Synthesis, Phase Transition, and Optical Studies of Ba2−xSrxZnWO6 (x = 1.00, 1.25, 1.50, 1.75, 2.00) Tungsten Double Perovskite Oxides
Abstract
1. Introduction
2. Materials and Experimental
2.1. Preparation of Materials
2.2. Sample Characterization
3. Results and Discussion
3.1. XRD
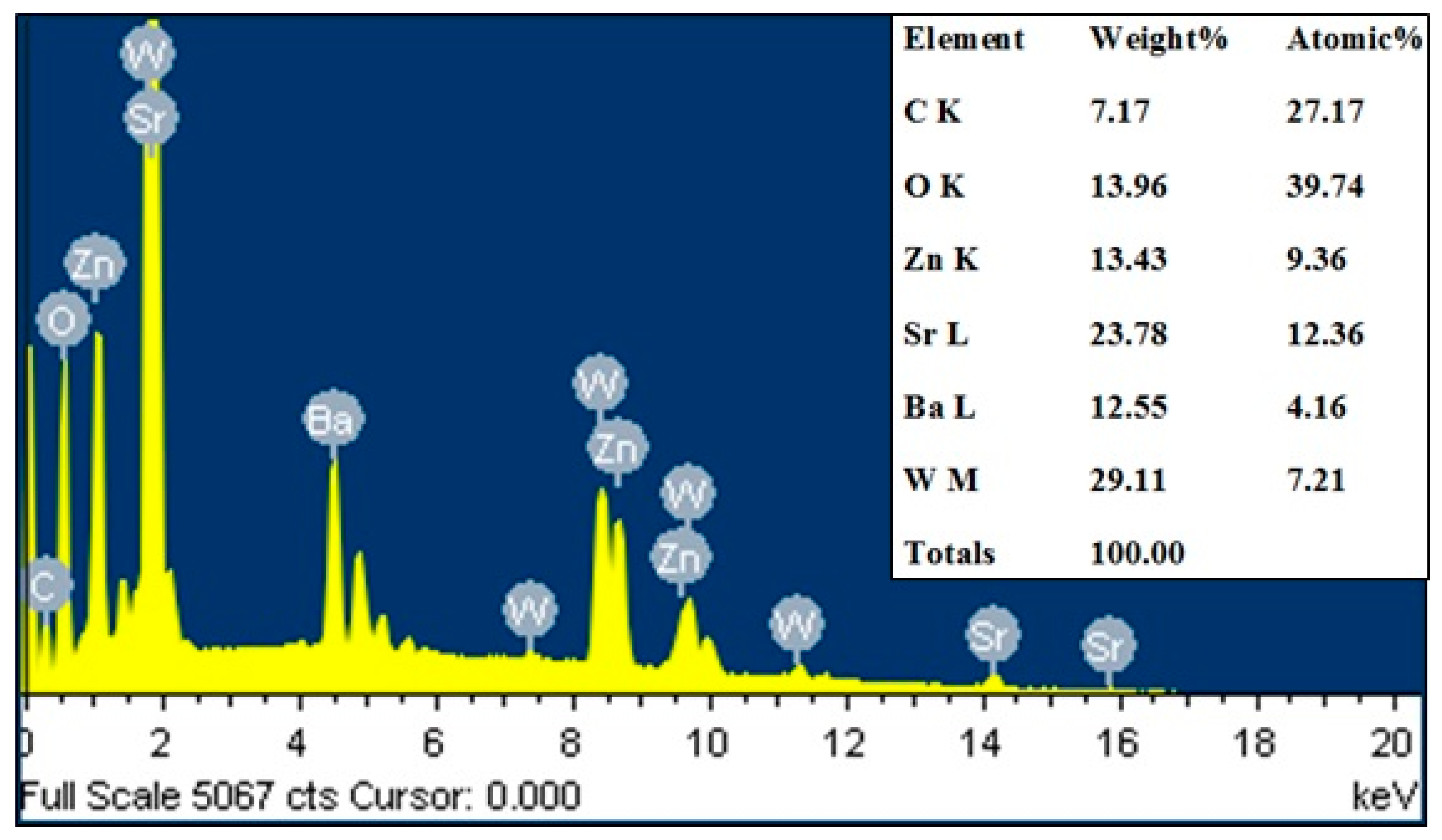
3.2. SEM and EDX
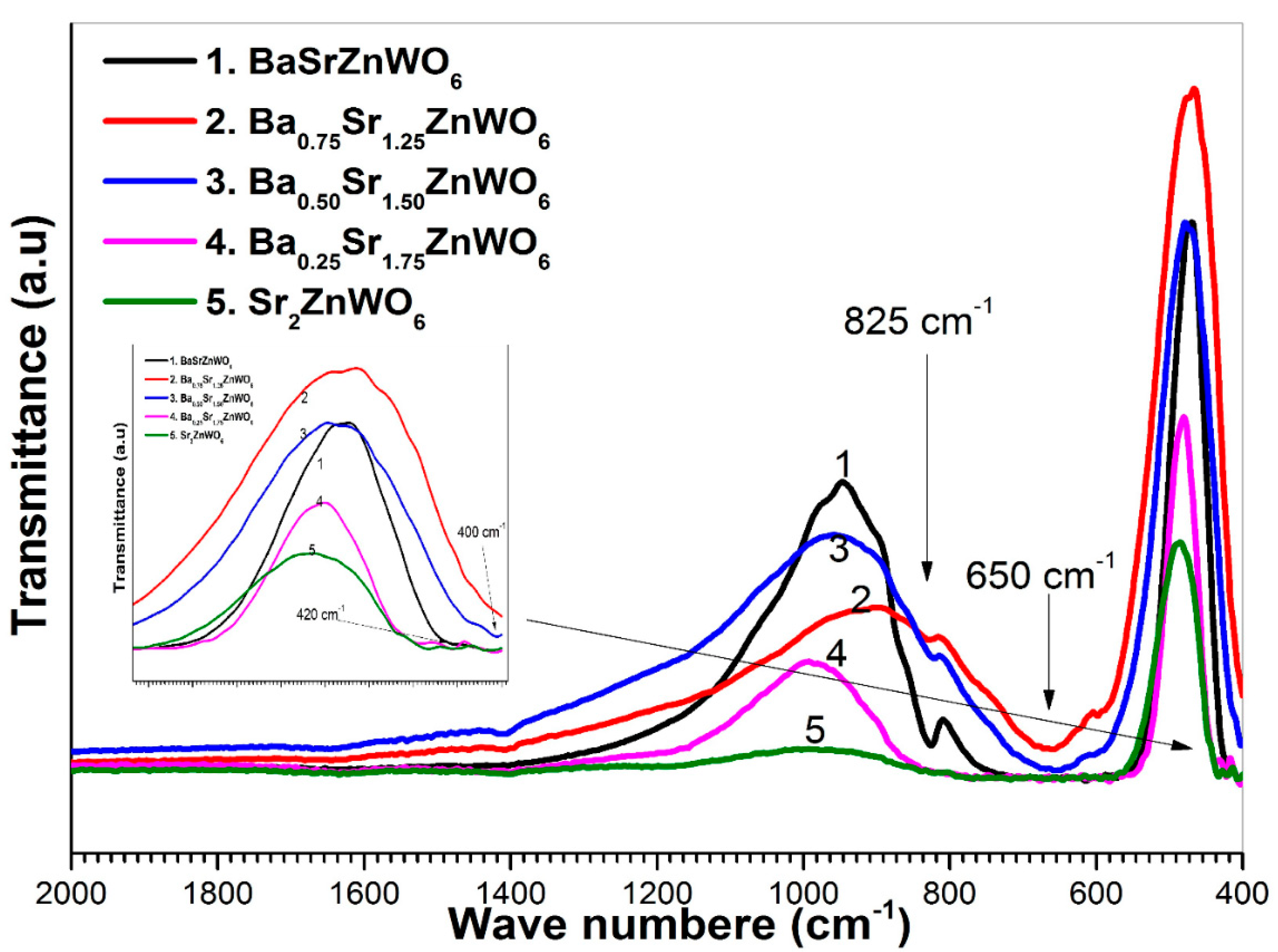
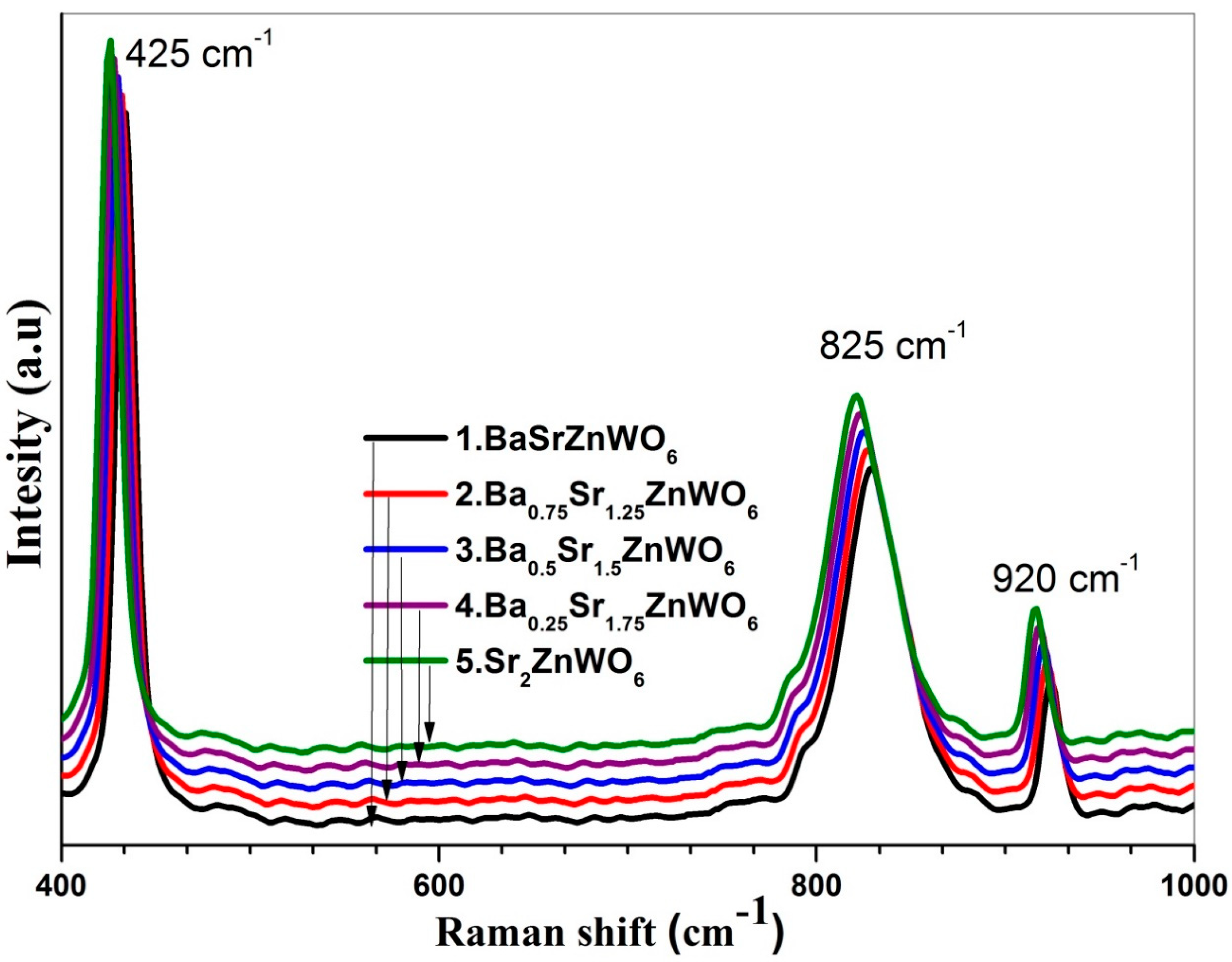
3.3. FTIR and RAMAN Spectroscopies
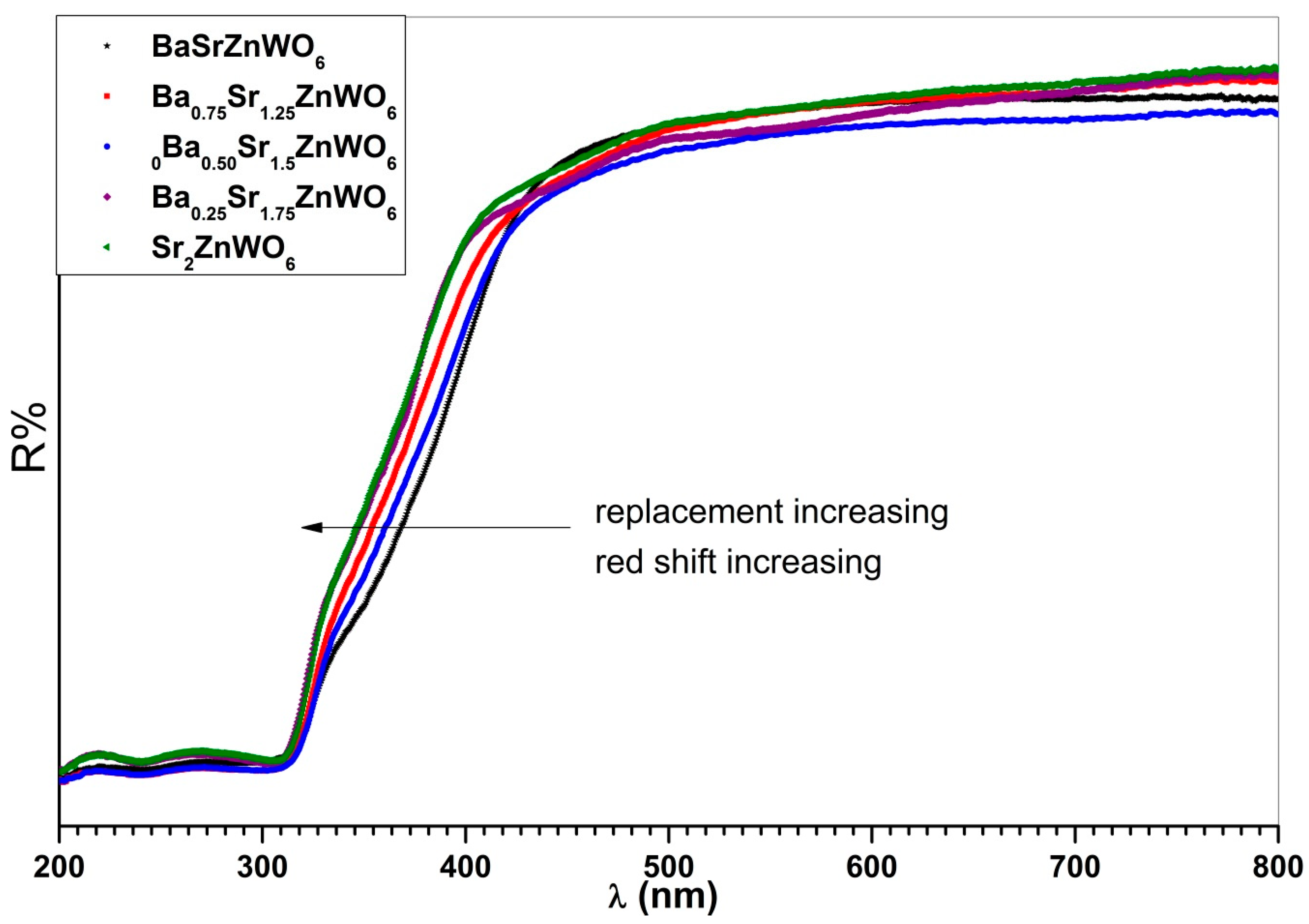
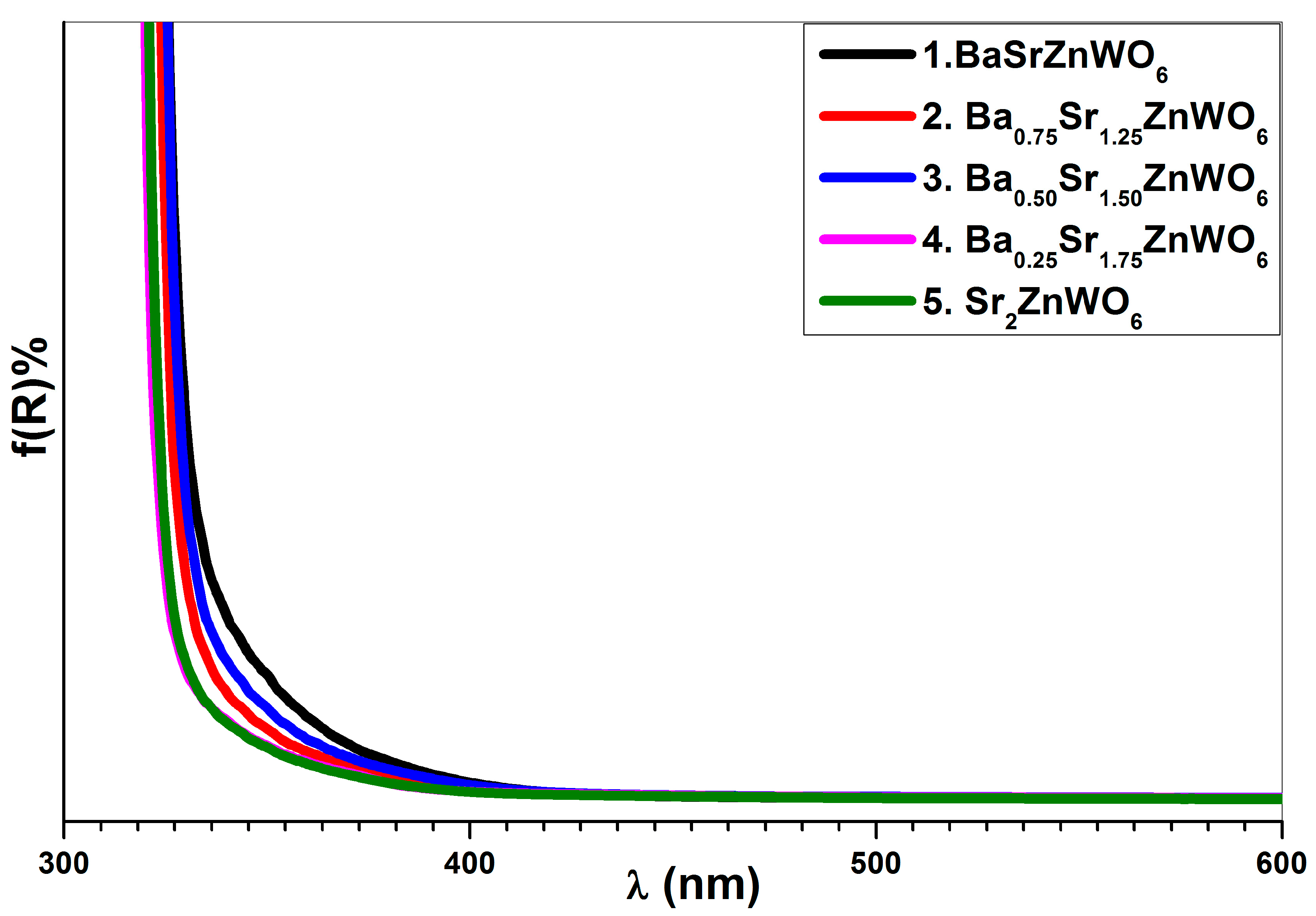
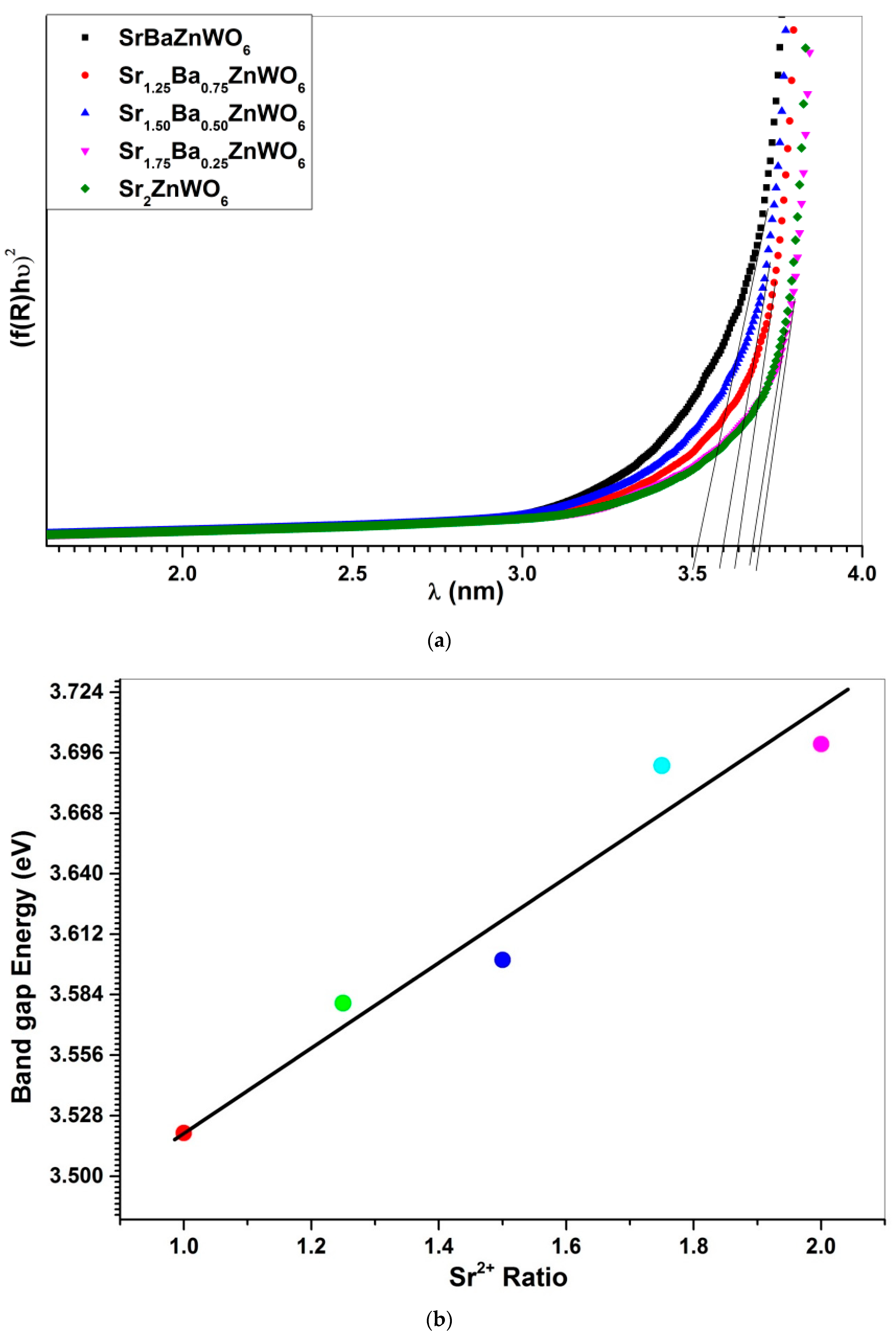
3.4. UV–Visible Diffuse Reflectance Spectroscopy
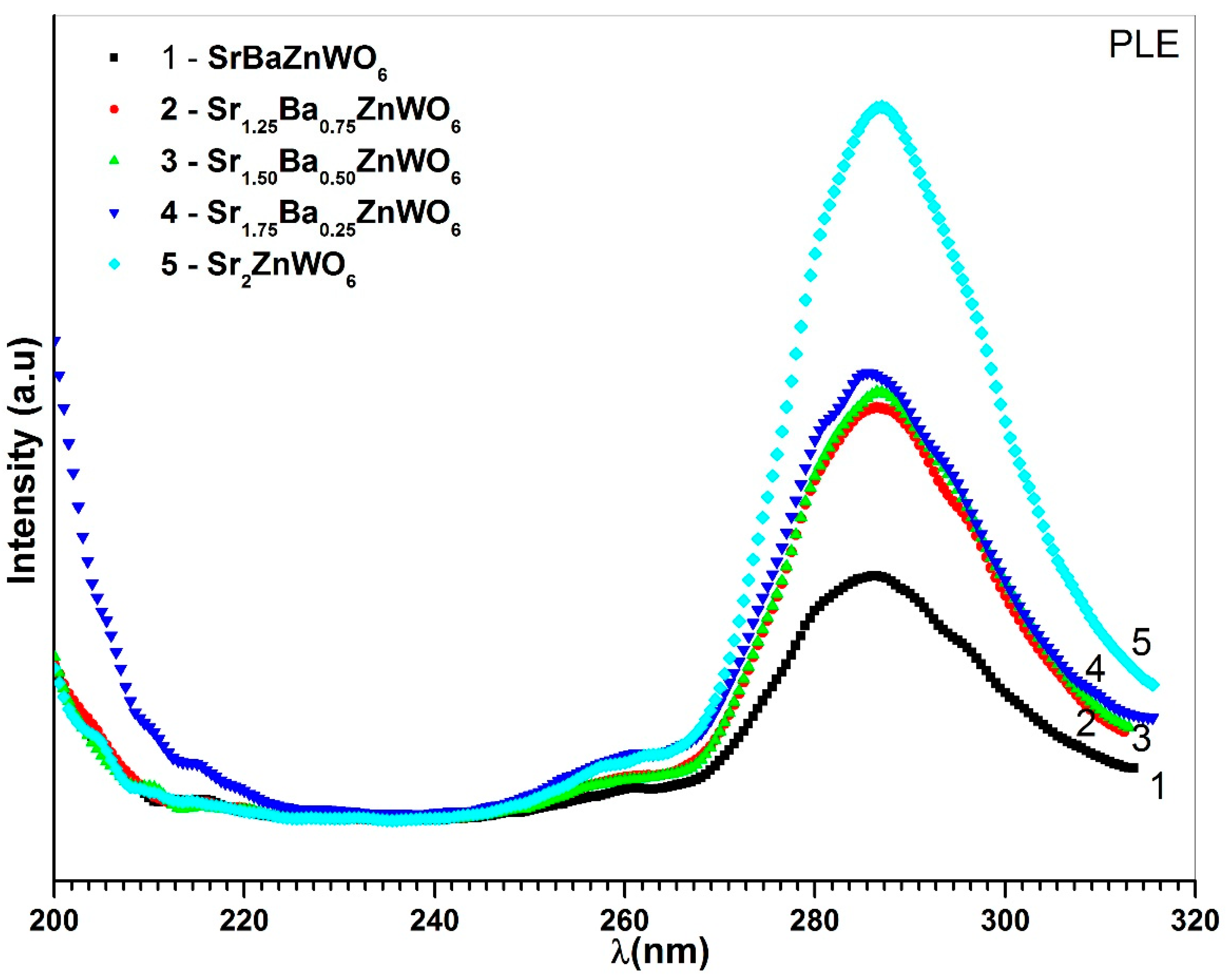
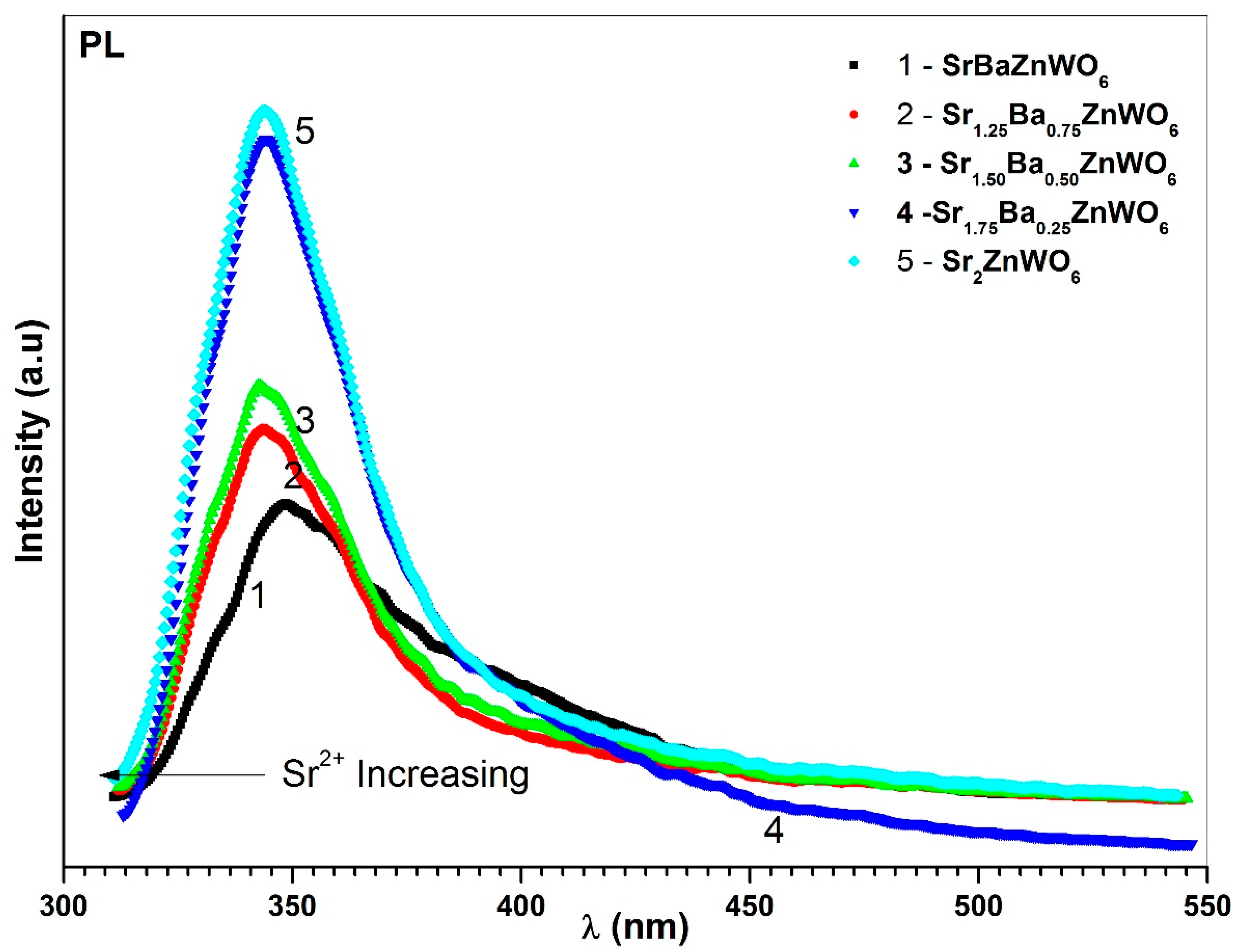
3.5. Photoluminescence Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- King, G.; Woodward, P. Cation ordering in perovskites. J. Mater. Chem. 2010, 20, 5785–5796. [Google Scholar] [CrossRef]
- Knapp, M.C.; Woodward, P. A-site cation ordering in AA′BB′O6 perovskites. J. Solid State Chem. 2006, 179, 1076–1085. [Google Scholar] [CrossRef]
- Zelewski, S.; Urban, J.M.; Surrente, A.; Maude, D.K.; Kuc, A.B.; Schade, L.; Johnson, R.D.; Dollmann, M.; Nayak, P.K.; Snaith, H.J.; et al. Revealing the nature of photoluminescence emission in the metal-halide double perovskite Cs2AgBiBr6. J. Mater. Chem. C 2019, 7, 8350–8356. [Google Scholar] [CrossRef]
- King, G.; Thimmaiah, S.; Dwivedi, A.; Woodward, P. Synthesis and characterization of new AA′BWO6 perovskites exhibiting simultaneous ordering of A-Site and B-Site cations. Chem. Mater. 2007, 19, 6451–6458. [Google Scholar] [CrossRef]
- Xia, Z.; Sun, J.; Du, H.; Chen, D.; Sun, J. Luminescence properties of double-perovskite Sr2Ca1−2xEuxNaxMoO6 red-emitting phosphors prepared by the citric acid-assisted sol–gel method. J. Mater. Sci. 2010, 45, 1553–1559. [Google Scholar] [CrossRef]
- Alsabah, Y.A.; AlSalhi, M.S.; Elbadawi, A.A.; Mustafa, E.M. Influence of Zn2+ and Ni2+ cations on the structural and optical properties of Ba2Zn1−xNixWO6 (0 ≤ x ≤ 1) tungsten double perovskites. J. Alloy. Compd. 2017, 701, 797–805. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.H.; Kanatzidis, M.G. Lead-free solid-state organic-inorganic halide perovskite solar cells. Nat. Photon. 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Qin, P.; Tanaka, S.; Ito, S.; Tetreault, N.; Manabe, K.; Nishino, H.; Nazeeruddin, M.K.; Grätzel, M. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 2014, 5, 3834. [Google Scholar] [CrossRef]
- Schaak, R.E.; Mallouk, T.E. Prying apart Ruddlesden− Popper phases: Exfoliation into sheets and nanotubes for assembly of perovskite thin films. Chem. Mater. 2000, 12, 3427–3434. [Google Scholar] [CrossRef]
- Uma, S.; Raju, A.R.; Gopalakrishnan, J. Bridging the Ruddlesden–Popper and the Dion–Jacobson series of layered perovskites: Synthesis of layered oxides, A 2–xLa2Ti 3–xNbxO10 (A = K, Rb), exhibiting ion exchange. J. Mater. Chem. 1993, 3, 709–713. [Google Scholar] [CrossRef]
- Daniels, L.M. Structures and Properties of Perovskites and Pyrochlores from Hydrothermal Synthesis; University of Warwick: Coventry, UK, 2015. [Google Scholar]
- Depianti, J.B.; Orlando, M.; Cavichini, A.; Corrêa, H.P.S.; Rodrigues, V.A.; Passamai, J.L.; O Piedade, E.L.; Belich, H.; Medeiros, E.F.; De Melo, F.C.L. Structural and magnetic investigation of Ca2MnReO6 doped with Ce. Cerâmica 2013, 59, 262–268. [Google Scholar] [CrossRef]
- Serrate, D.; De Teresa, J.M.; Ibarra, M.R. Double perovskites with ferromagnetism above room temperature. J. Phys. Condens. Matter 2006, 19, 023201. [Google Scholar] [CrossRef]
- Alsabah, Y.A.; Elbadawi, A.A.; Mustafa, E.M.; Siddig, M.A. The effect of replacement of Zn2+ cation with Ni2+ cation on the structural properties of Ba2Zn1–xNixWO6 double perovskite oxides (X= 0, 0.25, 0.50, 0.75, 1). J. Mater. Sci. Chem. Eng. 2016, 4, 61. [Google Scholar]
- Bugaris, D.E.; Hodges, J.; Huq, A.; Loye, H.-C.Z. Crystal growth, structures, and optical properties of the cubic double perovskites Ba2MgWO6 and Ba2ZnWO6. J. Solid State Chem. 2011, 184, 2293–2298. [Google Scholar] [CrossRef]
- Manoun, B.; Igartua, J.M.; Lazor, P. High temperature Raman spectroscopy studies of the phase transitions in Sr2NiWO6 and Sr2MgWO6 double perovskite oxides. J. Mol. Struct. 2010, 971, 18–22. [Google Scholar] [CrossRef]
- Colis, S.; Pourroy, G.; Panissod, P.; Mény, C.; Dinia, A. Correlation between magnetic properties and nuclear magnetic resonance observations in Sr2FeMoO6 double perovskite. J. Magn. Magn. Mater. 2004, 272–276, 2018–2020. [Google Scholar] [CrossRef]
- Jose, R.; John, A.M.; James, J.; Nair, K.; Kurian, K.; Koshy, J. Superconducting Bi(2223) films (TC(0) = 110 K) by dip-coating on Ba2LaZrO5.5: A newly developed perovskite ceramic substrate. Mater. Lett. 1999, 41, 112–116. [Google Scholar] [CrossRef]
- Zhu, H.; Fang, M.; Huang, Z.; Min, X.; Chen, K.; Guan, M.; Tang, C.; Zhang, L.; Wang, M. Novel chromium doped perovskites A2ZnTiO6 (A = Pr, Gd): Synthesis, crystal structure and photocatalytic activity under simulated solar light irradiation. Appl. Surf. Sci. 2017, 393, 348–356. [Google Scholar] [CrossRef]
- Gandhi, A.; Keshri, S. Microwave dielectric properties of double perovskite ceramics. Ceram. Int. 2015, 41, 3693–3700. [Google Scholar] [CrossRef]
- Lan, C.; Zhao, S.; Xu, T.; Ma, J.; Hayase, S.; Ma, T. Investigation on structures, band gaps, and electronic structures of lead free La2NiMnO6 double perovskite materials for potential application of solar cell. J. Alloy. Compd. 2016, 655, 208–214. [Google Scholar] [CrossRef]
- Gateshki, M.; Igartua, J.M. Crystal structures and phase transitions of the double-perovskite oxides Sr2CaWO6 and Sr2 MgWO6. J. Phys. Condens. Matter 2004, 16, 6639. [Google Scholar] [CrossRef]
- Khalyavin, D.D.; Han, J.; Senos, A.M.; Mantas, P.Q. Synthesis and dielectric properties of tungsten-based complex perovskites. J. Mater. Res. 2003, 18, 2600–2607. [Google Scholar] [CrossRef]
- Reddy, M.P.; Zhou, X.; Jing, L.; Huang, Q. Microwave sintering, characterization and magnetic properties of double perovskite La2CoMnO6 nanoparticles. Mater. Lett. 2014, 132, 55–58. [Google Scholar] [CrossRef]
- Faik, A.; Igartua, J.M.; Pizarro, J.L. Synthesis, structures and temperature-induced phase transitions of the Sr2Cd1−xCaxWO6 (0 ≤ x ≤ 1) double perovskite tungsten oxides. J. Mol. Struct. 2009, 920, 196–201. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Zhang, X.X.; Wang, X.C. Antibacterial and absorbent acrylonitrile-vinylidene chloride copolymer fibres. J. Text. Inst. 2010, 101, 128–134. [Google Scholar] [CrossRef]
- Grothe, H.; Tizek, H.; Waller, D.; Stokes, D.J. The crystallization kinetics and morphology of nitric acid trihydrate. Phys. Chem. Chem. Phys. 2006, 8, 2232–2239. [Google Scholar] [CrossRef]
- Kavitha, V.T.; Jose, R.; Ramakrishna, S.; Wariar, P.R.S.; Koshy, J. Combustion synthesis and characterization of Ba2NdSbO6 nanocrystals. Bull. Mater. Sci. 2011, 34, 661–665. [Google Scholar] [CrossRef]
- Wei, H.-J.; Xing, D.; Wu, G.-Y.; Jin, Y.; Gu, H.-M. Optical properties of human normal small intestine tissue determined by Kubelka-Munk method in vitro. World J. Gastroenterol. 2003, 9, 2068–2072. [Google Scholar] [CrossRef]
- Elbadawi, A.A.; Yassin, O.; Siddig, M.A. Effect of the cation size disorder at the A-Site on the structural properties of SrAFeTiO6 double perovskites (A = La, Pr or Nd). J. Mater. Sci. Chem. Eng. 2015, 3, 21–29. [Google Scholar]
- Manoun, B.; Ezzahi, A.; Benmokhtar, S.; Ider, A.; Lazor, P.; Bih, L. X-ray diffraction and Raman spectroscopy studies of temperature and composition induced phase transitions in Ba2−xSrxZnWO6 (0 ≤ x ≤ 2) double perovskite oxides. J. Alloy. Compd. 2012, 533, 43–52. [Google Scholar] [CrossRef]
- Zhou, Q.; Kennedy, B.J.; Elcombe, M.M. Composition and temperature dependent phase transitions in Co–W double perovskites, a synchrotron X-ray and neutron powder diffraction study. J. Solid State Chem. 2007, 180, 541–548. [Google Scholar] [CrossRef]
- Mostafa, M.; Ata-Allah, S.; Youssef, A.; Refai, H. Electric and AC magnetic investigation of the manganites La0.7Ca0.3Mn0.96In0.04xAl(1−x)0.04O3; (0.0 ≤ x ≤ 1.0). J. Magn. Magn. Mater. 2008, 320, 344–353. [Google Scholar] [CrossRef]
- Elbadawi, A.; Yassin, O.; Gismelseed, A.A. Effect of the internal pressure and the anti-site disorder on the structure and magnetic properties of ALaFeTiO6 (A = Ca, Sr, Ba) double perovskite oxides. J. Magn. Magn. Mater. 2013, 326, 1–6. [Google Scholar] [CrossRef]
- Rodrigues, J.; Bezerra, D.; Hernandes, A. Calculation of the optical phonons in ordered Ba2MgWO6 perovskite using short-range force field model. J. Raman Spectrosc. 2018, 49, 1822–1829. [Google Scholar] [CrossRef]
- Diao, C.-L. First-principle calculation and assignment for vibrational spectra of Ba (Mg 1/2 W 1/2) O 3 microwave dielectric ceramic. J. Am. Ceram. Soc. 2013, 96, 2898–2905. [Google Scholar] [CrossRef]
- Xiao, N.; Shen, J.; Xiao, T.; Wu, B.; Luo, X.; Li, L.; Wang, Z.; Zhou, X.-J. Sr2CaWxMo1−xO6:Eu3+, Li+: An emission tunable phosphor through site symmetry and excitation wavelength. Mater. Res. Bull. 2015, 70, 684–690. [Google Scholar] [CrossRef]
- Xu, D.; Yang, Z.; Sun, J.; Gao, X.; Du, J. Synthesis and luminescence properties of double-perovskite white emitting phosphor Ca3WO6:Dy3+. J. Mater. Sci. Mater. Electron. 2016, 27, 8370–8377. [Google Scholar] [CrossRef]
- Chen, F.; Zeng, G.; Yang, Q.; Li, X.; Zeng, G. Facile synthesis of visible-light-active BiOI modified Bi2MoO6 photocatalysts with highly enhanced photocatalytic activity. Ceram. Int. 2016, 42, 2515–2525. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to solid state physics. Am. J. Phys. 1967, 35, 547–548. [Google Scholar] [CrossRef]
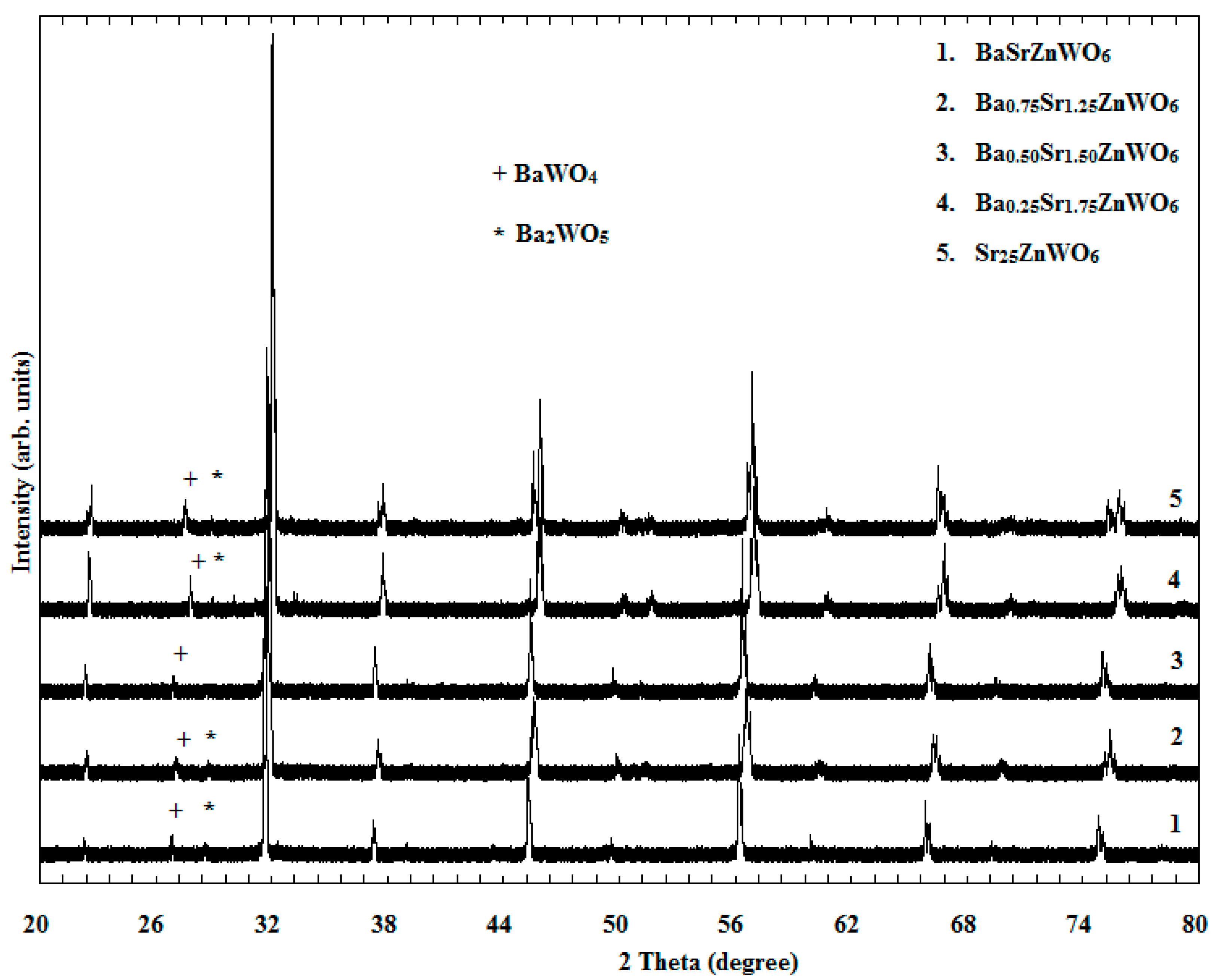
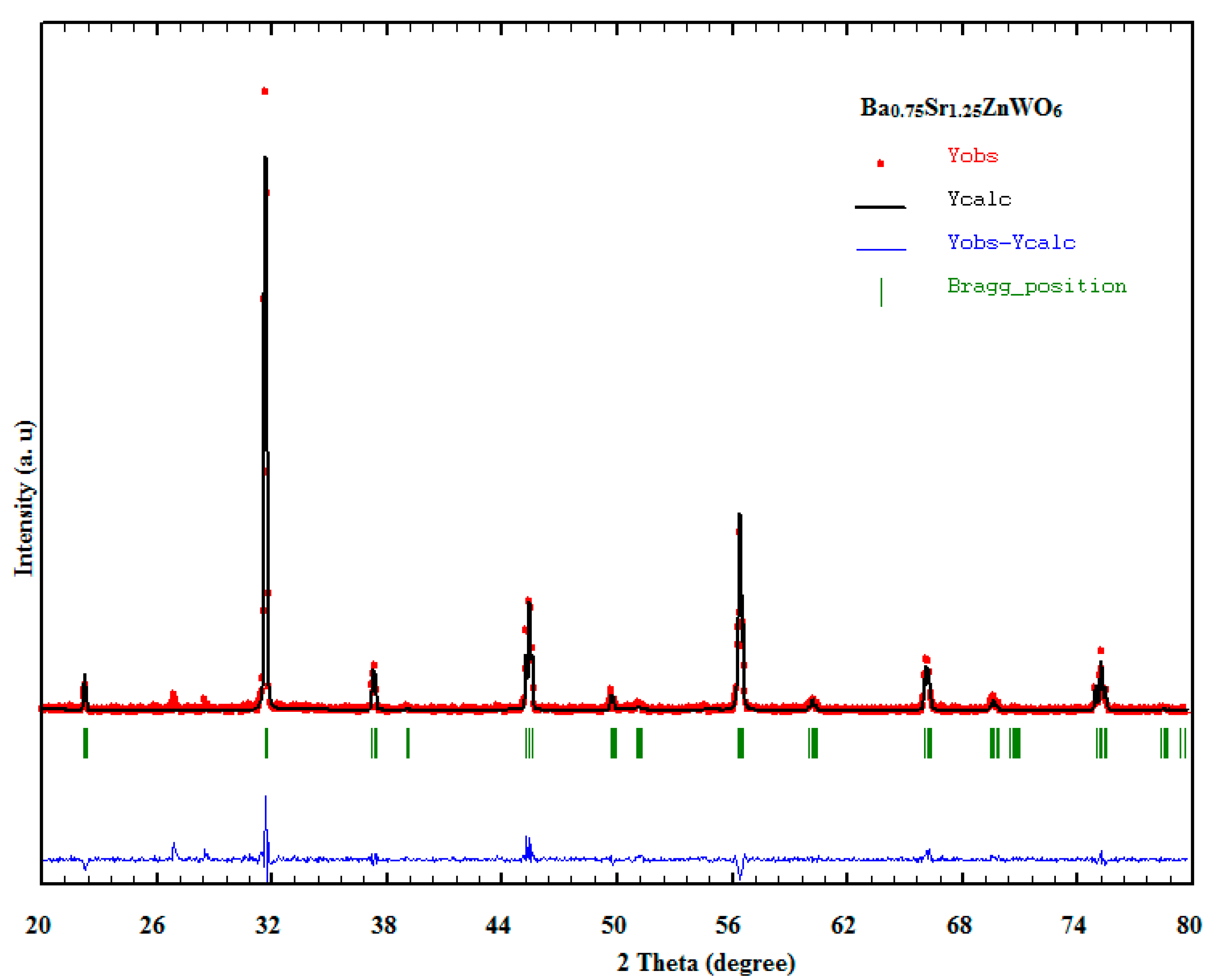

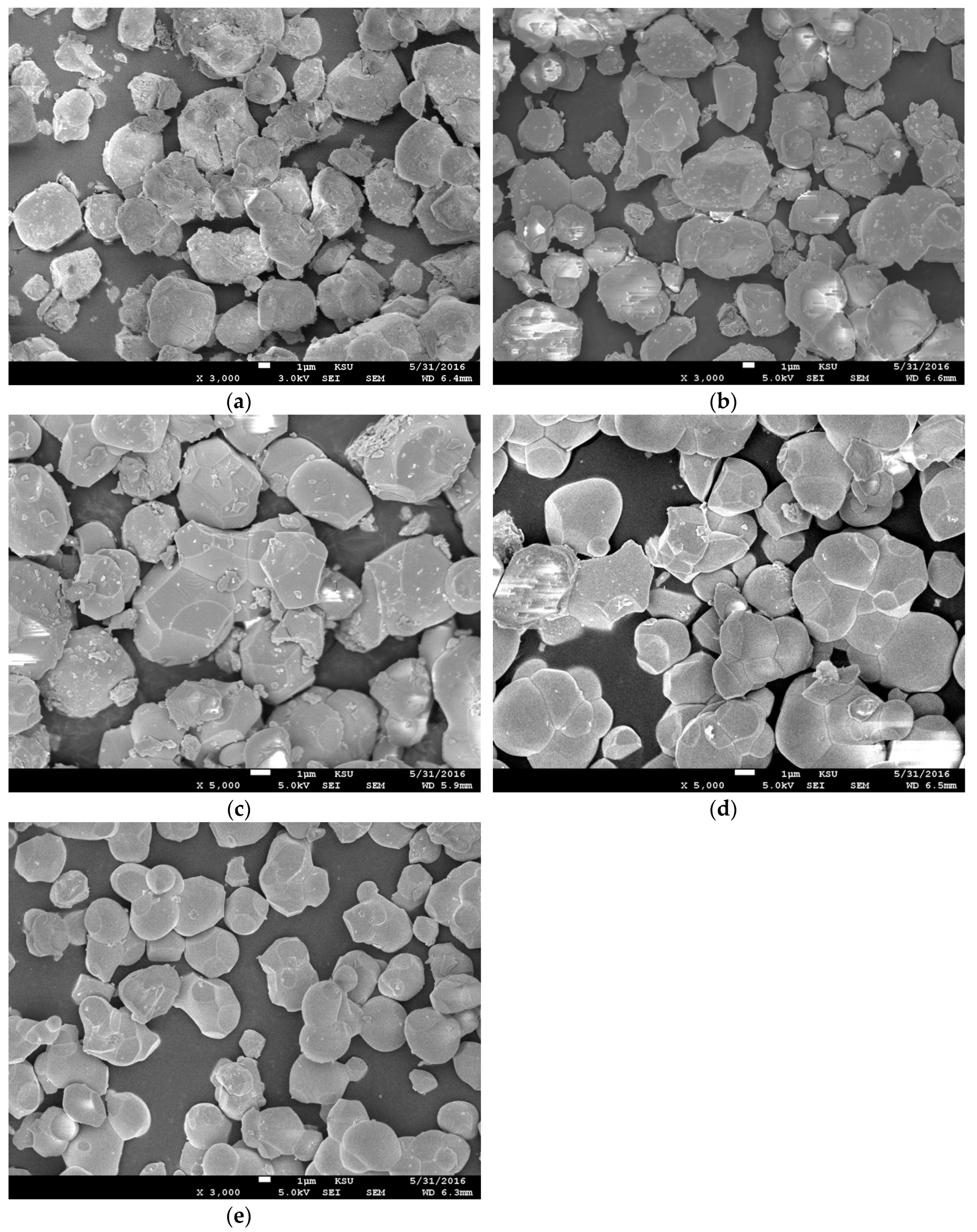
| Composition | BaSrZnWO6 | Ba0.75Sr1.25ZnWO6 | Ba0.50Sr1.50ZnWO6 | Ba0.25Sr1.75ZnWO6 | Sr2ZnWO6 | |
|---|---|---|---|---|---|---|
| Program | Fullprof | Fullprof | Fullprof | Fullprof | Fullprof | |
| λKα1 (Å) | 1.5406 Å | 1.5406 Å | 1.5406 Å | 1.5406 Å | 1.5406 Å | |
| Atom number | 6 | 6 | 6 | 6 | 6 | |
| 2θ Range (°) | 20–80 | 20–80 | 20–80 | 20–80 | 20–80 | |
| Step scan | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | |
| Zero point (° 2θ) | 0.205620 | 0.196440 | 0.181520 | 0.198830 | 0.296540 | |
| Caglioti parametes | U V W | 0.016(9) | 0.330(9) | 0.006(9) | 0.199(3) | 0.096(1) |
| 0.005(7) | -0.235(8) | 0.003(8) | −0.089(3) | −0.079(2) | ||
| 0.010(1) | 0.077(1) | 0.003(1) | 0.002(1) | 0.017(1) | ||
| 1.44 | 1.65 | 1.45 | 1.55 | 1.61 | ||
| RB | 7.52027 | 8.44375 | 5.08192 | 7.62028 | 8.54376 | |
| RF | 9.40813 | 8.62935 | 6.60579 | 9.50814 | 8.72936 | |
| Crystal Structure | Cubic | Tetragonal | Tetragonal | Tetragonal | Monoclinic | |
| Space group | Fm-3m | I 4/m | I 4/m | I 4/m | P21/n | |
| a (Å) | 8.039(1) | 5.642(2) | 5.667(1) | 5.610(1) | 5.639(1) | |
| b (Å) | 8.039(1) | 5.642(2) | 5.667(1) | 5.610(1) | 5.636(1) | |
| C (Å) | 8.039(1) | 8.011(3) | 8.020(2) | 7.951(1) | 7.944(1) | |
| α (Å) | 90 | 90 | 90 | 90 | 90 | |
| β (Å) | 90 | 90 | 90 | 90 | 90.315 | |
| γ (Å) | 90 | 90 | 90 | 90 | 90 | |
| V (Å3) | 519.600(8) | 255.077(8) | 257.580(7) | 250.262(6) | 252.557(4) | |
| Tolerance factor (T) | 1.002 | 0.993 | 0.983 | 0.974 | 0.964 | |
| Crystalline size (D) (nm3) | 84.59 | 59.53 | 61.64 | 71.38 | 57.56 | |
| Atom | X | Y | Z | B (Å2) | Occ |
|---|---|---|---|---|---|
| BaSrZnWO6 | |||||
| Ba2+ | 0.250(3) | 0.250(3) | 0.250(6) | 0.691(2) | 0.250 |
| Sr2+ | 0.250(3) | 0.250(3) | 0.250(4) | 0.691(3) | 0.250 |
| Zn2+ | 0.500(8) | 0.500(4) | 0.500(3) | 1.055(4) | 0.250 |
| W6+ | 0.000 | 0.000 | 0.000 | 0.365(3) | 0.250 |
| O12− | 0.000 | 0.000 | 0.248(2) | 1.400(2) | 1.000 |
| O22− | 0.000 | 0.000 | 0.248(9) | 0.600(6) | 0.500 |
| Ba0.75Sr1.25ZnWO6 | |||||
| Ba12+ | 0.000 | 0.500(3) | 0.250(2) | 1.857(3) | 0.188 |
| Ba22+ | 0.000 | 0.500(4) | 0.250(3) | 1.857(5) | 0.313 |
| Zn2+ | 0.000 | 0.000 | 0.000 | 0.992(4) | 0.250 |
| W6+ | 0.000 | 0.000 | 0.500(3) | 0.187(3) | 0.250 |
| O12− | 0.319(4) | 0.227(3) | 0.000 | 1.770(4) | 1.000 |
| O22− | 0.000 | 0.000 | 0.258(2) | 1.770(4) | 0.500 |
| Ba0.50Sr1.50ZnWO6 | |||||
| Ba2+ | 0.000 | 0.500(2) | 0.250(3) | 0.600(4) | 0.125 |
| Sr2+ | 0.000 | 0.500(2) | 0.250(2) | 0.600(4) | 0.375 |
| Zn2+ | 0.000 | 0.000 | 0.000 | 1.900(4) | 0.250 |
| W6+ | 0.000 | 0.000 | 0.500(3) | 0.941(3) | 0.250 |
| O12− | 0.332(7) | 0.213(3) | 0.000 | 0.800(3) | 1.000 |
| O22− | 0.000 | 0.000 | 0.251(5) | 0.800(7) | 0.500 |
| Ba0.25Sr1.75ZnWO6 | |||||
| Ba2+ | 0.000 | 0.500(2) | 0.250(3) | 1.868(4) | 0.063 |
| Sr2+ | 0.000 | 0.500(5) | 0.250(4) | 1.868(7) | 0.438 |
| Zn2+ | 0.000 | 0.000 | 0.000 | 0.161(4) | 0.250 |
| W6+ | 0.000 | 0.000 | 0.500(3) | 0.140(6) | 0.250 |
| O12− | 0.330(2) | 0.202(2) | 0.000 | 1.666(3) | 1.000 |
| O22− | 0.000 | 0.000 | 0.363(2) | 1.298(3) | 0.500 |
| Sr2ZnWO6 | |||||
| Sr22+ | 0.008(4) | 0.515(2) | 0.250(7) | 0.577(2) | 0.500 |
| Zn2+ | 0.000 | 0.000 | 0.000 | 0.402(3) | 0.250 |
| W6+ | 0.500(1) | 0.500(4) | 0.000 | 0.402(3) | 0.250 |
| O12− | 0.267(4) | 0.018(3) | 0.003(3) | 1.000(4) | 0.500 |
| O22− | 0.250(4) | −0.006(3) | −0.009(4) | 1.000(4) | 0.500 |
| O22− | −0.051(3) | 0.250(2) | 0.117(4) | 1.000(6) | 0.500 |
| Elements | X = 1.00 | X = 1.25 | X = 1.50 | X = 1.75 | X = 2.00 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | |
| C K | 9.29 | 32.75 | 7.17 | 27.17 | 9.32 | 33.71 | 10.82 | 34.77 | 43.32 | 73.24 |
| O K | 14.58 | 38.61 | 13.96 | 39.74 | 12.93 | 35.12 | 15.59 | 37.60 | 13.24 | 16.80 |
| Zn K | 11.26 | 7.30 | 13.43 | 9.36 | 15.03 | 9.99 | 14.05 | 8.30 | 18.86 | 5.86 |
| Sr L | 16.15 | 7.81 | 23.78 | 12.36 | 18.99 | 9.42 | 29.54 | 13.01 | 10.79 | 2.50 |
| Ba L | 2.88 | 1.34 | 12.55 | 4.16 | 17.61 | 5.57 | 0.37 | 0.10 | 2.09 | 0.31 |
| W M | 21.04 | 6.49 | 29.11 | 7.21 | 26.13 | 6.18 | 29.62 | 6.22 | 11.70 | 1.29 |
| Totals | 100.0 | 100.00 | 100.00 | 100.00 | 100.00 | |||||
| X Ratio | X = 1 | X = 1.25 | X = 1.50 | X = 1.75 | X = 2 |
|---|---|---|---|---|---|
| Cut-off wavelength (nm) | 388 | 345 | 343 | 337 | 326 |
| Eg (eV) by cutoff wavelength | 3.44 | 3.59 | 3.61 | 3.67 | 3.8 |
| Eg (eV) by Tauc plot | 3.50 | 3.58 | 3.60 | 3.69 | 3.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsabah, Y.A.; AlSalhi, M.S.; Mustafa, E.M.; Elbadawi, A.A.; Devanesan, S.; Siddig, M.A. Synthesis, Phase Transition, and Optical Studies of Ba2−xSrxZnWO6 (x = 1.00, 1.25, 1.50, 1.75, 2.00) Tungsten Double Perovskite Oxides. Crystals 2020, 10, 299. https://doi.org/10.3390/cryst10040299
Alsabah YA, AlSalhi MS, Mustafa EM, Elbadawi AA, Devanesan S, Siddig MA. Synthesis, Phase Transition, and Optical Studies of Ba2−xSrxZnWO6 (x = 1.00, 1.25, 1.50, 1.75, 2.00) Tungsten Double Perovskite Oxides. Crystals. 2020; 10(4):299. https://doi.org/10.3390/cryst10040299
Chicago/Turabian StyleAlsabah, Yousef A., Mohamad S. AlSalhi, Eltayeb M. Mustafa, Abdelrahman A. Elbadawi, Sandhanasamy Devanesan, and Mohamed A. Siddig. 2020. "Synthesis, Phase Transition, and Optical Studies of Ba2−xSrxZnWO6 (x = 1.00, 1.25, 1.50, 1.75, 2.00) Tungsten Double Perovskite Oxides" Crystals 10, no. 4: 299. https://doi.org/10.3390/cryst10040299
APA StyleAlsabah, Y. A., AlSalhi, M. S., Mustafa, E. M., Elbadawi, A. A., Devanesan, S., & Siddig, M. A. (2020). Synthesis, Phase Transition, and Optical Studies of Ba2−xSrxZnWO6 (x = 1.00, 1.25, 1.50, 1.75, 2.00) Tungsten Double Perovskite Oxides. Crystals, 10(4), 299. https://doi.org/10.3390/cryst10040299








