Synthesis, Characterization and Catalytic Evaluation of Chromium Oxide Deposited on Titania–Silica Mesoporous Nanocomposite for the Ethane Dehydrogenation with CO2
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Catalyst Evaluation
3. Results and Discussion
3.1. Catalysts Characterization
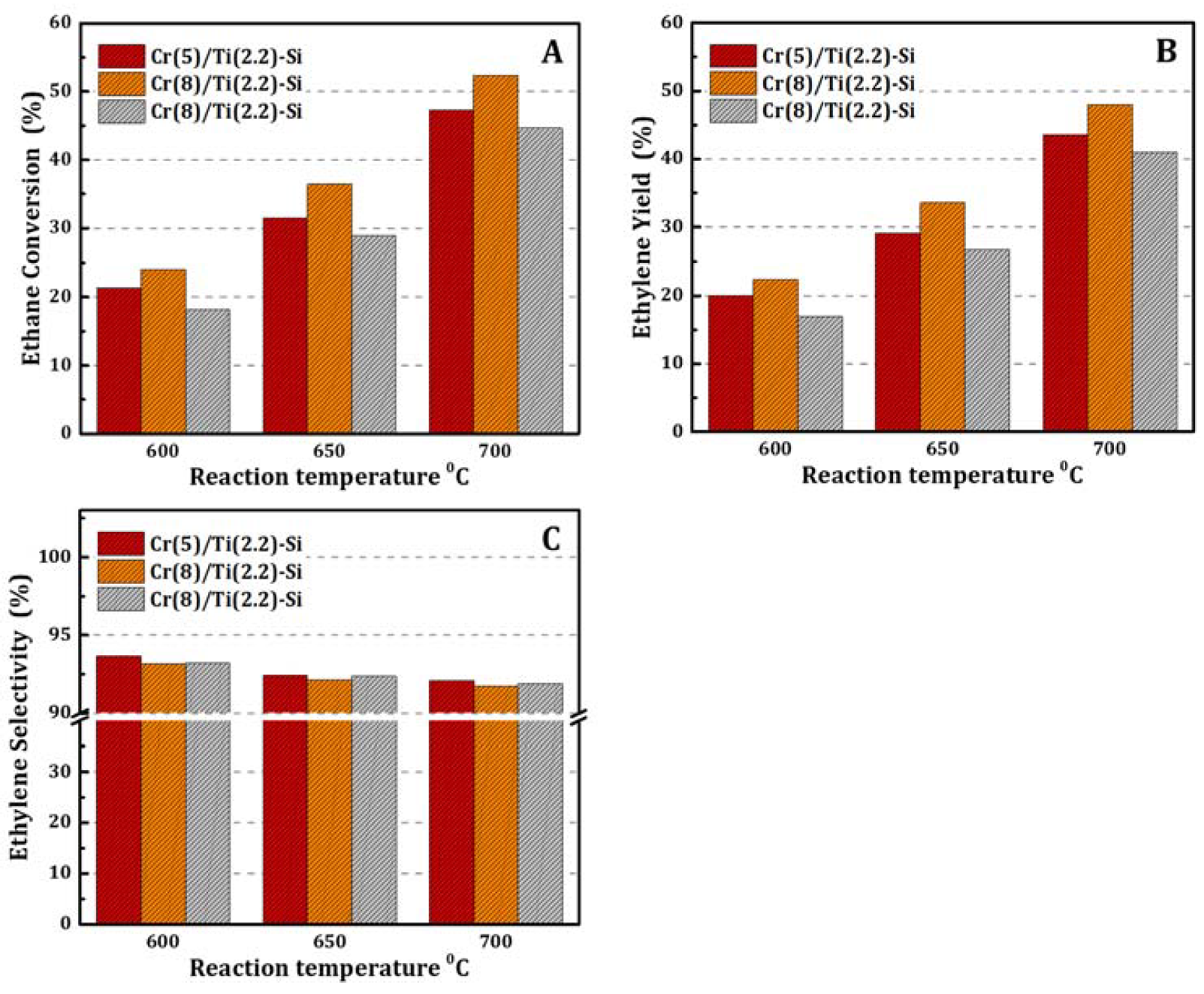
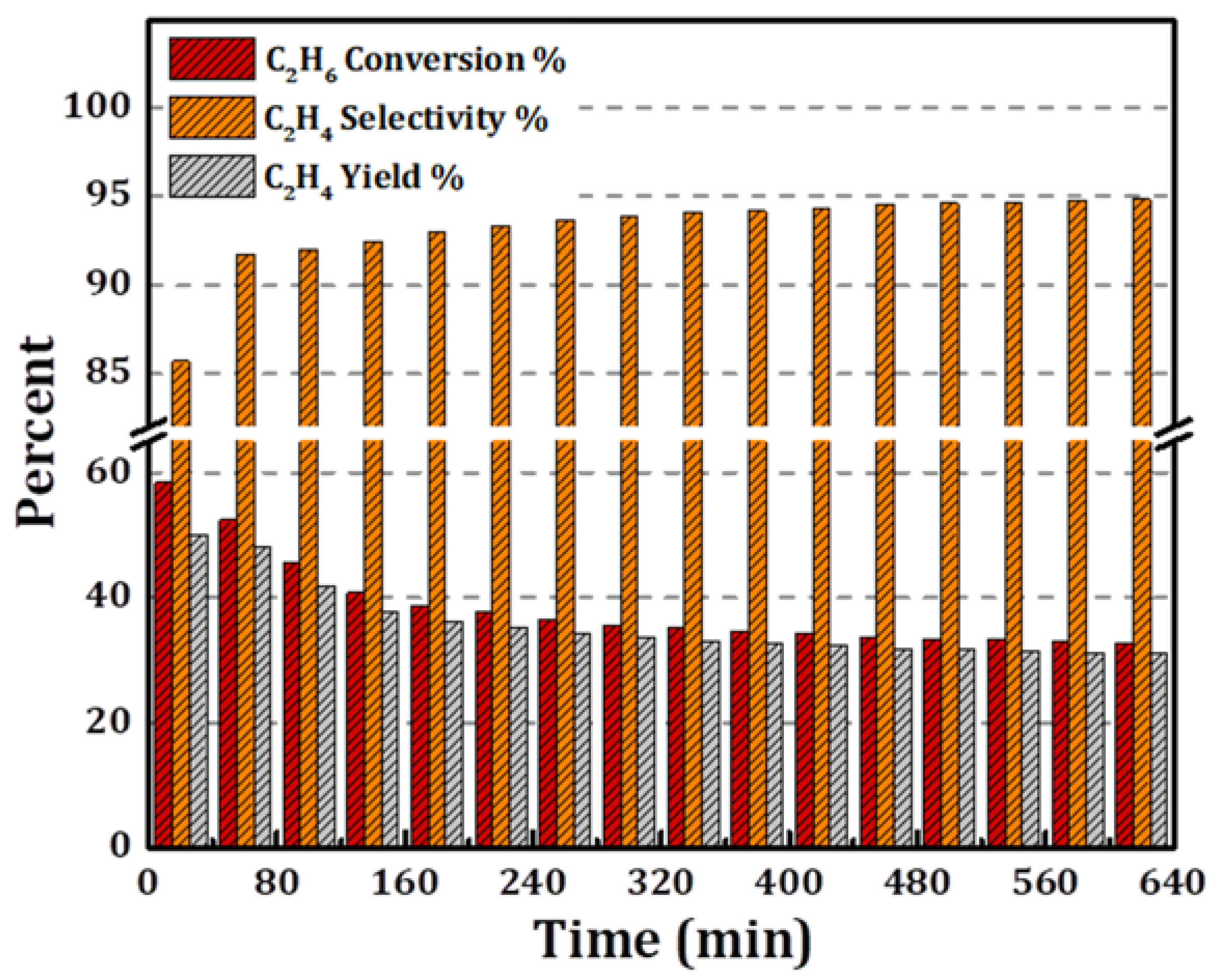
3.2. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gärtner, C.A.; van Veen, A.C.; Lercher, J.A. Oxidative Dehydrogenation of Ethane: Common Principles and Mechanistic Aspects. ChemCatChem 2013, 5, 3196–3217. [Google Scholar] [CrossRef]
- Koirala, R.; Buechel, R.; Pratsinis, S.E.; Baiker, A. Silica is preferred over various single and mixed oxides as support for CO2-assisted cobalt-catalyzed oxidative dehydrogenation of ethane. Appl. Catal. A 2016, 527, 96–108. [Google Scholar] [CrossRef]
- Bhasin, M.M.; McCain, J.H.; Vora, B.V.; Imai, T.; Pujadó, P.R. Dehydrogenation and oxydehydrogenation of paraffins to olefins. Appl. Catal. A 2001, 221, 397–419. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef]
- Zimmermann, R.W.H. Ethylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2009; Volume 13, pp. 465–529. [Google Scholar]
- Dharia, D.; Letzsch, W.; Kim, H.; McCue, D.; Chapin, L. Increase light olefins production: New methods based on proven FCC technology provide additional olefins source. Hydrocarb. Process 2004, 83, 61–66. [Google Scholar]
- Grace Chan, K.; Inal, F.; Senkan, S. Suppression of coke formation in the steam cracking of alkanes: Ethane and propane. Ind. Eng. Chem. Res. 1998, 37, 901–907. [Google Scholar] [CrossRef]
- Resasco, D.E.; Haller, G.L. Catalytic dehydrogenation of lower alkanes. In Catalysis; Spivey, J.J., Agarwal, S.K., Eds.; The Royal Society of Chemistry: London, UK, 1994; Volume 11, pp. 379–411. [Google Scholar]
- Bañares, M.A. Supported metal oxide and other catalysts for ethane conversion: A review. Catal. Today 1999, 51, 319–348. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F. The oxidative dehydrogenation of ethane and propane as an alternative way for the production of light olefins. Catal. Today 1995, 24, 307–313. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.H. Catalytic Conversion of Alkanes to Olefins by Carbon Dioxide Oxidative Dehydrogenation: A Review. Energy Fuel 2004, 18, 1126–1139. [Google Scholar] [CrossRef]
- Mimura, N.; Okamoto, M.; Yamashita, H.; Oyama, S.T.; Murata, K. Oxidative Dehydrogenation of Ethane over Cr/ZSM-5 Catalysts Using CO2 as an Oxidant. J. Phys. Chem. B 2006, 110, 21764–21770. [Google Scholar] [CrossRef]
- Wang, S.; Murata, K.; Hayakawa, T.; Hamakawa, S.; Suzuki, K. Dehydrogenation of ethane with carbon dioxide over supported chromium oxide catalysts. Appl. Catal. A 2000, 196, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Wu, S.; Zhang, K.; Zhou, G.; Liu, J.; Zhen, K.; Wu, T.; Cheng, T. Cr-MCM-41 molecular sieves crystallized at room temperature for reaction of ethane with CO2. J. Nat. Gas Chem. 2005, 14, 207–212. [Google Scholar]
- Al-Awadi, A.S.; El-Toni, A.M.; Alhoshan, M.; Khan, A.; Shar, M.A.; Abasaeed, A.E.; Al-Zahrani, S.M. Synergetic Impact of Secondary Metal Oxides of Cr-M/MCM41 Catalyst Nanoparticles for Ethane Oxidative Dehydrogenation Using Carbon Dioxide. Crystals 2019, 10, 7. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, F.; Zhang, Y.; Miao, C.; Hua, W.; Yue, Y.; Gao, Z. Oxidative dehydrogenation of ethane with CO2 over Cr supported on submicron ZSM-5 zeolite. Chin. J. Catal. 2015, 36, 1242–1248. [Google Scholar] [CrossRef]
- Rahmani, F.; Haghighi, M.; Amini, M. The beneficial utilization of natural zeolite in preparation of Cr/clinoptilolite nanocatalyst used in CO2-oxidative dehydrogenation of ethane to ethylene. J. Ind. Eng. Chem. 2015, 31, 142–155. [Google Scholar] [CrossRef]
- Rahmani, F.; Haghighi, M.; Mahboob, S. CO2-enhanced dehydrogenation of ethane over sonochemically synthesized Cr/clinoptilolite-ZrO2 nanocatalyst: Effects of ultrasound irradiation and ZrO2 loading on catalytic activity and stability. Ultrason. Sonochem. 2016, 33, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Michorczyk, P.; Pietrzyk, P.; Ogonowski, J. Preparation and characterization of SBA-1–supported chromium oxide catalysts for CO2 assisted dehydrogenation of propane. Microporous Mesoporous Mater. 2012, 161, 56–65. [Google Scholar] [CrossRef]
- Ramesh, Y.; Thirumala Bai, P.; Hari Babu, B.; Lingaiah, N.; Rama Rao, K.S.; Prasad, P.S.S. Oxidative dehydrogenation of ethane to ethylene on Cr2O3/Al2O3–ZrO2 catalysts: The influence of oxidizing agent on ethylene selectivity. Appl. Petrochem. Res. 2014, 4, 247–252. [Google Scholar] [CrossRef]
- Deng, S.; Li, H.; Li, S.; Zhang, Y. Activity and characterization of modified Cr2O3/ZrO2 nano-composite catalysts for oxidative dehydrogenation of ethane to ethylene with CO2. J. Mol. Catal. A Chem. 2007, 268, 169–175. [Google Scholar] [CrossRef]
- Asghari, E.; Haghighi, M.; Rahmani, F. CO2 Oxidative Dehydrogenation of Ethane to Ethylene over Cr/MCM-41 Nanocatalyst Synthesized via Hydrothermal/Impregnation Methods: Influence of Chromium Content on Catalytic Properties and Performance. J. Mol. Catal. A Chem. 2016, 418, 115–124. [Google Scholar] [CrossRef]
- Botavina, M.A.; Martra, G.; Agafonov, Y.A.; Gaidai, N.A.; Nekrasov, N.V.; Trushin, D.V.; Coluccia, S.; Lapidus, A.L. Oxidative dehydrogenation of C3–C4 paraffins in the presence of CO2 over CrOx/SiO2 catalysts. Appl. Catal. A 2008, 347, 126–132. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Niemczyk, M. Investigation of catalytic activity of CrSBA-1 materials obtained by direct method in the dehydrogenation of propane with CO2. Appl. Catal. A 2010, 374, 142–149. [Google Scholar] [CrossRef]
- Jóźwiak, W.K.; Ignaczak, W.; Dominiak, D.; Maniecki, T.P. Thermal stability of bulk and silica supported chromium trioxide. Appl. Catal. A 2004, 258, 33–45. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Zhang, Y. Mesoporous silica-supported chromium catalyst: Characterization and excellent performance in dehydrogenation of propane to propylene with carbon dioxide. Catal. Commun. 2007, 8, 565–570. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Kuśtrowski, P.; Chmielarz, L. Chromium oxide supported on MCM-41 as a highly active and selective catalyst for dehydrogenation of propane with CO2. Appl. Catal. A 2008, 349, 62–69. [Google Scholar] [CrossRef]
- Shi, X.; Ji, S.; Wang, K. Oxidative Dehydrogenation of Ethane to Ethylene with Carbon dioxide over Cr–Ce/SBA-15 Catalysts. Catal. Lett. 2008, 125, 331–339. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, Y.; Gao, Z. Chromium Oxide Supported on Mesoporous SBA-15 as Propane Dehydrogenation and Oxidative Dehydrogenation Catalysts. Catal. Lett. 2002, 83, 19–25. [Google Scholar] [CrossRef]
- Wang, Y.; Ohishi, Y.; Shishido, T.; Zhang, Q.; Yang, W.; Guo, Q.; Wan, H.; Takehira, K. Characterizations and catalytic properties of Cr-MCM-41 prepared by direct hydrothermal synthesis and template-ion exchange. J. Catal. 2003, 220, 347–357. [Google Scholar] [CrossRef]
- Wang, Y.; Ohishi, Y.; Shishido, T.; Zhang, Q.; Takehira, K. Cr-MCM-41 for selective dehydrogenation of lower alkanes with carbon dioxide. In Studies in Surface Science and Catalysis; Park, S.-E., Ryoo, R., Ahn, W.-S., Lee, C.W., Chang, J.-S., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 146, pp. 725–728. [Google Scholar]
- Takehira, K.; Ohishi, Y.; Shishido, T.; Kawabata, T.; Takaki, K.; Zhang, Q.; Wang, Y. Behavior of active sites on Cr-MCM-41 catalysts during the dehydrogenation of propane with CO2. J. Catal. 2004, 224, 404–416. [Google Scholar] [CrossRef]
- Al-Awadi, A.S.; El-Toni, A.M.; Alhoshan, M.; Khan, A.; Labis, J.P.; Al-Fatesh, A.; Abasaeed, A.E.; Al-Zahrani, S.M. Impact of precursor sequence of addition for one-pot synthesis of Cr-MCM-41 catalyst nanoparticles to enhance ethane oxidative dehydrogenation with carbon dioxide. Ceram. Int. 2019, 45, 1125–1134. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kajita, C.; Ide, Y.; Okamura, M.; Kato, S.; Kasuya, H.; Ikenaga, N.O.; Kobayashi, T.; Suzuki, T. Promoting effect of carbon dioxide on the dehydrogenation and aromatization of ethane over gallium-loaded catalysts. Catal. Lett. 2000, 64, 215–221. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kajita, C.; Okumura, K.; Ikenaga, N.-O.; Nishitani-Gamo, M.; Ando, T.; Kobayashi, T.; Suzuki, T. Role of Carbon Dioxide in the Dehydrogenation of Ethane over Gallium-Loaded Catalysts. J. Catal. 2001, 203, 87–93. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, J.; Xu, H.; Yue, Y.; Hua, W.; Shen, W. Dehydrogenation of ethane to ethylene over a highly efficient Ga2O3/HZSM-5 catalyst in the presence of CO2. Appl. Catal. A 2009, 356, 148–153. [Google Scholar] [CrossRef]
- Koirala, R.; Buechel, R.; Krumeich, F.; Pratsinis, S.E.; Baiker, A. Oxidative Dehydrogenation of Ethane with CO2 over Flame-Made Ga-Loaded TiO2. ACS Catal. 2015, 5, 690–702. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J. Dehydrogenation of propane to propene over gallium oxide in the presence of CO2. Appl. Catal. A 2003, 251, 425–433. [Google Scholar] [CrossRef]
- Valenzuela, R.X.; Bueno, G.; Cortés Corberán, V.; Xu, Y.; Chen, C. Selective oxidehydrogenation of ethane with CO2 over CeO2-based catalysts. Catal. Today 2000, 61, 43–48. [Google Scholar] [CrossRef]
- Valenzuela, R.X.; Bueno, G.; Solbes, A.; Sapiña, F.; Martínez, E.; Cortés Corberán, V. Nanostructured ceria-based catalysts for oxydehydrogenation of ethane with CO2. Top. Catal. 2001, 15, 181–188. [Google Scholar] [CrossRef]
- Li, H.; Yue, Y.; Miao, C.; Xie, Z.; Hua, W.; Gao, Z. Dehydrogenation of ethylbenzene and propane over Ga2O3–ZrO2 catalysts in the presence of CO2. Catal. Commun. 2007, 8, 1317–1322. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, Q.; Xu, B.; He, D. Oxidative dehydrogenation of ethane over Co–BaCO3 catalysts using CO2 as oxidant: Effects of Co promoter. Catal. Lett. 2007, 117, 140–145. [Google Scholar] [CrossRef]
- Elkabouss, K.; Kacimi, M.; Ziyad, M.; Ammar, S.; Bozon-Verduraz, F. Cobalt-exchanged hydroxyapatite catalysts: Magnetic studies, spectroscopic investigations, performance in 2-butanol and ethane oxidative dehydrogenations. J. Catal. 2004, 226, 16–24. [Google Scholar] [CrossRef]
- Myint, M.; Yan, B.; Wan, J.; Zhao, S.; Chen, J.G. Reforming and oxidative dehydrogenation of ethane with CO2 as a soft oxidant over bimetallic catalysts. J. Catal. 2016, 343, 168–177. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, A.; Li, X.; Gong, W. Oxidative dehydrogenation of ethane with CO2 over catalyst under pulse corona plasma. Catal. Today 2004, 89, 97–102. [Google Scholar] [CrossRef]
- Peng, X.; Zhu, J.; Yao, L.; Hu, C. Effect of methane co-feeding on the selectivity of ethylene produced from oxidative dehydrogenation of ethane with CO2 over a Ni-La/SiO2 catalyst. J. Energy Chem. 2013, 22, 653–658. [Google Scholar] [CrossRef]
- Xu, L.; Liu, J.; Yang, H.; Xu, Y.; Wang, Q.; Lin, L. Regeneration behaviors of Fe/SiO2 and Fe–Mn/SiO2 catalysts for C2H6 dehydrogenation with CO2 to C2H4. Catal. Lett. 1999, 62, 185–189. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kajita, C.; Ikenaga, N.-O.; Nishitani-Gamo, M.; Ando, T.; Suzuki, T. Dehydrogenation of light alkanes over oxidized diamond-supported catalysts in the presence of carbon dioxide. Catal. Today 2003, 84, 149–157. [Google Scholar] [CrossRef]
- Ogonowski, J.; Skrzyńska, E. Conversion of Lower Hydrocarbons in the Presence of Carbon Dioxide: The Theoretic Analysis and Catalytic Tests over Active Carbon Supported Vanadium Oxide. Catal. Lett. 2008, 124, 52–58. [Google Scholar] [CrossRef]
- Qiao, A.; Kalevaru, V.N.; Radnik, J.; Martin, A. Oxidative dehydrogenation of ethane to ethylene over Ni–Nb–M–O catalysts: Effect of promoter metal and CO2-admixture on the performance. Catal. Today 2016, 264, 144–151. [Google Scholar] [CrossRef]
- Solymosi, F.; Németh, R. The oxidative dehydrogenation of ethane with CO2 over Mo2C/SiO2 catalyst. Catal. Lett. 1999, 62, 197–200. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Zhuang, J.-H. Supported indium oxide as novel efficient catalysts for dehydrogenation of propane with carbon dioxide. Appl. Catal. A 2010, 377, 35–41. [Google Scholar] [CrossRef]
- Zhu, J.; Qin, S.; Ren, S.; Peng, X.; Tong, D.; Hu, C. Na2WO4/Mn/SiO2 catalyst for oxidative dehydrogenation of ethane using CO2 as oxidant. Catal. Today 2009, 148, 310–315. [Google Scholar] [CrossRef]
- Wu, R.; Xie, P.; Cheng, Y.; Yue, Y.; Gu, S.; Yang, W.; Miao, C.; Hua, W.; Gao, Z. Hydrothermally prepared Cr2O3–ZrO2 as a novel efficient catalyst for dehydrogenation of propane with CO2. Catal. Commun. 2013, 39, 20–23. [Google Scholar] [CrossRef]
- Hakuli, A.; Harlin, M.E.; Backman, L.B.; Krause, A.O.I. Dehydrogenation of i-Butane on CrOx/SiO2 Catalysts. J. Catal. 1999, 184, 349–356. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Deng, J.; Dai, H.; He, H.; Au, C.T. Preparation, characterization, and catalytic activity of chromia supported on SBA-15 for the oxidative dehydrogenation of isobutane. Appl. Catal. A 2009, 355, 192–201. [Google Scholar] [CrossRef]
- Kim, D.S.; Tatibouet, J.-M.; Wachs, I.E. Surface structure and reactivity of CrO3/SiO2 catalysts. J. Catal. 1992, 136, 209–221. [Google Scholar] [CrossRef]
- Baek, J.; Yun, H.J.; Yun, D.; Choi, Y.; Yi, J. Preparation of Highly Dispersed Chromium Oxide Catalysts Supported on Mesoporous Silica for the Oxidative Dehydrogenation of Propane Using CO2: Insight into the Nature of Catalytically Active Chromium Sites. ACS Catal. 2012, 2, 1893–1903. [Google Scholar] [CrossRef]
- Puurunen, R.L.; Weckhuysen, B.M. Spectroscopic Study on the Irreversible Deactivation of Chromia/Alumina Dehydrogenation Catalysts. J. Catal. 2002, 210, 418–430. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García-Sancho, C.; Mérida-Robles, J.M.; Santamaría-González, J.; Moreno-Tost, R.; Maireles-Torres, P. V and V–P containing Zr-SBA-15 catalysts for dehydration of glycerol to acrolein. Catal. Today 2015, 254, 43–52. [Google Scholar] [CrossRef]
- Klimova, T.; Gutiérrez, O.; Lizama, L.; Amezcua, J. Advantages of ZrO2- and TiO2–SBA-15 mesostructured supports for hydrodesulfurization catalysts over pure TiO2, ZrO2 and SBA-15. Microporous Mesoporous Mater. 2010, 133, 91–99. [Google Scholar] [CrossRef]
- Lizama, L.; Amezcua, J.C.; Klimova, T. Development of New Hybrid TiO2/SBA-15 Mesoporous Molecular Sieves and Their Use as Supports for Deep Hydrodesulfurization NiMo Catalysts. In Studies in Surface Science and Catalysis; Gédéon, A., Massiani, P., Babonneau, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 174, pp. 1351–1354. [Google Scholar]
- Zhao, X.; Wang, X. Oxidative dehydrogenation of ethane to ethylene by carbon dioxide over Cr/TS-1 catalysts. Catal. Commun. 2006, 7, 633–638. [Google Scholar] [CrossRef]
- Al-Awadi, A.S.; El-Toni, A.M.; Al-Zahrani, S.M.; Abasaeed, A.E.; Alhoshan, M.; Khan, A.; Labis, J.P.; Al-Fatesh, A. Role of TiO2 nanoparticle modification of Cr/MCM41 catalyst to enhance Cr-support interaction for oxidative dehydrogenation of ethane with carbon dioxide. Appl. Catal. A 2019, 584, 117114. [Google Scholar] [CrossRef]
- Bai, P.T.; Srinath, S.; Upendar, K.; Sagar, T.V.; Lingaiah, N.; Rao, K.R.; Prasad, P.S. Oxidative dehydrogenation of ethane with carbon dioxide over Cr2O3/SBA-15 catalysts: The influence of sulfate modification of the support. Appl. Petrochem. Res. 2017, 7, 107–118. [Google Scholar]
- Jehng, J.-M.; Wachs, I.E.; Weckhuysen, B.M.; Schoonheydt, R.A. Surface chemistry of silica–titania-supported chromium oxide catalysts. J. Chem. Soc. Faraday Trans. 1995, 91, 953–961. [Google Scholar] [CrossRef]
- Al-Awadi, A.S.; Al-Zahrani, S.M.; El-Toni, A.M.; Abasaeed, A.E. Dehydrogenation of Ethane to Ethylene by CO2 over Highly Dispersed Cr on Large-Pore Mesoporous Silica Catalysts. Catalysts 2020, 10, 97. [Google Scholar] [CrossRef]
- Moulder, J.F. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics: Eden Prairie, MN, USA, 1995; pp. 230–232. [Google Scholar]
- Wu, D.; Ma, J.; Bao, Y.; Cui, W.; Hu, T.; Yang, J.; Bai, Y. Fabrication of Porous Ag/TiO2/Au Coatings with Excellent Multipactor Suppression. Sci. Rep. 2017, 7, 43749. [Google Scholar] [CrossRef]
- Yurddaskal, M.; Dikici, T.; Yildirim, S.; Yurddaskal, M.; Toparli, M.; Celik, E. Fabrication and characterization of nanostructured anatase TiO2 films prepared by electrochemical anodization and their photocatalytic properties. J. Alloys Compd. 2015, 651, 59–71. [Google Scholar] [CrossRef]
- Ayari, F.; Mhamdi, M.; Álvarez-Rodríguez, J.; Ruiz, A.R.G.; Delahay, G.; Ghorbel, A. Selective catalytic reduction of NO with NH3 over Cr-ZSM-5 catalysts: General characterization and catalysts screening. Appl. Catal. B Environ. 2013, 134, 367–380. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Zeńczak, K. Activity of chromium oxide deposited on different silica supports in the dehydrogenation of propane with CO2—A comparative study. J. Mol. Catal. A Chem. 2011, 349, 1–12. [Google Scholar] [CrossRef]
- Cavani, F.; Koutyrev, M.; Trifirò, F.; Bartolini, A.; Ghisletti, D.; Iezzi, R.; Santucci, A.; Del Piero, G. Chemical and Physical Characterization of Alumina-Supported Chromia-Based Catalysts and Their Activity in Dehydrogenation of Isobutane. J. Catal. 1996, 158, 236–250. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Wachs, I.E.; Schoonheydt, R.A. Surface Chemistry and Spectroscopy of Chromium in Inorganic Oxides. Chem. Rev. 1996, 96, 3327–3350. [Google Scholar] [CrossRef]
- Rao, T.V.M.; Yang, Y.; Sayari, A. Ethane dehydrogenation over pore-expanded mesoporous silica supported chromium oxide: 1. Catalysts preparation and characterization. J. Mol. Catal. A Chem. 2009, 301, 152–158. [Google Scholar] [CrossRef]
- Sun, B.; Reddy, E.P.; Smirniotis, P.G. Effect of the Cr6+ concentration in Cr-incorporated TiO2-loaded MCM-41 catalysts for visible light photocatalysis. Appl. Catal. B Environ. 2005, 57, 139–149. [Google Scholar] [CrossRef]
- Sun, B.; Reddy, E.P.; Smirniotis, P.G. TiO2-loaded Cr-modified molecular sieves for 4-chlorophenol photodegradation under visible light. J. Catal. 2006, 237, 314–321. [Google Scholar] [CrossRef]
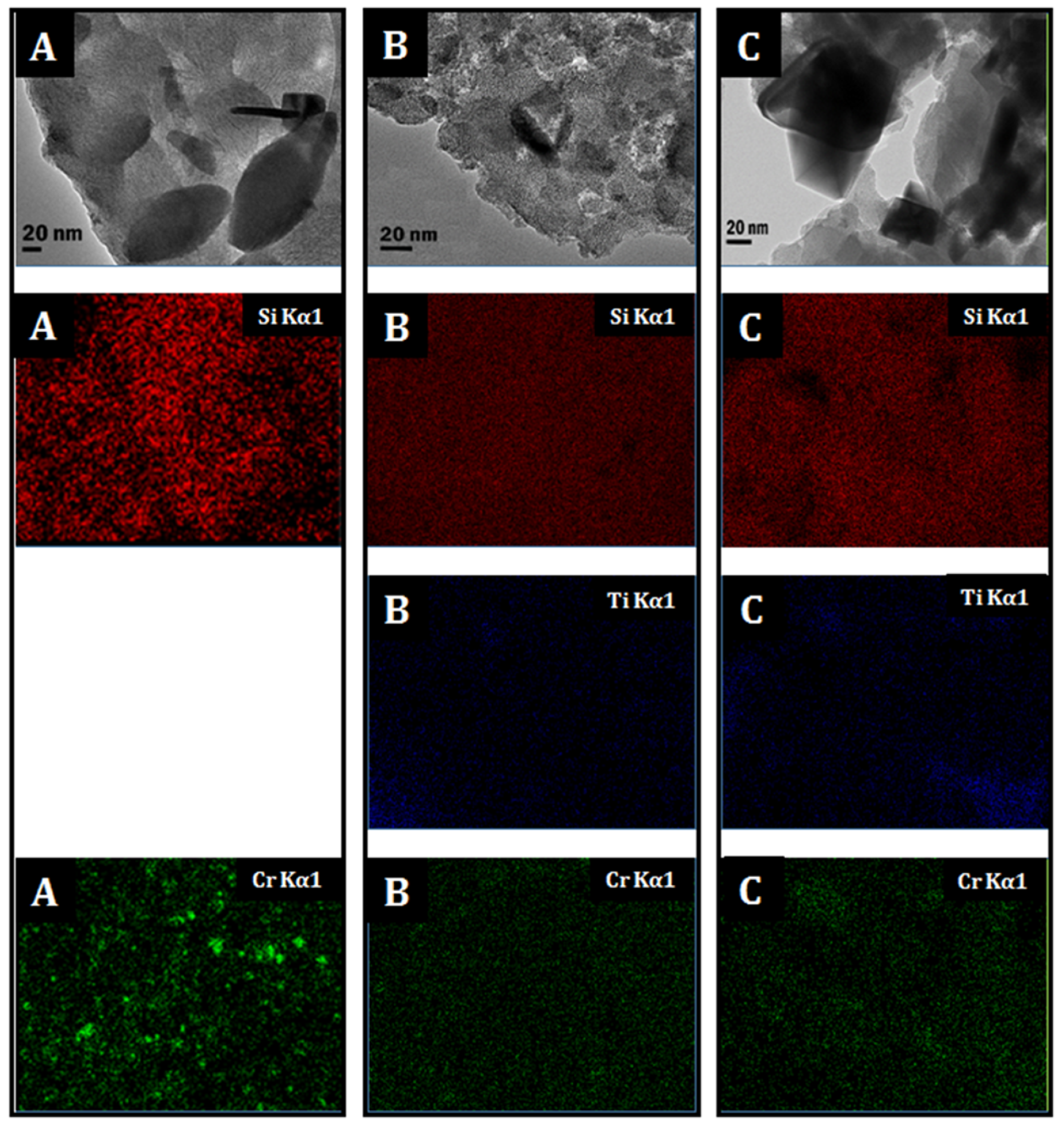

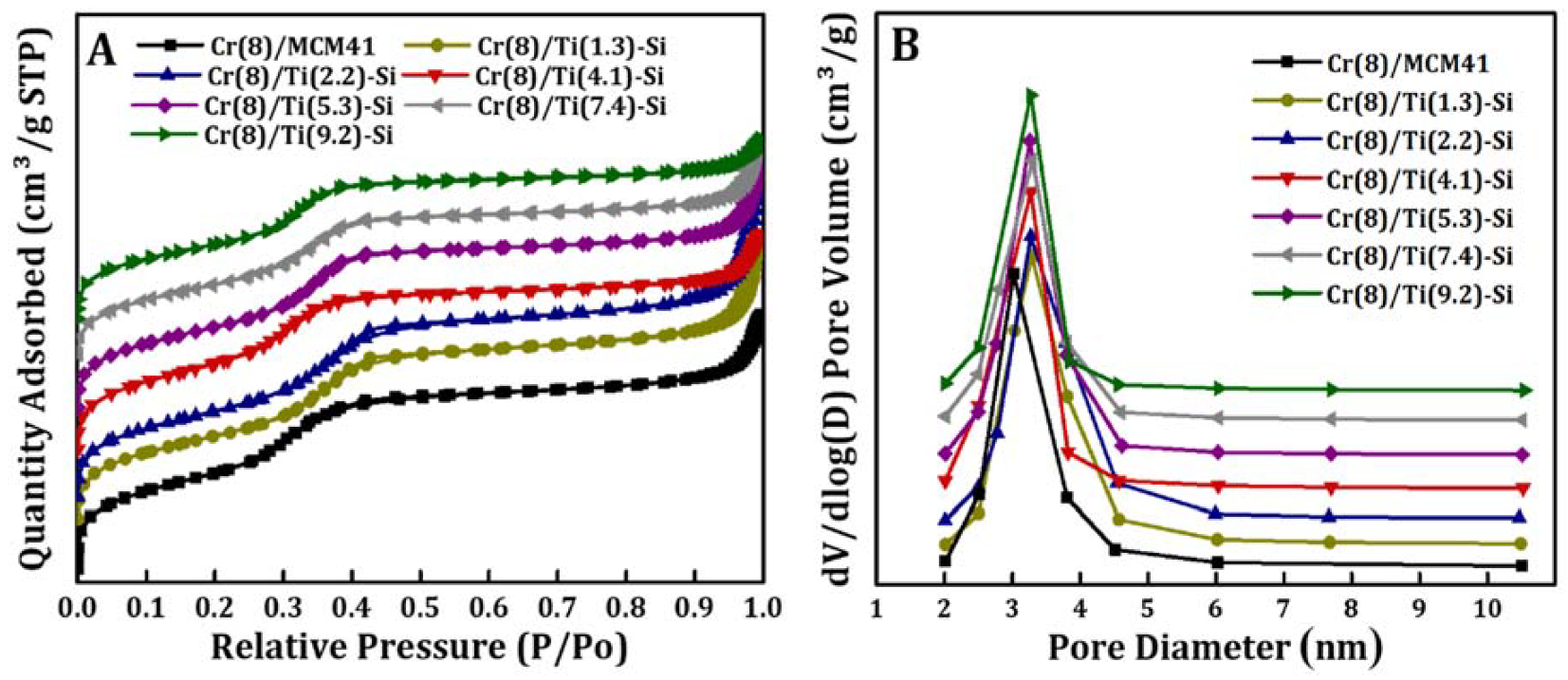




| Catalyst | BET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Cr Surface Density (Cr/nm2) b | Cr Particle Size a (nm) |
|---|---|---|---|---|---|
| Cr(8)/MCM-41 | 794 | 0.86 | 3.02 | 1.170 | 24.14 |
| Cr(8)/Ti(1.3)–Si | 750 | 0.98 | 3.29 | 1.235 | 20.03 |
| Cr(8)/Ti(2.2)–Si | 742 | 0.90 | 3.27 | 1.249 | 20.63 |
| Cr(8)/Ti(4.1)–Si | 730 | 0.87 | 3.27 | 1.269 | 20.61 |
| Cr(8)/Ti(5.3)–Si | 717 | 0.85 | 3.26 | 1.292 | 20.89 |
| Cr(8)/Ti(7.4)–Si | 643 | 0.75 | 3.29 | 1.441 | 21.35 |
| Cr(8)/Ti(9.2)–Si | 621 | 0.63 | 3.28 | 1.491 | 21.82 |
| Catalyst | Cr 2p3/2 Binding Energy (eV) | Cr(VI)/Cr(III) | |
|---|---|---|---|
| Cr(VI) | Cr(III) | ||
| Cr(8)/MCM-41 | 575.77 | 577.84 | 0.882 |
| Cr(8)/Ti(2.2)–Si | 575.74 | 577.47 | 2.062 |
| Cr(8)/Ti(5.3)–Si | 575.49 | 576.98 | 1.451 |
| Cr(8)/Ti(9.2)–Si | 575.41 | 576.92 | 1.052 |
| Catalysts | H2 Uptake (µmol/g) | H2/Cr Total (mol/mol) | First Cr(VI) Peak Area | Second Cr(VI) Peak Area | Second Cr(VI) Peak Area/Total Area |
|---|---|---|---|---|---|
| Cr(8)/MCM-41 | 386.14 | 0.251 | 2.06 | 1.13 | 0.35 |
| Cr(8)/Ti(2.2)–Si | 384.16 | 0.249 | 0.86 | 2.52 | 0.75 |
| Cr(8)/Ti(4.1)–Si | 368.63 | 0.240 | 1.63 | 2.29 | 0.58 |
| Cr(8)/Ti(5.3)–Si | 359.13 | 0.233 | 1.73 | 2.12 | 0.55 |
| Cr(8)/Ti(7.4)–Si | 342.48 | 0.223 | 1.60 | 0.73 | 0.31 |
| Cr(8)/Ti(9.2)–Si | 327.26 | 0.213 | 2.11 | 0.66 | 0.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Awadi, A.S.; El-Toni, A.M.; Al-Zahrani, S.M.; Abasaeed, A.E.; Khan, A. Synthesis, Characterization and Catalytic Evaluation of Chromium Oxide Deposited on Titania–Silica Mesoporous Nanocomposite for the Ethane Dehydrogenation with CO2. Crystals 2020, 10, 322. https://doi.org/10.3390/cryst10040322
Al-Awadi AS, El-Toni AM, Al-Zahrani SM, Abasaeed AE, Khan A. Synthesis, Characterization and Catalytic Evaluation of Chromium Oxide Deposited on Titania–Silica Mesoporous Nanocomposite for the Ethane Dehydrogenation with CO2. Crystals. 2020; 10(4):322. https://doi.org/10.3390/cryst10040322
Chicago/Turabian StyleAl-Awadi, Abdulrhman S., Ahmed Mohamed El-Toni, Saeed M. Al-Zahrani, Ahmed E. Abasaeed, and Aslam Khan. 2020. "Synthesis, Characterization and Catalytic Evaluation of Chromium Oxide Deposited on Titania–Silica Mesoporous Nanocomposite for the Ethane Dehydrogenation with CO2" Crystals 10, no. 4: 322. https://doi.org/10.3390/cryst10040322
APA StyleAl-Awadi, A. S., El-Toni, A. M., Al-Zahrani, S. M., Abasaeed, A. E., & Khan, A. (2020). Synthesis, Characterization and Catalytic Evaluation of Chromium Oxide Deposited on Titania–Silica Mesoporous Nanocomposite for the Ethane Dehydrogenation with CO2. Crystals, 10(4), 322. https://doi.org/10.3390/cryst10040322







