Characterization of Biominerals in Cacteae Species by FTIR
Abstract
:1. Introduction
2. Material and Methods
2.1. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.2. Polarized and Brightfield Microscope Analysis
2.3. Field Emission Scanning Electron Microscopy (FE-SEM) and Energy Dispersive X-ray Spectrometry (EDS) Analysis
3. Results
3.1. Analysis by FTIR
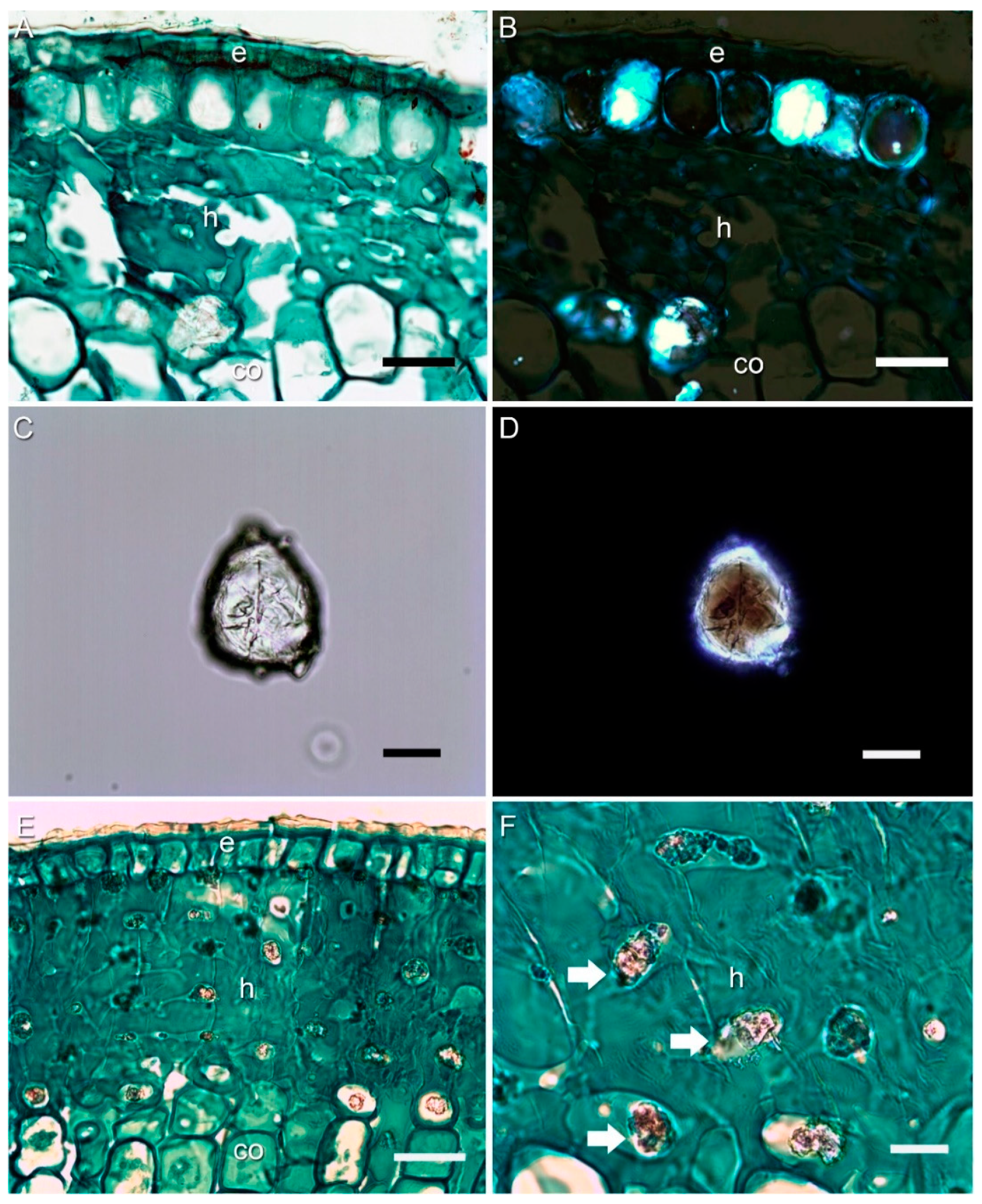
3.2. Anatomy with Light and Polarized Microscopy
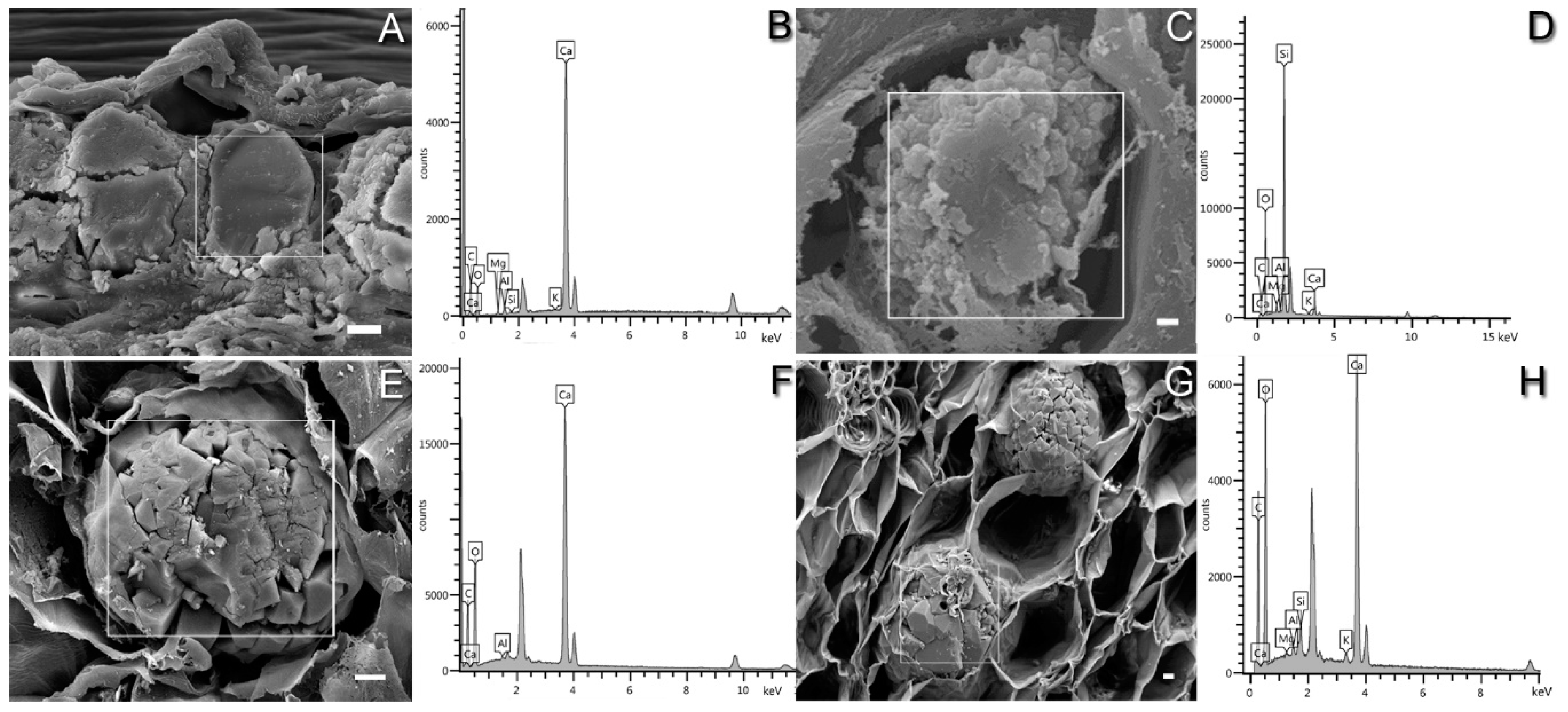
3.3. FE-SEM-EDS Analysis
4. Discussion
4.1. Biominerals Identification
4.1.1. Calcium Oxalate
4.1.2. Silica–Amorphous Hydrated Silica
4.1.3. Calcium Carbonate
4.1.4. Unknown Biominerals
4.2. Possible Functions of Biominerals in Different Tissues
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skinner, H.C.W. Biominerals. Mineral. Mag. 2005, 69, 621–641. [Google Scholar] [CrossRef]
- Arnott, H.J. Studies of Calcification in Plants. In Calcified Tissues; Fleish, H., Blackwood, H.J.J., Owen, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1966; pp. 152–157. ISBN 978-3-642-85843-7. [Google Scholar]
- Arnott, H.J. Three Systems of Biomineralization in Plants with Comments on the Associated Organic Matrix. In Biological Mineralization and Demineralization; Nancollas, G.H., Ed.; Springer: Berlin, Germany, 1982; pp. 199–218. ISBN 978-3-642-68574-3. [Google Scholar]
- Bauer, P.; Elbaum, R.; Weiss, I.M. Calcium and Silicon Mineralization in Land Plants: Transport, Structure and Function. Plant Sci. 2011, 180, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, V.R.; Nakata, P.A. Calcium Oxalate in Plants: Formation and Function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef]
- McNair, J.B. The Interrelation between Substances in Plants: Essential Oils and Resins, Cyanogen and Oxalate. Am. J. Bot. 1932, 19, 255–272. [Google Scholar] [CrossRef]
- Skinner, H.C.W.; Jahren, A.H. Biomineralization. In Treatise on Geochemistry; Holland, D.H., Turekian, K.K., Eds.; Elsevier Sciences: Amsterdam, The Netherlands, 2003; Volume 8, pp. 1–69. ISBN 9780080437514. [Google Scholar]
- Cheavin, W.H.S. The Crystals and Cystolites Found in Plant Cells. Part 1: Crystals. Microscope 1938, 2, 155–158. [Google Scholar]
- Schleiden, M.J. Beiträge zur Anatomie der Cacteen. Mem l’Acadèmie Imp des Sci St. Pétersbg 1845, 4, 335–380. [Google Scholar]
- Bárcenas-Argüello, M.L.; Gutiérrez-Castorena, M.C.-D.-C.; Terrazas, T. The Polymorphic Weddellite Crystals in Three Species of Cephalocereus (Cactaceae). Micron 2015, 77, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hartl, W.P.; Barbier, B.; Klapper, H.M.; Barthlott, W. Dimorphism of Calcium Oxalate Crystals in Stem Tissues of RHIPSALIDEAE (Cactaceae)—A Contribution to the Systematics and Taxonomy of the Tribe. Bot. Jahrb. Syst. Pflanzengesch. Pflanzengeogr. 2003, 124, 287–302. [Google Scholar] [CrossRef]
- Hartl, W.P.; Klapper, H.; Barbier, B.; Ensikat, H.J.; Dronskowski, R.; Müller, P.; Ostendorp, G.; Tye, A.; Bauer, R.; Barthlott, W. Diversity of Calcium Oxalate Crystals in Cactaceae. Can. J. Bot. 2007, 85, 501–517. [Google Scholar] [CrossRef]
- Monje, P.V.; Baran, E.J. On the Formation of Weddellite in Chamaecereus Silvestrii, a Cactaceae Species from Northern Argentina. Z. Naturforsch. C J. Biosci. 1996, 51, 426–428. [Google Scholar] [CrossRef]
- Monje, P.V.; Baran, E.J. On the Formation of Whewellite in the Cactaceae Species Opuntia Microdasys. Z. Naturforsch. C J. Biosci. 1997, 52, 267–269. [Google Scholar] [CrossRef]
- Monje, P.V.; Baran, E.J. Characterization of Calcium Oxalates Generated as Biominerals in Cacti. Plant Physiol. 2002, 128, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E.R.; Smith, B.N. Crystal Morphology and 13Carbon/12Carbon Composition of Solid Oxalate in Cacti. Plant Physiol. 1979, 64, 966–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monje, P.V.; Baran, E.J. Evidence of Formation of Glushinskite as a Biomineral in a Cactaceae Species. Phytochemistry 2005, 66, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.G.; Bryant, V.M. Phytolith Taxonomy in Selected Species of Texas Cacti. In Phytolith Systematics; Springer: Boston, MA, USA, 1992; Volume 1, pp. 215–238. ISBN 9781489911575. [Google Scholar]
- Monje, P.V.; Baran, E.J. First Evidences of the Bioaccumulation of α-Quartz in Cactaceae. J. Plant Physiol. 2000, 157, 457–460. [Google Scholar] [CrossRef]
- Loza-Cornejo, S.; Terrazas, T. Epidermal and Hypodermal Characteristics in North American Cactoideae (Cactaceae). J. Plant Res. 2003, 116, 27–35. [Google Scholar] [CrossRef]
- Terrazas, T.; Loza-Cornejo, S.; Arreola-Nava, H.J. Anatomía Caulinar de las Especies del Género Stenocereus (Cactaceae). Acta Botánica Venez. 2005, 28, 321–336. [Google Scholar]
- Gibson, A.C.; Horak, K.E. Systematic Anatomy and Phylogeny of Mexican Columnar Cacti. Ann. Mo. Bot. Gard. 1978, 65, 999. [Google Scholar] [CrossRef]
- De La Rosa-Tilapa, A.; Vázquez-Sánchez, M.; Terrazas, T. Stem Anatomy of Turbinicarpus s.l. (Cacteae, Cactaceae) and Its Contribution to Systematics. Plant Biosyst. 2019, 153, 600–609. [Google Scholar] [CrossRef]
- Frausto-Reyes, C.; Loza-Cornejo, S.; Terrazas, T.; De La Luz Miranda-Beltrán, M.; Aparicio-Fernández, X.; López-Mací, B.M.; Morales-Martínez, S.E.; Ortiz-Morales, M. Raman Spectroscopy Study of Calcium Oxalate Extracted from Cacti Stems. Appl. Spectrosc. 2014, 68, 1260–1265. [Google Scholar] [CrossRef]
- López-Macías, B.M.; Morales-Martínez, S.E.; Loza-Cornejo, S.; Reyes, C.F.; Terrazas, T.; Patakfalvi, R.J.; Ortiz-Morales, M.; Miranda-Beltrán, M.D.l.L. Variability and Composition of Calcium Oxalate Crystals in Embryos-Seedlings-Adult Plants of the Globose Cacti Mammillaria Uncinata. Micron 2019, 125, 102731. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: Oxford, UK, 1999; ISBN 0195089561. [Google Scholar]
- Loza-Cornejo, S.; Terrazas, T. Anatomía del tallo y de la raíz de dos Especies de Wilcoxia Britton & Rose (Cactaceae) del Noreste de México. Bot. Sci. 1996, 59, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Durak, T.; Depciuch, J. Effect of Plant Sample Preparation and Measuring Methods on ATR-FTIR Spectra Results. Environ. Exp. Bot. 2020, 169, 103915. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared spectroscopy: Fundamentals and Applications; ANTS (Analytical Techniques in the Sciences); John Wiley & Sons, Ltd.: Chichester, UK, 2004; ISBN 978-0-470-85428-0. [Google Scholar]
- Mascarenhas, M.; Dighton, J.; Arbuckle, G.A. Characterization of Plant Carbohydrates and Changes in Leaf Carbohydrate Chemistry due to Chemical and Enzymatic Degradation Measured by Microscopic ATR FT-IR Spectroscopy. Appl. Spectrosc. 2000, 54, 681–686. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, V.; Kumar, R. Distribution of Phytoliths in Plants: A Review. Geol. Ecol. Landsc. 2019, 3, 123–148. [Google Scholar] [CrossRef] [Green Version]
- Conti, C.; Casati, M.; Colombo, C.; Possenti, E.; Realini, M.; Gatta, G.D.; Merlini, M.; Brambilla, L.; Zerbi, G. Synthesis of Calcium Oxalate Trihydrate: New Data by Vibrational Spectroscopy and Synchrotron X-Ray Diffraction. Spectrochim. Acta Part AMol. Biomol. Spectrosc. 2015, 150, 721–730. [Google Scholar] [CrossRef]
- Petit, I.; Belletti, G.D.; Debroise, T.; Llansola-Portoles, M.J.; Lucas, I.T.; Leroy, C.; Bonhomme, C.; Bonhomme-Coury, L.; Bazin, D.; Daudon, M.; et al. Vibrational Signatures of Calcium Oxalate Polyhydrates. Chem. Sel. 2018, 3, 8801–8812. [Google Scholar] [CrossRef]
- Zancajo, V.M.R.; Diehn, S.; Filiba, N.; Goobes, G.; Kneipp, J.; Elbaum, R. Spectroscopic Discrimination of Sorghum Silica Phytoliths. Front. Plant Sci. 2019, 10, 1571. [Google Scholar] [CrossRef] [Green Version]
- Corrales-Ureña, Y.R.; Villalobos-Bermúdez, C.; Pereira, R.; Camacho, M.; Estrada, E.; Argüello-Miranda, O.; Vega-Baudrit, J.R. Biogenic Silica-Based Microparticles Obtained as a Sub-Product of the Nanocellulose Extraction Process from Pineapple Peels. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Wright, C.R.; Waddell, E.A.; Setzer, W.N. Accumulation of Silicon in Cacti Native to the United States: Characterization of Silica Bodies and Cyclic Oligosiloxanes in Stenocereus Thurberi, Opuntia Littoralis, Opuntia Ficus-Indica, and Opuntia Stricta. Nat. Prod. Commun. 2014, 9, 873–878. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Veneklaas, E.J.; Kuo, J.; Lambers, H. Physiological and Ecological Significance of Biomineralization in Plants. Trends Plant Sci. 2014, 19, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.; Hirsch, A.; Siegel, S.; Li, C.; Aichmayer, B.; Politi, Y.; Fratzl, P.; Weiner, S.; Addadi, L. Plant Cystoliths: A Complex Functional Biocomposite of Four Distinct Silica and Amorphous Calcium Carbonate Phases. Chem. Eur. J. 2012, 18, 10262–10270. [Google Scholar] [CrossRef] [PubMed]
- Palacio, S.; Aitkenhead, M.; Escudero, A.; Montserrat-Martí, G.; Maestro, M.; Robertson, A.H.J. Gypsophile Chemistry Unveiled: Fourier Transform Infrared (FTIR) Spectroscopy Provides New Insight into Plant Adaptations to Gypsum Soils. PLoS ONE 2014, 9, e107285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monje, P.V.; Baran, E.J. Complex Biomineralization Pattern in Cactaceae. J. Plant Physiol. 2004, 161, 121–123. [Google Scholar] [CrossRef]
- Berg, R.H. A Calcium Oxalate-Secreting Tissue in Branchlets of the Casuarinaceae. Protoplasma 1994, 183, 29–36. [Google Scholar] [CrossRef]
- Lersten, N.R.; Horner, H.T. Unique Calcium Oxalate “Duplex” and “Concretion” Idioblasts in Leaves of Tribe Naucleeae (Rubiaceae). Am. J. Bot. 2011, 98, 1–11. [Google Scholar] [CrossRef]
- He, H.; Bleby, T.M.; Veneklaas, E.J.; Lambers, H.; Kuo, J. Morphologies and Elemental Compositions of Calcium Crystals in Phyllodes and Branchlets of Acacia Robeorum (Leguminosae: Mimosoideae). Ann. Bot. 2012, 109, 887–896. [Google Scholar] [CrossRef]
- Vahur, S.; Teearu, A.; Peets, P.; Joosu, L.; Leito, I. ATR-FT-IR Spectral Collection of Conservation Materials in the Extended Region of 4000–80 cm−1. Anal. Bioanal. Chem. 2016, 408, 3373–3379. [Google Scholar] [CrossRef]
- De la Rosa-Tilapa, A. Structure and Composition of Biominerals in the Stem of the Cacteae Tribe (Cactaceae). Master’s Thesis, National Autonomous University of Mexico, Mexico City, Mexico, 2020. [Google Scholar]
- Pierantoni, M.; Tenne, R.; Brumfeld, V.; Kiss, V.; Oron, D.; Addadi, L.; Weiner, S. Plants and Light Manipulation: The Integrated Mineral System in Okra Leaves. Adv. Sci. 2017, 4, 1600416. [Google Scholar] [CrossRef]
- Volk, G.M.; Lynch-Holm, V.J.; Kostman, T.A.; Goss, L.J.; Franceschi, V.R. The Role of Druse and Raphide Calcium Oxalate Crystals in Tissue Calcium Regulation in Pistia Stratiotes Leaves. Plant Biol. 2002, 4, 34–45. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Zakharenko, A.M.; Zemchenko, I.V.; Haider, M.S.; Ali, M.A.; Imtiaz, M.; Chung, G.; Tsatsakis, A.; Sun, S.; Golokhvast, K.S. Phytolith Formation in Plants: From Soil to Cell. Plants 2019, 8, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodson, M.J.; Sangster, A.G. The interaction between silicon and aluminium in Sorghum bicolor (L.) Moench: Growth analysis and x-ray microanalysis. Ann. Bot. 1993, 72, 389–400. [Google Scholar] [CrossRef]



| Species | Collection Number | Location |
|---|---|---|
| Astrophytum asterias (Zucc.) Lem. | TT1020 | La Esperanza, Tamaulipas |
| TT846 | San Carlos Tamaulipas | |
| Ariocarpus retusus subsp. trigonus (F.A.C.Weber) E.F.Anderson & W.A.Fitz Maur. | TT1005 | La Soledad, Tamaulipas |
| TT879 | Moctezuma, San Luis Potosí | |
| Echinocactus texensis Hopffer | TT1021 | La Esperanza, Tamaulipas |
| TT851 | Tula, Tamaulipas | |
| Mammillaria melanocentra subsp. rubrograndis (Repp. & A.B. Lau) D.R. Hunt | TT1050 | Ejido Huizache, Tamaulipas |
| Mammillaria sphaerica A. Dietr. | TT1051 | Ejido Huizache, Tamaulipas |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Rosa-Tilapa, A.; Maceda, A.; Terrazas, T. Characterization of Biominerals in Cacteae Species by FTIR. Crystals 2020, 10, 432. https://doi.org/10.3390/cryst10060432
De la Rosa-Tilapa A, Maceda A, Terrazas T. Characterization of Biominerals in Cacteae Species by FTIR. Crystals. 2020; 10(6):432. https://doi.org/10.3390/cryst10060432
Chicago/Turabian StyleDe la Rosa-Tilapa, Alejandro, Agustín Maceda, and Teresa Terrazas. 2020. "Characterization of Biominerals in Cacteae Species by FTIR" Crystals 10, no. 6: 432. https://doi.org/10.3390/cryst10060432





