1. Introduction
Impairment of the cardiac pump function due to the damage of myocardial tissue may manifest in severe heart failure. In many cases, the treatment of choice is the surgical reconstruction, in which the damaged areas are resected and the defect is covered with synthetic patch materials such as Dacron [
1,
2] or biological tissues such as autologous pericardium [
3,
4]. However, autologous pericardium cannot always be obtained in sufficient quantities and the currently available synthetic substitutes have no regenerative potential and do not actively contribute to myocardial contraction.
Innovative biological prostheses for myocardial reconstruction with a potential for physiological remodelling may overcome these limitations [
5]. However, many grafts lack sufficient vascularisation, which is a prerequisite for the viability of biological prostheses. In addition to its indispensable viability, the ideal heart muscle replacement should primarily fulfill the general requirements for bioartificial prostheses, such as high immunological compatibility, good integrability into the host tissue, the absence of pathogens, regenerative capacity, lifetime durability, unlimited availability, ease of use, and not least cost-efficient manufacture and storage. Moreover, specific properties such as coherent contractility, sufficient mechanical stability, and low diastolic pressure are also required for consideration in the development of bioartificial myocardial tissue. Therefore, synchronised electrophysiology with a physiological spread of action potentials and refractory periods appropriate to the stimulus conduction of the native tissue is mandatory for the good functional integration of the graft.
Autologous vascularised tissues following a physiological remodelling after transplantation into the myocardium can exhibit specific cardiac features and functions and, therefore, could eventually meet these requirements. In animal tests, the applicability of small intestine tissue for the transmural right ventricular reconstruction of the myocardium was demonstrated [
6,
7,
8]. As early as one month following transplantation, Tudorache et al. were able to detect evidence of cardiomyocytes in the transplanted small intestine tissue employing a swine model. Thereby, they demonstrated the feasibility of the conversion of heterotopic tissue into a functional myocardial prosthesis [
6]. However, due to its reduced wall thickness, the small intestine does not offer sufficient mechanical stability for its use for the reconstruction of the left ventricular myocardium. The high-pressure conditions of the left ventricle could cause a formation of dangerous aneurysms in the grafts, with the risk of life-threatening rupture, thrombus formation, and further impairment of the cardiac pumping function. In the present study, therefore, stomach tissue was employed for the reconstruction of the left ventricular myocardium, since stomach features higher mechanical stability in comparison with the tunica muscularis of the small intestine. However, even a patch of stomach tissue is initially not stable enough to reliably withstand an intracardiac blood pressure of up to 240 mm of mercury. Thus, the initially delicate stomach tissue could be stabilised temporarily by degradable scaffolds. This concept of transiently supporting vascularised biological grafts by degradable scaffolds would allow for exploiting their essential regenerative potential in a broader spectrum of applications.
We have already demonstrated the epicardial applicability and biocompatibility of degradable structures of magnesium alloy LA63 in a swine model [
9]. The degradation kinetics of magnesium structures depend on the alloy composition and geometry. Especially when using other than high-purity magnesium (99.99%), natural impurities in the alloy can cause galvanic corrosion due to the formation of intermetallic phases. Elements like Fe or Mn, e.g., act as cathodic sites within the alloy. Hence, a confluent coating of the magnesium alloy surface is required to prevent an electrolyte from triggering corrosion within the scaffold. A disadvantageously inhomogenous pitting corrosion can be alleviated or at least delayed by a magnesium fluoride layer. Adding lithium and aluminum to form a magnesium alloy (LA63) further decreases the corrosion rate of the scaffold. Lithium alkalizes the corrosion layer and thereby decelerates the oxidation of the magnesium implants. Aluminum forms a protective oxide layer. Thus, using high-purity magnesium, selecting suitable alloys, shaping stable scaffold geometries, and coating the grafts’ surface can be utilized to adjust the degradation rate in order to match the expected in vivo remodelling rate of the vascularised biological stomach tissue. Ideally, the physiological transformation processes that lead to sufficient mechanical stability of the biological graft should take place at precisely the magnesium structure’s pace of degradation. The long-term therapeutic goal would be a stable and functionally integrated biological myocardial prosthesis after the complete and biocompatible degradation of the stabilising scaffold.
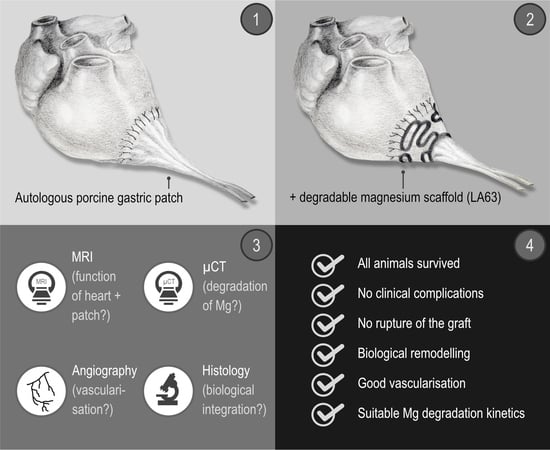
Therefore, this pilot study aims to investigate the function, integration, and biocompatibility of a left ventricular full wall myocardial replacement with a patch of autologous vascularised stomach tissue, temporarily stabilised by magnesium alloy LA63 structures coated with magnesium fluoride.
2. Materials and Methods
All experiments were carried out in accordance with the European Convention on Animal Welfare and were approved by the licensing authority (Lower Saxony State Office for Consumer Protection and Food Safety (LAVES), Lower Saxony) in accordance with Section 8, Paragraph 1 of the Animal Welfare Act, German Civil Code 1. IS 01484 (experiment #08/1604).
After manufacturing the degradable magnesium scaffolds (
Figure 1 and
Figure 2), a left ventricular transmural defect in six Lewe minipigs was covered with a vascularised segment of the autologous stomach (
Figure 1 and
Figure 3). The native vascular supply of the stomach via the left epigastric vein and artery was maintained. To stabilise this biological graft, magnesium structures were fixed to the epicardium over the reconstructed area (
Figure 1,
Figure 2 and
Figure 3).
Subsequently, the degradation of the magnesium structures, the biological reactions of the surrounding cardiac muscle to the metallic implant, and the integration and vascularisation of the biological graft as well as the heart function were examined three months and six months following transplantation.
2.1. Manufacturing of Magnesium Scaffolds
The construction of the magnesium scaffolds has been described in detail elsewhere [
10]; the scaffolds were made from sheets of magnesium alloy LA63 (6 wt% lithium, 3 wt% aluminium) with a thickness of 1 mm. The scaffolds were shaped by abrasive water jet cutting using a high pressure water jet pump (Type 20 XW Waternife, Flow, Kent, WA, USA) under a maximum water pressure of 400 MPa and a maximum flow rate of 7.8 l/m at ambient temperature. The water jet nozzle diameter was 0.17 mm using a focusing tube of 0.6 mm. Garnet mesh #120 (GMA Garnet (Europe) GmbH, Hamburg, Germany) was added to the water jet as an abrasive with a flow rate of 150 g/min (
Figure 2). The scaffolds’ surfaces were then transformed to magnesium fluoride as a coating layer with 40% hydrofluoric acid (Carl-Roth GmbH & Co. KG, Karlsruhe, Germany) for 96 h. The magnesium scaffolds were subsequently sterilized with gamma radiation at a dose of 25 kGy at room temperature for 8 h with a cobalt gammaradiation source according to DIN EN ISO 11137-2 (German Institute for Standardization, European Norm, International Organization for Standadization). The scaffolds were then stored in a sterile package at room temperature until implantation.
2.2. Implantation
The anaesthetic regimen and preoperative and postoperative measures applied to the six 35 kg Lewe minipigs to prevent arrhythmia, infection, pain, or gastric ulcers, as well as the wound closure, have already been described in detail elsewhere [
9].
A median laparotomy was performed to prepare an approximately 4 × 4 cm large segment of the stomach originating from the gastrosplenic ligament. The laparotomy was then extended to a left lateral thoracotomy in the fourth intercostal space. After systemic heparinization (400 IU/kg; heparin sodium 25,000, Ratiopharm, Ulm, Germany), an extracorporeal circulation (Stöckert S3, Sorin Group Germany GmbH, Munich, Germany) was applied via the carotid artery and the right atrium. The body temperature of the animal was cooled down to 28 °C.
First, a transmural and nearly circular defect measuring approximately 4 × 4 cm was produced in the anterolateral wall of the left ventricle. The diaphragm was incised, and the prepared segment of the stomach, including its native vascular supply via the left epigastric artery, was positioned through this incision into the thoracic cavity. The stomach was then closed with a continuous suture (Polyprolene 2.0, Ethicon, Hamburg, Germany). The stomach segment was mechanically freed from its mucosa with a scalpel and drawn through the central ellipsoid opening of the magnesium scaffold. The circular transmural defect of the left ventricle was then covered with the stomach segment. The vascularised stomach segment was fixed with a continuous suture (Polypronlene 4.0, Ethicon, Hamburg, Germany) to the myocardium. To stabilise the stomach segment against the high intraventricular pressure, the magnesium scaffold—still hanging loosely around the pedicle—was pushed forward until it rested with its outer edges on the epicardium and finally sutured with 4 simple interrupted stitches (Polyprolene 4.0, Ethicon, Hamburg, Germany) to the epicardium, each approximately 5 mm beyond the border zone between the stomach and myocardium (
Figure 3).
After the transposition of the stomach segment and the fixation of the magnesium scaffold, the systemic heparinisation was antagonised with 400 IU/kg of protamine (Medapharma, Wangen-Brüttisellen, Switzerland). Finally, a precise bleeding control, rewarming to 37 °C, weaning from the heart-lung machine, and wound closure were performed. For this purpose, the ribs were adapted (Mersilene 2.0, Ethicon, Hamburg, Germany), the muscle layers closed with a continuous suture (Vicryl 2.0, Ethicon, Hamburg, Germany), and, after performing a skin closure according to the Donati technique (CBX1 Vicryl, Ethicon, Hamburg, Germany), the wound was covered with aluminium spray (Almapharm, Wildpoldsried, Germany).
After this two-cavity operation, three animals were scheduled for euthanasia after three months (3M group), and another three animals after six months (6M group).
2.3. Cardiac Magnetic Resonance Imaging (MRI)
The laboratory animals were examined by MRI directly before euthanasia 3 (3M group) and 6 (6M group) months following surgery, respectively. To immobilise the animals during examination, they were sedated with intravenous Propofol Lipuro (1 mL/kg/h, Braun, Melsungen, Germany), intubated, ventilated, and then placed into the MRI scanner in the left lateral position. The MRI scan was performed in a 1.5 Tesla MR scanner (Genesis Signa CVI, GE Healthcare, Braunschweig, Germany). The objective of the scan was to evaluate the morphology, function, and tissue characteristics of the left ventricle, particularly of the patch region. For the quantitative assessment of the left ventricular volumes and ejection fraction, as well as for the visual evaluation of the morphology and wall motion an ECG-gated, a breath-hold balanced steady-state free precession (SSFP) gradient-echo sequence (FIESTA) in standard short-axis view and two and four-chamber view was used. The following parameters were used: TR 3.9 ms, TE 1.6 ms, flip angle of 40 degrees, acquisition matrix 224 × 224, reconstructed matrix 512 × 512, and slice thickness 8 mm.
For the visual differentiation between the vital myocardium and fibrotic areas, an ECG-gated breath-hold contrast-enhanced T1-weighted inversion recovery gradient echo (IRGE) in the standard view was used. For this, the exposures were made about 10 to 20 min after the intravenous administration of a gadolinium-based MRI contrast agent (Gadobutrol; Gadovist, Bayer Vital GmbH, Leverkusen, Germany; dose of 0.15 mmol/kg body weight). The measurements were made using the following parameters: TR 7.2 ms, TE 3.1 ms, flip angle 20 degrees, acquisition matrix 256 × 192, reconstructed matrix 256 × 256, and slice thickness 8mm. The inversion time (IT) was correspondingly adjusted to zero the signal intensity of the healthy myocardium and was between 200–300 ms.
For the quantitative evaluation of the left ventricular volume and ejection fraction, the software CVI42 version 5.1.2 (Circle Cardiovascular Imaging Inc., Calgary, Canada) was used. The endocardial and epicardial contours in the end-systolic and end-diastolic phase were traced in the short-axis layers above the left ventricle from the atrioventricular junction to the apex. The morphology of the left ventricle, the regional wall motion, and the extent and distribution of the late enhancement were visually assessed.
2.4. Angiography
After three months (3M group) and after six months (6M group), the perfusion of the transplanted gastric segment was examined by angiography immediately before the euthanasia of the animals. Under the general anaesthesia already described in detail elsewhere [
9], a median sternotomy was performed to expose the heart with the transplanted stomach segment and the magnesium structure or its residues. After the preparation of the pedicle of the heterotopically transplanted stomach segment, the inherent left epigastric artery was exposed. The artery was incised and a cannula (Vasofix
® Safety, 22G, B. Braun, Melsungen, Germany) was inserted in the antegrade direction and used for the application of a non-ionic contrast medium (Imeron 350
®, bracco-Byk Gulden, Konstanz, Germany). An X-ray was performed using the C-arm system (Ziehm Imaging GmbH, Nürnberg, Germany).
2.5. Explantation of the Heart, Including the Heterotopically Applied Segment of the Stomach and the Remains of the Degradable Magnesium Scaffold
To explant the heart with the transplanted stomach segment and the stabilising scaffold or its remains, first the adhesions between the epicardium, pericardium, stomach patch, and visceral pleura were thoroughly removed. The euthanasia of the test animals was then performed by an injection of pentobarbiturate (450 mg/kg body weight, WDT, Garbsen, Germany). The whole heart was removed after severing the pedicle from the stomach segment, the ascending aorta, the superior and inferior vena cava, and the pulmonary vessels. The transplanted stomach segment was separated from the myocardium, leaving a rim of about 1 cm.
2.6. μ-CT (Micro Computertomography)
Immediately after explantation, the isolated portion of the left ventricular myocardium, including the stomach patch and magnesium scaffold or its remains, were examined in the μCT (μ-CT81, Scanco Medical AG, Brüttisellen, Switzerland). The exact procedure and the parameters have already been described in detail elsewhere [
9]: Each layer was scanned for 1 s at a voltage of 55 kV and a current of 72 μA. The thickness of the sections was 36 microns in each case.
All the magnesium structures had been scanned prior to implantation to determine the initial volume. The volume of the magnesium structures remaining in the explant was determined, and the μ-CT data were reconstructed in a three-dimensional image with the proprietary software of the computer tomograph (Scanco Medical AG, Brüttisellen, Switzerland).
2.7. Histology
The implanted regions in contact to the surrounding cardiac wall of the left ventricle were dissected and carefully relieved from the metallic material. After fixation with 2.5% glutaraldehyde (Polysciences, Warrington, WA, USA) in 0.1 M of sodium cacodylate of pH 7.3 (Th. Geyer, Hamburg, Germany) for 4 h at room temperature, the specimens were rinsed in 0.1 M of sodium cacodylate and postfixed with 2% osmium tetroxide (Polysciences Europe GmbH, Hirschberg an der Bergstrasse, Germany) in 0.1 M of sodium cacodylate. After dehydration in graded ethanols (Baker, Deventer, The Netherlands), they were embedded in epoxide resin (Serva GmbH, Heidelberg, Germany). The semithin sections were stained with 1% Toluidine Blue (Merck KGaA, Darmstadt, Germany) and analyzed with an Axiovert 200M microscope (Carl Zeiss AG, Oberkochen, Germany). Representative micrographs were documented with Axiovision 4.8.2 and processed with Adobe Photoshop CS6 (Adobe Systems GmbH, Munich, Germany).
4. Discussion
Since the 1930s, there have been attempts to support damaged heart muscle with regenerative biological grafts or even to reconstruct it [
11]. Until now, however, due to the low mechanical stability of biological grafts, transmural myocardial repair has been limited to the right ventricle or atrium [
6,
8,
12,
13]. The reconstruction of the right ventricle with stomach tissue has already been clinically implemented as a last resort. In contrast, the treatment of the dysfunctional left ventricle with biological myocardial prostheses primarily aimed to improve the pumping function and vascularisation of the scarred myocardium so far. The tissues were placed merely epicardially on the damaged area in these cases [
11,
14,
15,
16,
17,
18,
19,
20,
21,
22]. Ruel et al. used a ligation of the circumflex coronary artery in a swine model to induce infarction and then supported the infarcted myocardium with vascularised stomach, which they fixed epicardially on the dysfunctional area [
21]. Unlike Ruel et al., our approach was to use a segment of vascularised stomach for the transmural reconstruction of a left ventricular defect in a demanding two-cavity surgical procedure, which all animals survived in good clinical condition. No test animal showed any clinical evidence of hemodynamic insufficiency. Presumably, the reduced physical activity due to indoor confinement may have masked the expected reduced performance of those animals with a critically low left ventricular ejection fraction. Nonetheless, a distinctive remodelling did take place; cellular granulation tissue was formed between the native myocardium and stomach, which was also penetrated by numerous capillaries and neocapillaries, thus anastomosing the vasculature of both tissues. We were even able to find a retrograde uptake of the contrast medium that we had injected via the left epigastric artery of the stomach graft in the native coronary vessels angiographically. Therefore, a functional connection of the vascular system of the stomach to the coronary arteries must also be assumed. It remains unclear to what extent this active remodelling, the vigorous scar tissue in the border zone between the myocardium and gastric patch, and the stabilising magnesium scaffold prevented the formation of an aneurysm.
The stabilising magnesium structure prevented the rupture of the delicate transplanted stomach tissue in all cases. Nevertheless, we still diagnosed an aneurysm after a period of three and six months. It cannot be ruled out that the epicardial surgical fixation of the structure was carried out inadequately or not in accordance with the actual mechanical stress conditions. Possibly, more extensive meshes are required spanning a larger area of the still-healthy myocardium. This extensive approach has been adopted with the Paracor heartnet, which is designed to prevent further dilatation in heart failure patients [
23,
24].
We not only found a loss of volume in the structure of 64.76% after three months and 73.42% after six months but also a substantial disintegration of the original scaffold using μ-CT scans and histological analyses of the explants. The corrosion of magnesium alloys is subject to a specific time [
25,
26]. Most magnesium alloys show greater stability in the initial phase, while, subsequently, the speed of corrosion increases. Thus, in a preliminary study, we were able to demonstrate one month after epicardial fixation in a swine model that the coated magnesium scaffold was structurally intact [
9].
The corrosion of the metallic implants is also dependent on the mechanical load and shear forces [
27,
28]. Gu et al. were able to determine a corrosion rate of up to 10 times higher for magnesium alloys when they are subjected to mechanical stress [
28]. The intense pressure fluctuations that the magnesium scaffold is exposed to during cardiac action in the early phase after implantation may thus have accelerated the degradation of the magnesium alloy. Finally, it cannot be ruled out that damage was inflicted to the protective layer of magnesium fluoride during implantation. Possible early cracks in the coating could have been starting points for the corrosion. After all, the magnesium structure was greatly bent during the implantation to adapt it to the cardiac geometry. This deformation may have also caused cracks in the magnesium fluoride layer and additionally posed an enormous strain on the magnesium grid [
29].
About one litre of hydrogen gas is produced as a degradation product in the corrosion of one gram of magnesium. In the present study, irregular accumulations of small bubbles could not be seen on the explants. The gas formation may pose a serious problem in orthopaedic implants made of magnesium because the implants are naturally fit in the smallest possible bone channels or boreholes with not enough room for the gas [
30]. Against this, gas formation does not limit the epicardial fixation of magnesium structures in our experiment. After all, the pericardial sac and the mediastinum offer enough space for the regional distribution of gas volumes resulting over a long period of corrosion [
9]. According to Kuhlmann et al., there is an exchange with the gases of the surrounding tissue, such as nitrogen gas, oxygen, and carbon dioxide, immediately after the formation of hydrogen gas [
31]. This diffusion reduces the concentration of hydrogen gas and any associated toxic effects on the adjacent cardiac tissue, which could be confirmed in the present as well as in the previous study [
9].
Toxic effects may also result from the degradation products of the magnesium alloy and its coating. In the present study, we used a magnesium alloy with lithium and aluminium (LA63) which was coated with magnesium fluoride (MgF
2). Magnesium itself is ubiquitously present in the body and is a co-factor and an important component of intracellular enzymatic reactions. The magnesium fluoride coating of the magnesium alloy is supposed to slow down and homogenise the corrosion [
32,
33,
34]. Fluoride is a physiologically occurring element in the body that has undisputedly good biocompatibility and is required, for example, for building bone structure. Lithium is used in magnesium alloys to alkalise the corrosion layer and thus slow down degradation [
30]. Aluminium, in the alloy with magnesium, forms a phase and a protective aluminium trioxide layer (Al
2O
3) on the alloy surface [
35], which, according to Pardo et al., has an anticorrosive effect [
36]. In addition, in proportions of up to 6 wt%, aluminium provides the magnesium alloy with higher mechanical stability, as Zheng et al. have summarised [
35]. Both lithium and aluminium, which, as components of drugs, are also ingested, only have adverse effects in high concentrations. For example, Feyerabend et al. found that lithium and aluminium have toxic effects on perivascular cells at a concentration of about 1000 µM [
37]. El Rahman et al. were able to show the neuropathological effects of accumulated aluminium in terms of Alzheimer’s disease in a rat model [
38]. However, the implanted magnesium scaffold in this study did not have a sufficiently toxic amount of lithium nor aluminium. Magnesium hydroxide, the main degradation product of the magnesium alloy LA63, first forms at the surface of the magnesium structure before it dissolves inside the tissue. Janning et al. confirmed, however, that the solubility of this compound is exceptionally low. Thus, there is also only a very slow precipitation in tissues with resulting biologically well-tolerated concentrations [
39]. A cellular granulation tissue as part of the foreign body response to the implanted magnesium, as seen in our study, additionally reduced a possible toxic effect of the metallic implant or its degradation products by forming a barrier against the surrounding tissues. Thus, the remnants of the magnesium structure or solid degradation products could not be detected macroscopically in the explanted hearts in this work.
Limitations
Due to the number of animals used in this study, no statement of statistical significance can be made here. Merely the principle approach and the feasibility of this innovative method for the treatment of advanced heart failure can be shown in this pilot study. Besides this, the state of the stabilising scaffold after just a short period in the respective experimental groups is unknown, since μ-CT scans have to be performed on the explant. The computed tomography cannot enable a clear differentiation between the original magnesium alloy and the corrosion products. The swelling of the originally flat and rather angular arms of the meander pattern can be explained by degradation products, mainly magnesium hydroxide. Therefore, the calculated volumes of the residual magnesium structures may still be overestimated six months after implantation.