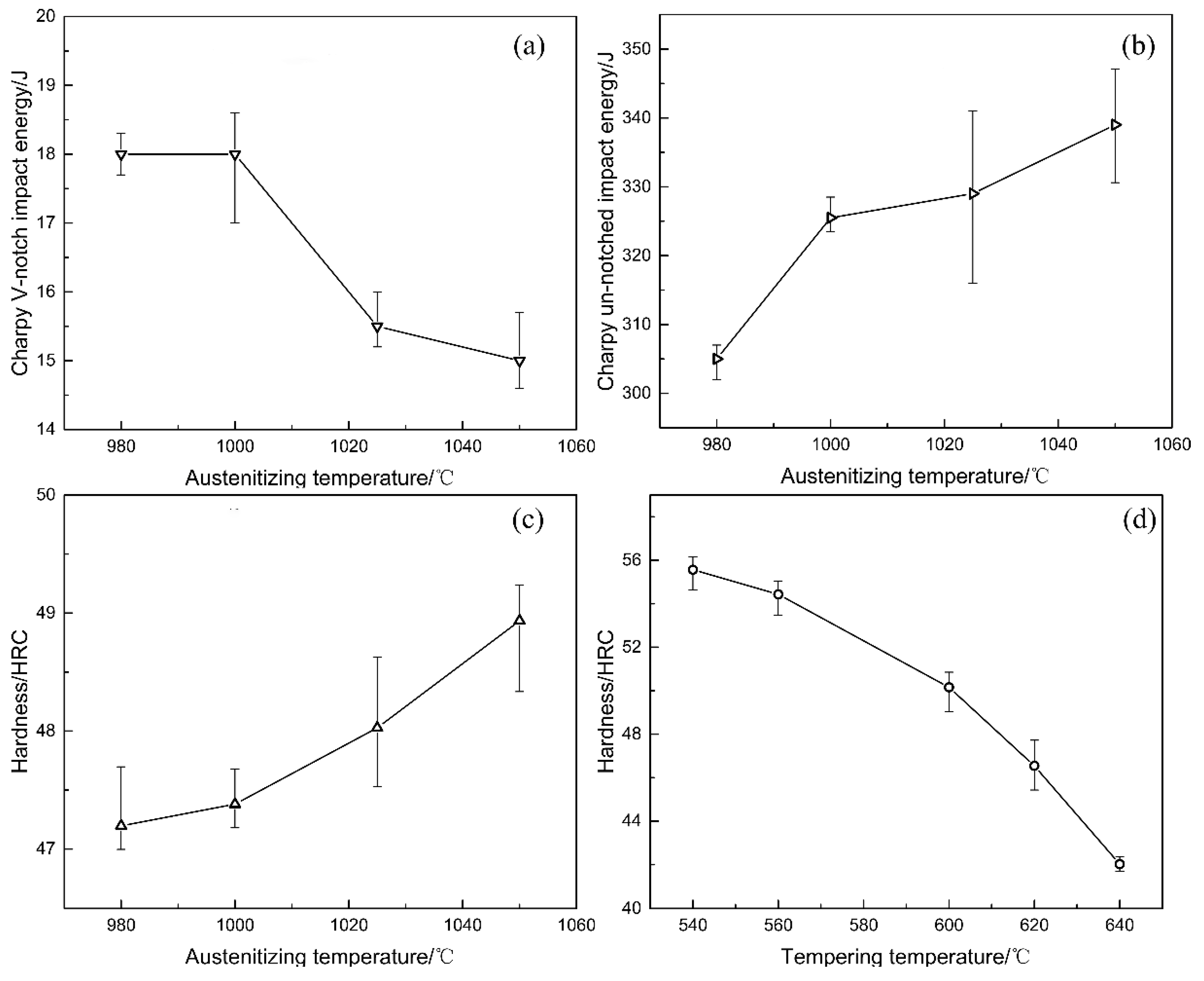
3.2. Mechanical Properties
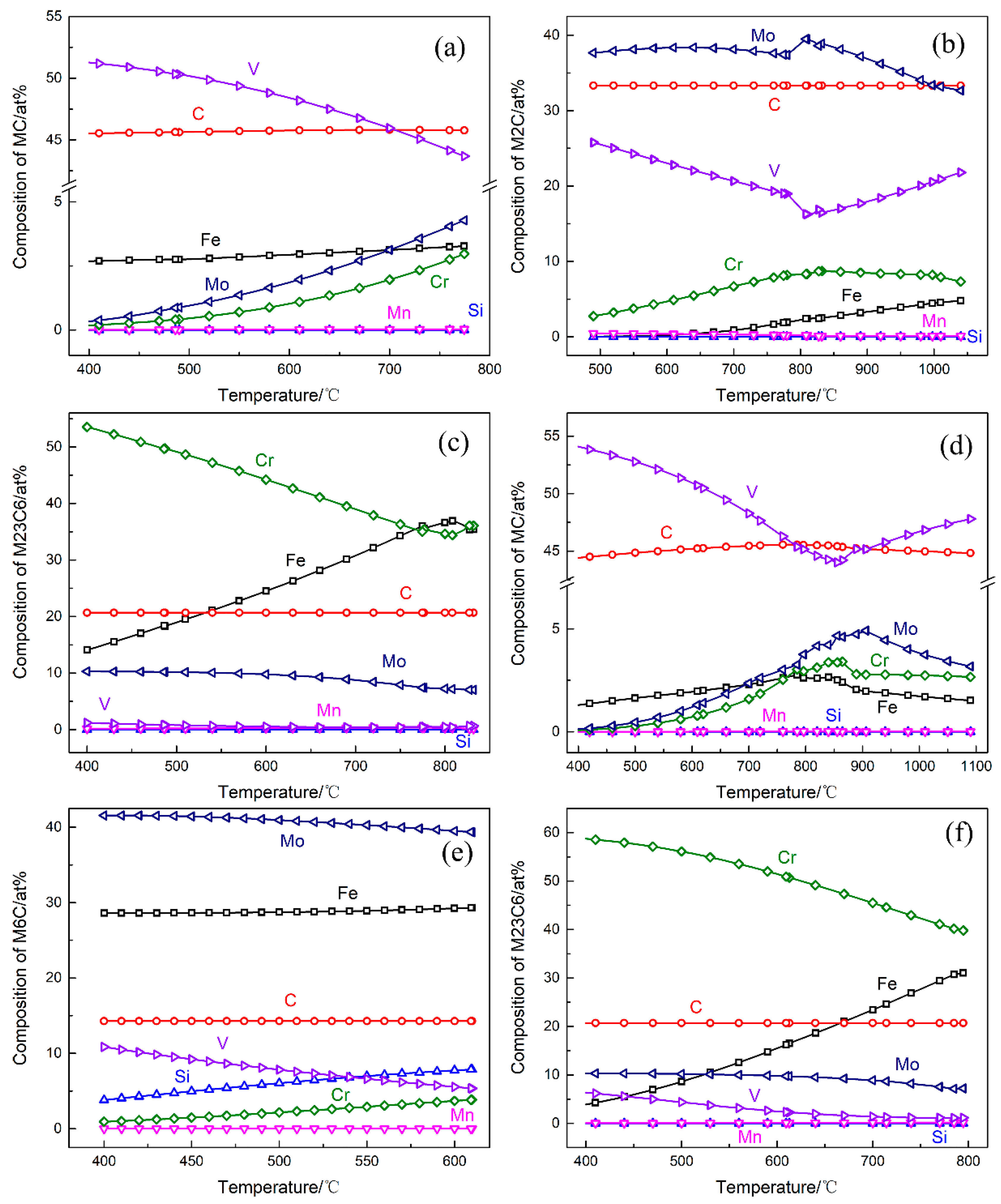
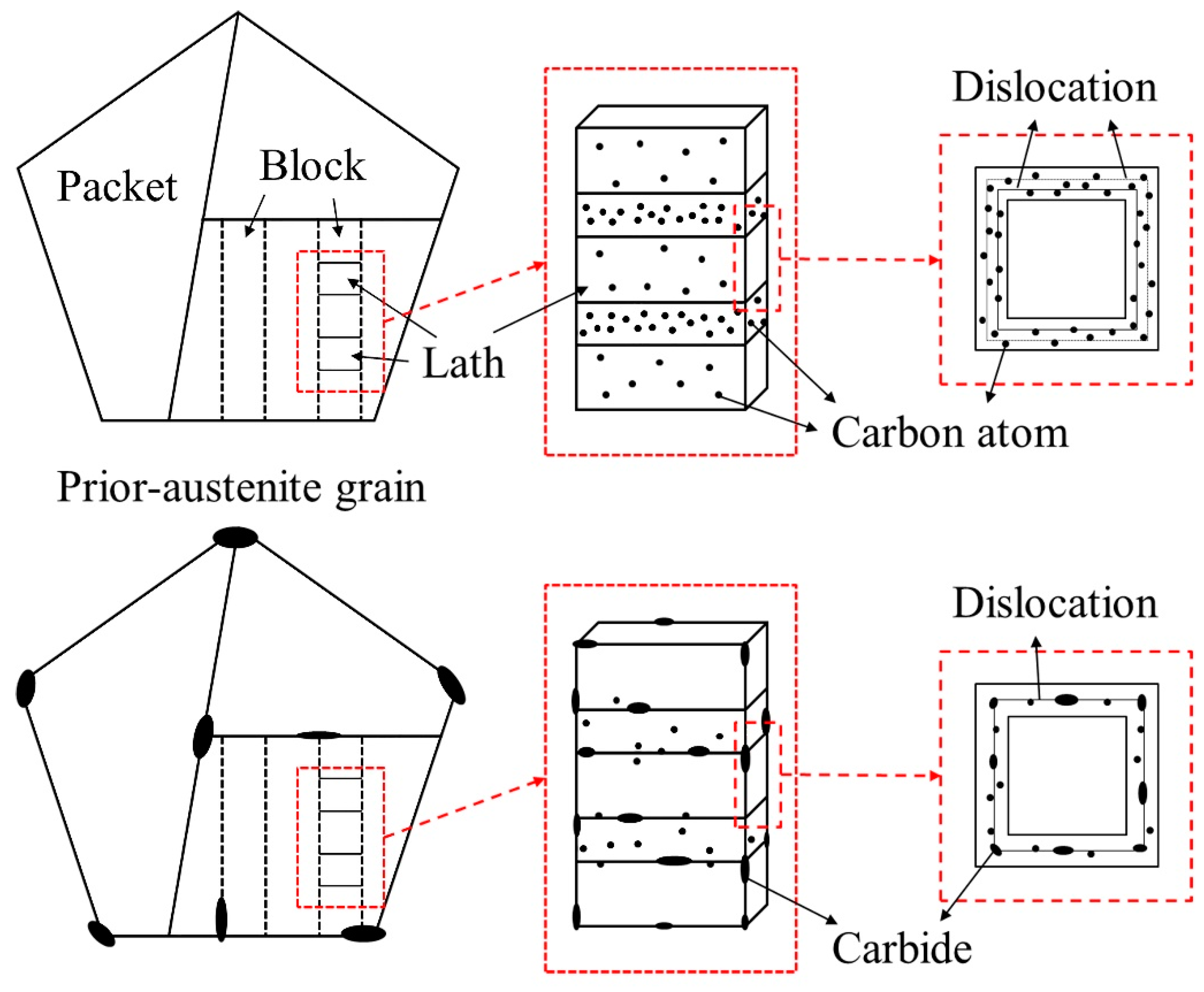
3.3. Microstructure
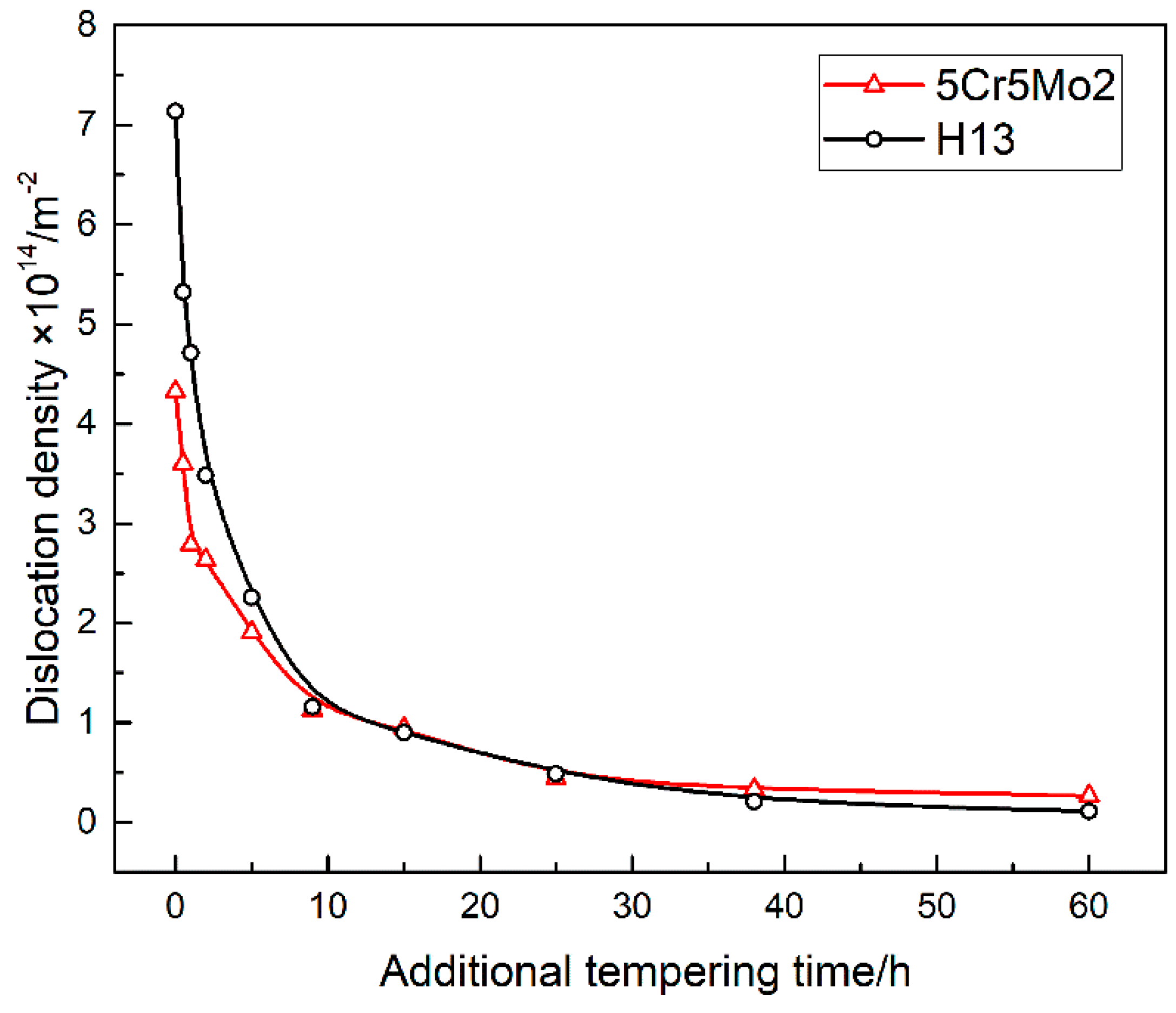
3.4. Dislocation Density
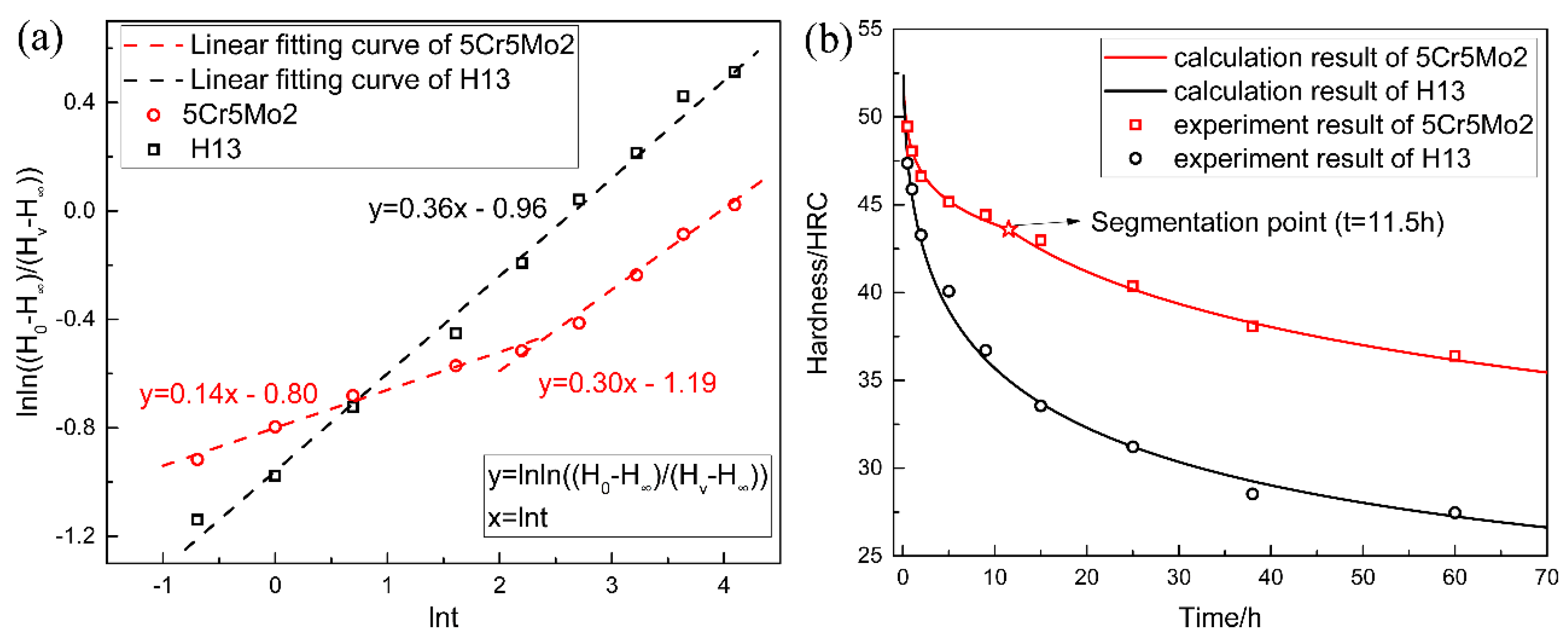
3.5. Tempering Kinetics and the Softening Model
4. Conclusions
- (1)
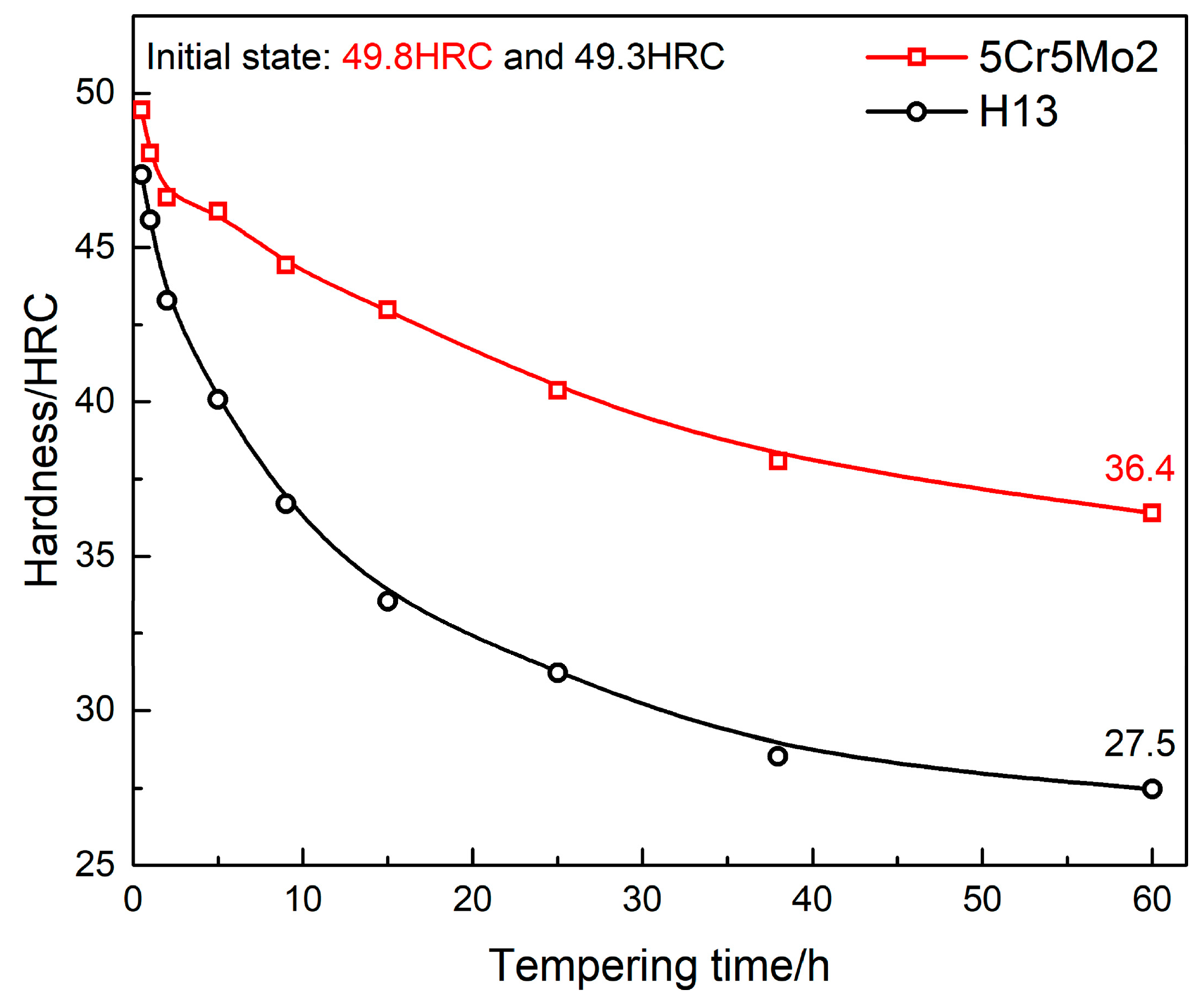
- The designed steel exhibits a superior softening resistance as compared with the common H13 steel. After tempering for 60 h, the hardness of the 5Cr5Mo2 and H13 steels decreased from 49.8 HRC and 49.3 HRC to 36.4 HRC and 27.3 HRC, respectively.
- (2)
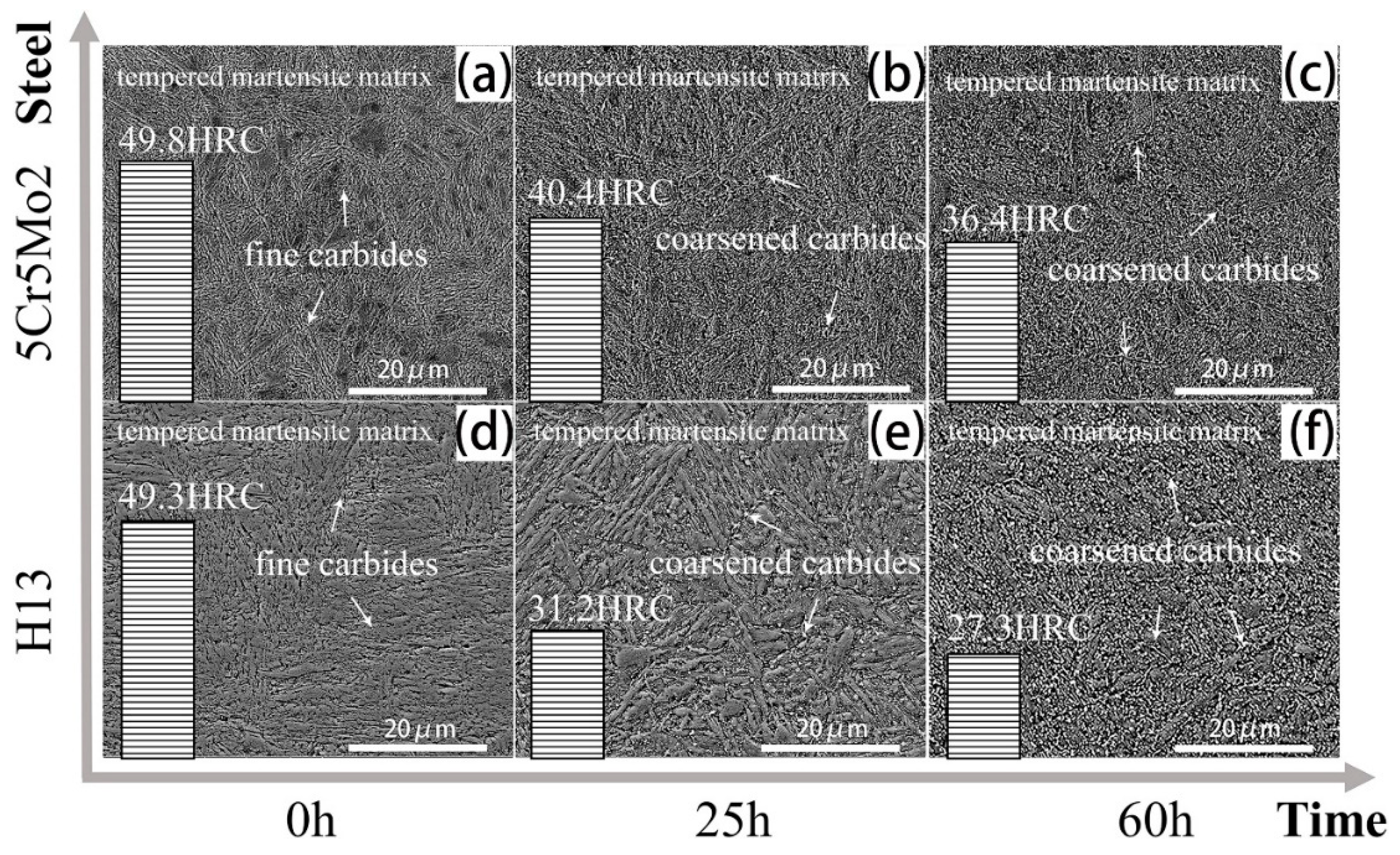
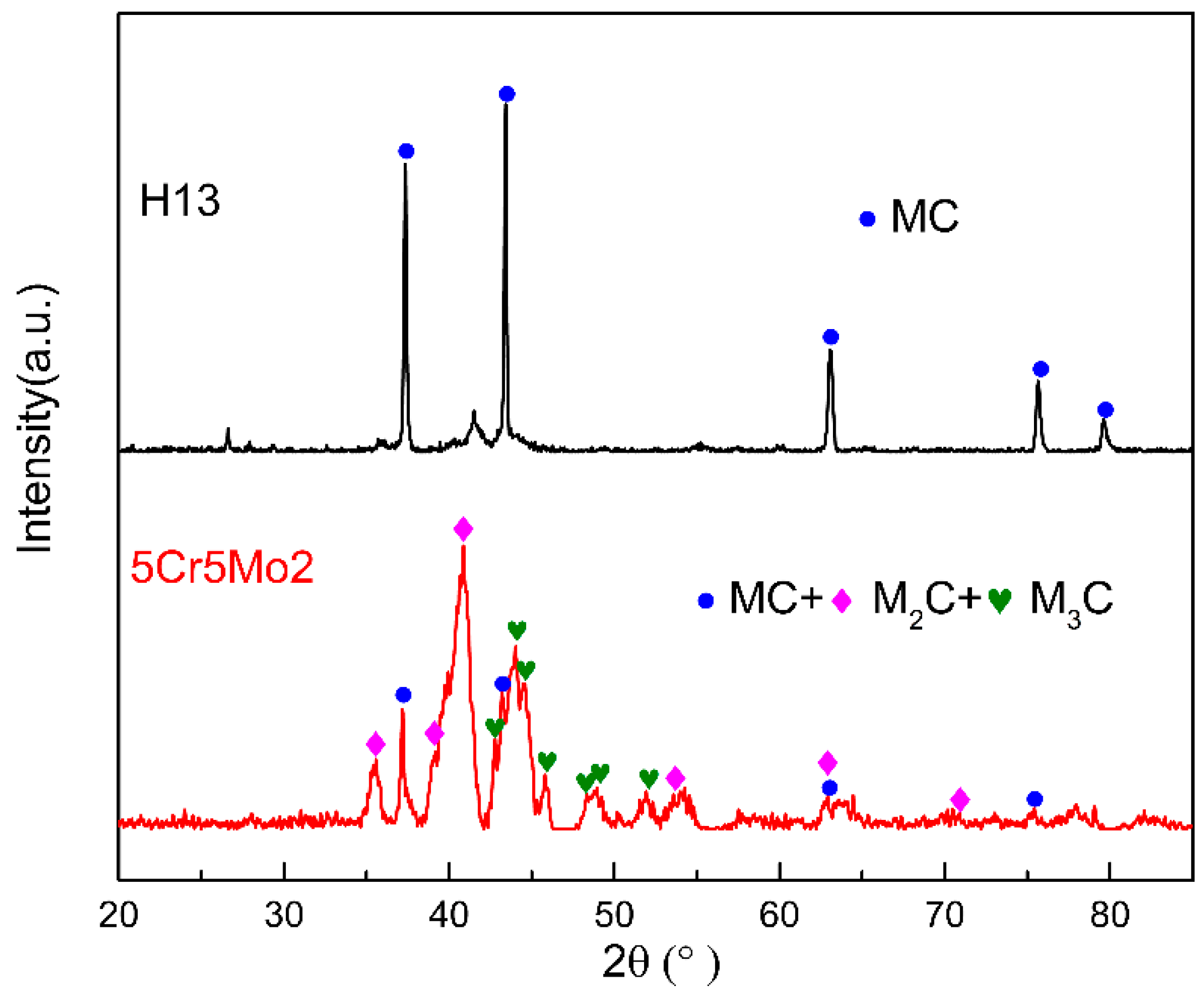
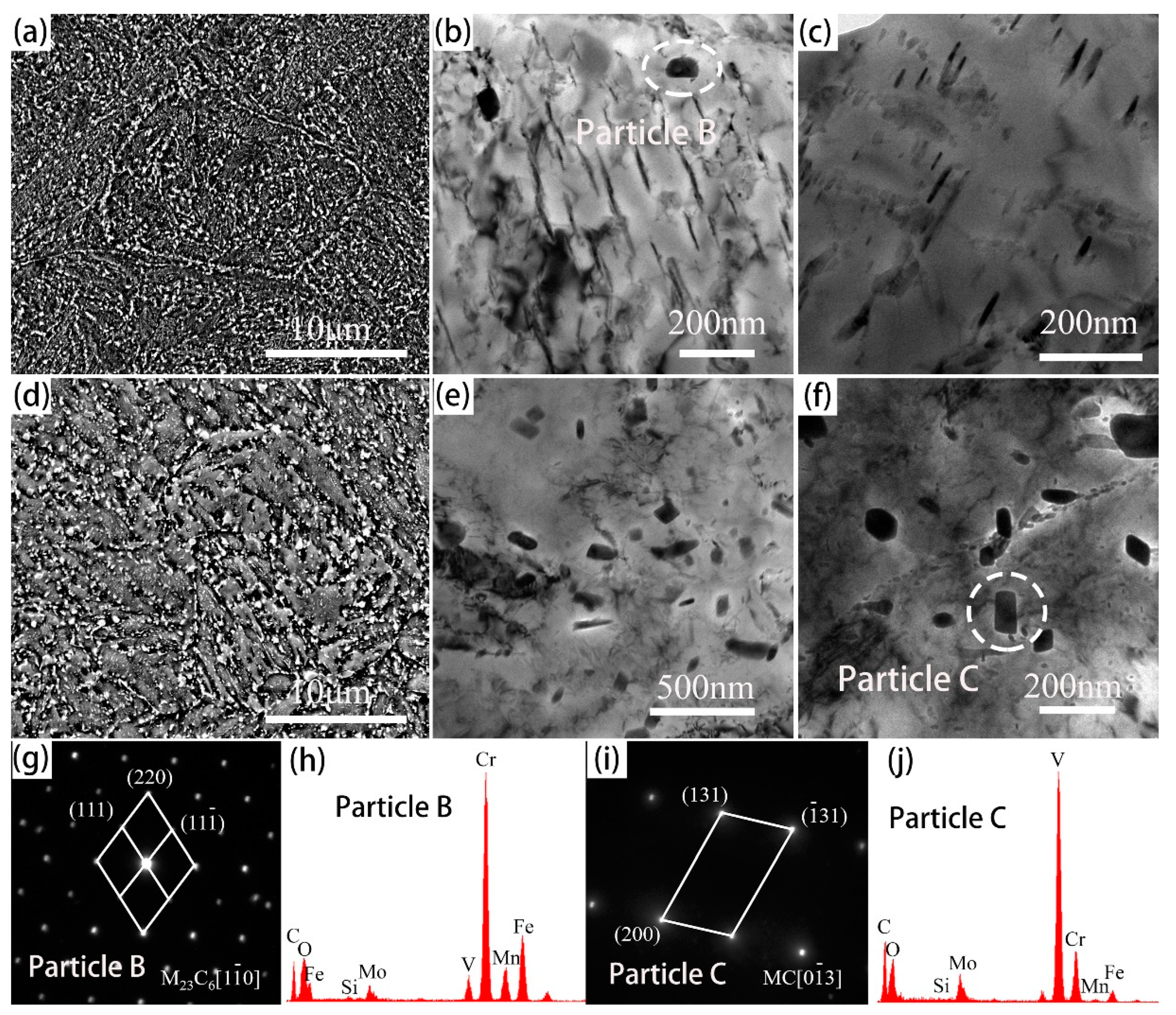
- The initial microstructure of the designed steel without the additional tempering primarily consisted of tempered martensite and fine alloy carbides. The typical secondary carbides in the 5Cr5Mo2 steel were elliptical vanadium carbides and fine acicular molybdenum carbides.
- (3)
- By the additional tempering, the smaller carbides were dissolved, and the coarsening of the selective carbide occurred. Martensites as well as dislocations were recovered in both steels. However, the initial characteristics of the martensite morphology were more pronounced in the 5Cr5Mo2 steel. Moreover, relatively finer carbides were retained in the 5Cr5Mo2 steel as compared to those in the H13 steel.
- (4)
- Initially, the dislocation density of the 5Cr5Mo2 steel was lower than that of the H13 steel due to its different pre-heat-treatment process. Nevertheless, after being exposed to an additional tempering lasting over 25 h, owing to the dislocation pinning by precipitation, the dislocation density exceeded that of the H13 steel.
- (5)
- By calculating the tempering kinetics of both steels, their softening equations were obtained and validated. The equations can be used to effectively predict the hardness of the dies after the prolonged service period at 600 °C.
Author Contributions
Funding
Conflicts of Interest
References
- Medvedeva, A.; Bergström, J.; Gunnarsson, S.; Andersson, J. High-temperature properties and microstructural stability of hot-work tool steels. Mater. Sci. Eng. A 2009, 523, 39–46. [Google Scholar] [CrossRef]
- Roberts, G.; Krauss, G.; Kennedy, R. Tool Steels, 5th ed.; ASM International: Novelty, OH, USA, 1998. [Google Scholar]
- Jilg, A.; Seifert, T. Temperature dependent cyclic mechanical properties of a hot work steel after time and temperature dependent softening. Mater. Sci. Eng. A 2018, 721, 96–102. [Google Scholar] [CrossRef]
- Chander, S.; Chawla, V. Failure of hot forging dies—An updated perspective. Mater. Today Proc. 2017, 4, 1147–1157. [Google Scholar] [CrossRef]
- Pešička, J.; Kužel, R.; Dronhofer, A.; Eggeler, G. The evolution of dislocation density during heat treatment and creep of tempered martensite ferritic steels. Acta Mater. 2003, 51, 4847–4862. [Google Scholar] [CrossRef]
- Zhang, Z.; Delagnes, D.; Bernhart, G. Microstructure evolution of hot-work tool steels during tempering and definition of a kinetic law based on hardness measurements. Mater. Sci. Eng. A 2004, 380, 222–230. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, X.; Shi, N.; Li, J.; Min, N. Microstructure evolution and kinetic analysis of DM hot-work die steels during tempering. Mater. Sci. Eng. A 2011, 528, 5696–5700. [Google Scholar] [CrossRef]
- Hu, X.; Li, L.; Wu, X.; Zhang, M. Coarsening behavior of M23C6 carbides after ageing or thermal fatigue in AISI H13 steel with niobium. Int. J. Fatigue 2006, 28, 175–182. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Xie, J. Improving strength and ductility of H13 die steel by pre-tempering treatment and its mechanism. Mater. Sci. Eng. A 2019, 752, 101–114. [Google Scholar] [CrossRef]
- Malheiros, L.R.C.; Rodriguez, E.A.P.; Arlazarov, A. Mechanical behavior of tempered martensite: Characterization and modeling. Mater. Sci. Eng. A 2017, 706, 38–47. [Google Scholar] [CrossRef]
- Michaud, P.; Delagnes, D.; Lamesle, P.; Mathon, M.H.; Levaillant, C. The effect of the addition of alloying elements on carbide precipitation and mechanical properties in 5% chromium martensitic steels. Acta Mater. 2007, 55, 4877–4889. [Google Scholar] [CrossRef]
- Delagnes, D.; Lamesle, P.; Mathon, M.H.; Mebarki, N.; Levaillant, C. Influence of silicon content on the precipitation of secondary carbides and fatigue properties of a 5%Cr tempered martensitic steel. Mater. Sci. Eng. A 2005, 394, 435–444. [Google Scholar] [CrossRef]
- Saha, D.C.; Biro, E.; Gerlich, A.P.; Zhou, Y. Effects of tempering mode on the structural changes of martensite. Mater. Sci. Eng. A 2016, 673, 467–475. [Google Scholar] [CrossRef]
- Gomes, C.; Kaiser, A.-L.; Bas, J.-P.; Aissaoui, A.; Piette, M. Predicting the mechanical properties of a quenched and tempered steel thanks to a “tempering parameter”. Rev. Metall. 2010, 107, 293–302. [Google Scholar] [CrossRef]
- Revilla, C.; López, B.; Rodriguez-Ibabe, J.M. Carbide size refinement by controlling the heating rate during induction tempering in a low alloy steel. Mater. Des. 2014, 62, 296–304. [Google Scholar] [CrossRef]
- Furuhara, T.; Kobayashi, K.; Maki, T. Control of cementite precipitation in lath martensite by rapid heating and tempering. ISIJ Int. 2004, 44, 1937–1944. [Google Scholar] [CrossRef]
- Hernandez, V.H.B.; Nayak, S.S.; Zhou, Y. Tempering of martensite in dual-phase steels and its effects on softening behavior. Metall. Mater. Trans. A 2011, 42, 3115–3129. [Google Scholar] [CrossRef]
- Nayak, S.S.; Hernandez, V.H.B.; Zhou, Y. Effect of chemistry on nonisothermal tempering and softening of dual-phase steels. Metall. Mater. Trans. A 2011, 42, 3242–3248. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Honeycombe, R. Tempering of Martensite. In Steels: Microstructure and Properties, 4th ed.; Gifford, C., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 237–270. [Google Scholar]
- Mote, V.D.; Purushotham, Y.; Dole, B.N. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theoret. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef]
- Dini, G.; Ueji, R.; Najafizadeh, A.; Monir-Vaghefi, S.M. Flow stress analysis of TWIP steel via the XRD measurement of dislocation density. Mater. Sci. Eng. A 2010, 527, 2759–2763. [Google Scholar] [CrossRef]
- Dragomir, I.C.; Li, D.S.; Castello-Branco, G.A.; Garmestani, H.; Snyder, R.L.; Ribarik, G.; Ungar, T. Evolution of dislocation density and character in hot rolled titanium determined by X-ray diffraction. Mater. Charact. 2005, 55, 66–74. [Google Scholar] [CrossRef]
- ABaghdadi, H.; Rajabi, A.; Selamat, N.F.M.; Sajuri, Z.; Omar, M.Z. Effect of post-weld heat treatment on mechanical behavior and dislocation density of friction stir welded Al6061. Mater. Sci. Eng. A 2019, 754, 728–734. [Google Scholar] [CrossRef]
- Liu, H.; Fu, P.; Liu, H.; Sun, C.; Sun, M.; Li, D. A novel large cross-section quenching and tempering mold steel matching excellent strength–hardness–toughness properties. Mater. Sci. Eng. A 2018, 737, 274–285. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Huang, B.-M.; Tsai, Y.-T.; Tsai, S.-P.; Chen, C.-Y.; Yang, J.-R. Microstructural evolutions of low carbon Nb/Mo-containing bainitic steels during high-temperature tempering. Mater. Charact. 2017, 131, 298–305. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, C.-H.; Heo, Y.-U.; Suh, D.-W. Stability of (Ti, M)C (M= Nb, V, Mo and W) carbide in steels using first-principles calculations. Acta Mater. 2012, 60, 208–217. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Guo, C.; Liu, W.; Yang, Z.; Sun, X.; Zhang, Z.; Jiang, F. Effect of molybdenum addition on the precipitation of carbides in the austenite matrix of titanium micro-alloyed steels. J. Mater. Sci. 2016, 51, 4996–5007. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yen, H.W.; Kao, F.H.; Li, W.C.; Huang, C.Y.; Yang, J.R.; Wang, S.H. Precipitation hardening of high-strength low-alloy steels by nanometer-sized carbides. Mater. Sci. Eng. A 2009, 499, 162–166. [Google Scholar] [CrossRef]
- Krajnik, P.; Kopač, J. Modern machining of die and mold tools. J. Mater. Process. Technol. 2004, 157–158, 543–552. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Honeycombe, R. Formation of Martensite, in Steels: Microstructure and Properties, 4th ed.; Gifford, C., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 135–177. [Google Scholar]
- Sakamoto, H.; Otsuka, K.; Shimizu, K. Rubber-like behavior in a Cu-Al-Ni alloy. Scr. Metall. 1977, 11, 607–611. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, K.B.; Yang, H.R.; Lee, J.B.; Kim, Y.S. Secondary hardening and fracture behavior in alloy steels containing Mo, W, and Cr. Metall. Mater. Trans. A 1997, 28, 775–784. [Google Scholar] [CrossRef]
- Williamson, G.K.; Smallman, R.E., III. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray debye-scherrer spectrum. Philos. Mag. 1956, 1, 34–46. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminum and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Johnson, W.A.; Mehl, R.F. Reaction kinetics in processes of nucleation and growth. Trans. Am. Inst. Min. Met. Eng. 1939, 135, 416–442. [Google Scholar]
- Avrami, M. Kinetics of phase change I. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change II. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase change, and microstructure kinetics of phase change III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Watté, P.; van Humbeeck, J.; Aernoudt, E.; Lefever, I. Strain ageing in heavily drawn eutectoid steel wires. Scripta Mater. 1996, 34, 89–95. [Google Scholar] [CrossRef]

| Steel | C | Cr | Mo | V | Si | Mn |
|---|---|---|---|---|---|---|
| 5Cr5Mo2 | 0.50 | 5.14 | 2.48 | 0.51 | 0.20 | 0.50 |
| H13 | 0.32 | 5.05 | 1.35 | 0.90 | 0.97 | 0.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, N.; Liu, H.; Fu, P.; Liu, H.; Sun, C.; Cao, Y.; Li, D. Microstructural Stability and Softening Resistance of a Novel Hot-Work Die Steel. Crystals 2020, 10, 238. https://doi.org/10.3390/cryst10040238
Du N, Liu H, Fu P, Liu H, Sun C, Cao Y, Li D. Microstructural Stability and Softening Resistance of a Novel Hot-Work Die Steel. Crystals. 2020; 10(4):238. https://doi.org/10.3390/cryst10040238
Chicago/Turabian StyleDu, Ningyu, Hongwei Liu, Paixian Fu, Hanghang Liu, Chen Sun, Yanfei Cao, and Dianzhong Li. 2020. "Microstructural Stability and Softening Resistance of a Novel Hot-Work Die Steel" Crystals 10, no. 4: 238. https://doi.org/10.3390/cryst10040238
APA StyleDu, N., Liu, H., Fu, P., Liu, H., Sun, C., Cao, Y., & Li, D. (2020). Microstructural Stability and Softening Resistance of a Novel Hot-Work Die Steel. Crystals, 10(4), 238. https://doi.org/10.3390/cryst10040238





