Migration, Distribution, and Crystallization of NaCl and Na2SO4 Solutions in Three Different Media
Abstract
:1. Introduction
2. Materials, Methods, and Experiments
2.1. Materials
2.2. Methods
2.2.1. Ultrafine Three-Dimensional Microscopic Analysis
2.2.2. Polarized Light Microscopic Analysis
2.2.3. Low-Field Nuclear Magnetic Resonance (Low-Field NMR)
2.2.4. Automatic High-Speed X-Ray Microtomography System (Micro-CT)
2.3. Experiments
3. Results and Analysis
3.1. Morphologies of Salt Crystals on the Nanofibrous Membrane
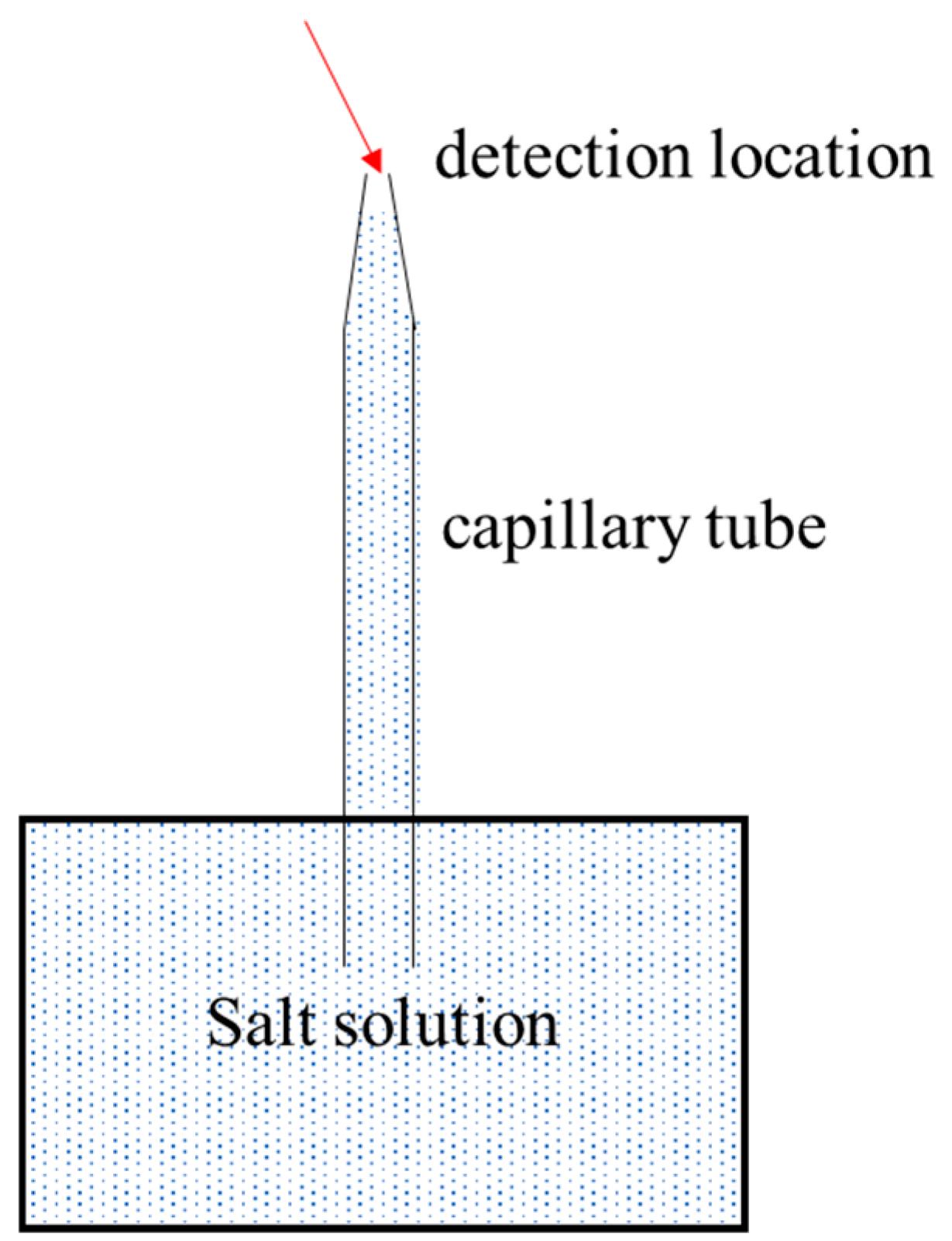
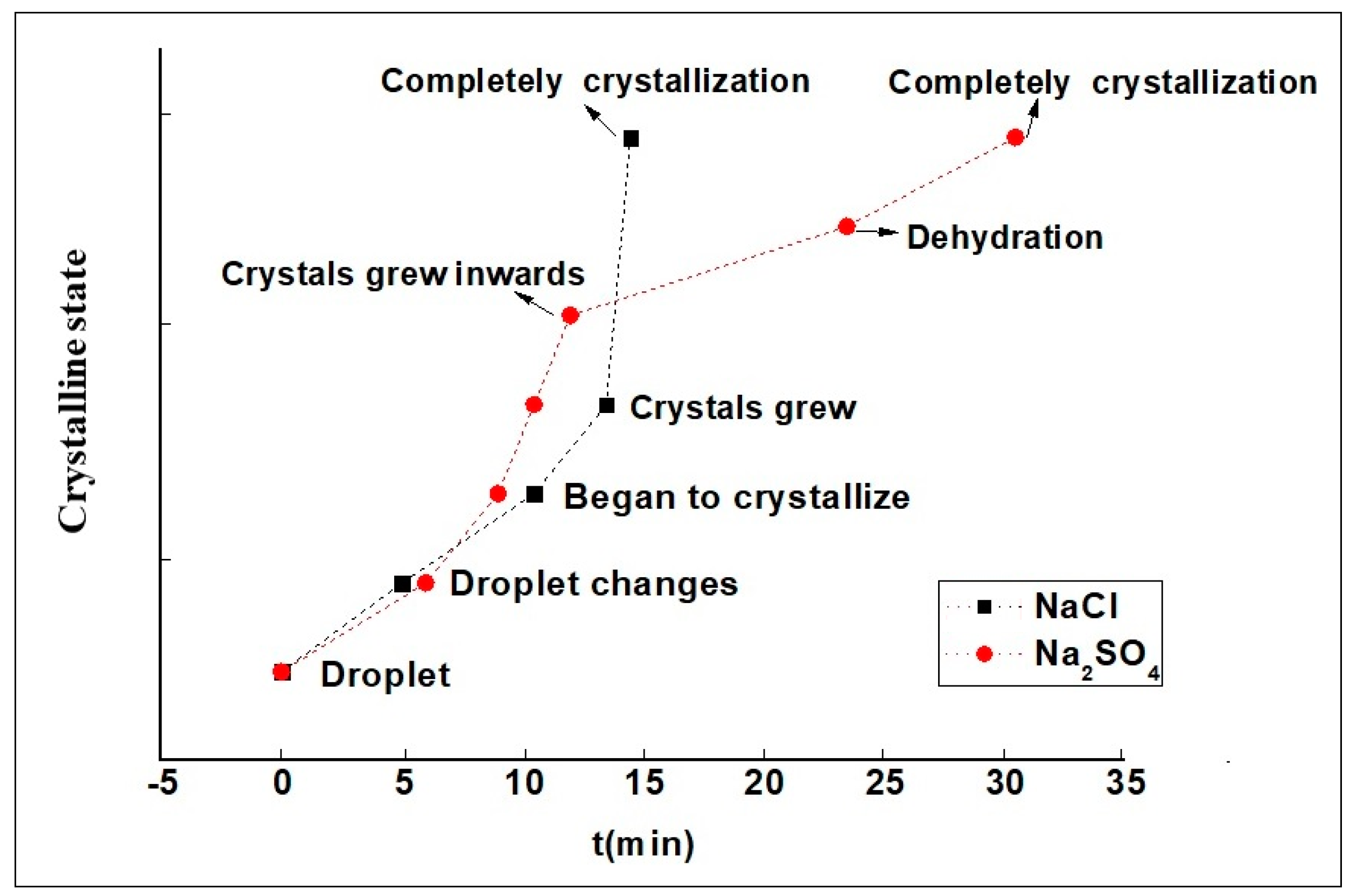
3.2. Migration Rate and Crystal Morphology of Salt Solutions in Capillary Tubes
3.3. Migration, Distribution, and Crystallization of Salt Solutions in Silicate Media
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rörig-Dalgaard, I. Development of a poultice for electrochemical desalination of porous building materials: Desalination effect and pH changes. Mater. Struct. 2013, 46, 959–970. [Google Scholar] [CrossRef]
- Francesco, C.; Timothy, W.; Flatt, R.J. Easy illustration of salt damage in stone. J. Chem. Educ. 2018, 95, 1615–1620. [Google Scholar]
- Vázquez, P.; Luque, A.; Alonso, F.J. Surface changes on crystalline stones due to salt crystallization. Environ. Earth Sci. 2013, 69, 1237–1248. [Google Scholar] [CrossRef]
- Jiang, X. Study on Capillary Transport Mechanism of Salts in Murals. Master’s Thesis, Lanzhou University, Lanzhou, China, 2014. [Google Scholar]
- Ma, Z.P.; Huang, J.Z. Chemical weathering of carbonate cement in sandstone and the related cultural relic diseases in Yungang grottoes. Carsologica Sinica 2005, 24, 71–76. [Google Scholar]
- Yan, S.J.; Fang, J.; Liu, J.H. Deterioration experiment with soluble salt on sandstone of Yungang grottoes and its model creation. Rock Soil Mech. 2013, 34, 3410–3416. [Google Scholar]
- Espinosa-Marzal, R.M.; Scherer, G.W. Advances in understanding damage by salt crystallization. Acc. Chem. Res. 2010, 43, 897–905. [Google Scholar] [CrossRef]
- Espinosa-Marzal, R.M.; Scherer, G.W. Impact of in-pore salt crystallization on transport properties. Environ. Earth Sci. 2013, 69, 2657–2669. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Navarro, C.; Linares-Fernandez, L.; Doehne, E. Effects of ferrocyanide ions on NaCl crystallization in porous stone. J. Cryst. Growth 2002, 243, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Veran-Tissoires, S.; Marcoux, M.; Prat, M. Salt crystallization at the surface of a heterogeneous porous medium. Europhys. Lett. 2012, 98, 1–5. [Google Scholar] [CrossRef]
- Webb, M.B.; Garofalini, S.H.; Scherer, G.W. Molecular dynamics investigation of solution structure between NaCl and quartz crystals. J. Phys. Chem. C 2011, 115, 19724–19732. [Google Scholar] [CrossRef]
- Webb, M.B.; Garofalini, S.H.; Scherer, G.W. Use of a dissociative potential to simulate hydration of Na+ and Cl− ions. J. Phys. Chem. B 2009, 113, 9886–9893. [Google Scholar] [CrossRef] [PubMed]
- Bonn, N.S.; Rafaı, S.; Bonn, D. Salt crystallization during evaporation: Impact of interfacial properties. Langmuir 2008, 24, 8599–8605. [Google Scholar] [CrossRef] [PubMed]
- Tamerlan, A.S.; Pel, L.; Kopinga, K. Crystallization pressure of sodium sulfate heptahydrate. Cryst. Growth Des. 2015, 15, 2087–2093. [Google Scholar]
- Désarnaud, J.; Grauby, O.; Bromblet, P.; Vallet, J.-M.; Baronnet, A. Growth and dissolution of crystal under load: New experimental results on KCl. Cryst Growth Des. 2013, 13, 1067–1074. [Google Scholar] [CrossRef]
- Flatt, R.J.; Steiger, M.; Scherer, G.W. A commented translation of the paper by C.W. Correns and W. Steinborn on crystallization pressure. Environ. Geol. 2007, 52, 187–203. [Google Scholar] [CrossRef]
- Steiger, M. Crystal growth in porous materials I: The crystallization pressure of large crystals. J. Cryst. Growth 2005, 282, 455–469. [Google Scholar] [CrossRef]
- Steiger, M. Crystal growth in porous materials II: Influence of crystal size on the crystallization pressure. J. Cryst. Growth 2005, 282, 470–481. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Desarnaud, J.; Derluyn, H.; Carmeliet, J.; Bonn, D.; Shahidzadeh, N. Hopper growth of salt crystals. J. Phys. Chem. Lett. 2018, 9, 2961–2966. [Google Scholar] [CrossRef]
- Xu, S.; Simmons, G.C.; Mahadevan, T.; Scherer, G.W.; Garofalini, S.H.; Pacheco, C. Transport of water in small pores. Langmuir 2009, 25, 5084–5090. [Google Scholar] [CrossRef]
- Rijniers, L.A.; Pel, L.; Huinink, H.P.; Kopinga, K. Salt crystallization as damage mechanism in porous building materials: A nuclear magnetic resonance study. Magn. Reson. Imag. 2005, 23, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Koniorczyk, M.; Gawin, D. Modelling of salt crystallization in building materials with microstructure-Poromechanical approach. Constr. Build. Mater. 2012, 36, 860–873. [Google Scholar] [CrossRef]
- Mucha, V.M.; Jungwirth, P. Salt crystallization from an evaporating aqueous solution by molecular dynamics simulations. J. Phys. Chem. B 2003, 33, 8271–8274. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, H.J.; Wang, L.Q. Process of salt crystallization in NaCl solution at efflorescence pottery. Sci. Sin. Technol. 2016, 46, 1–8. [Google Scholar]
- Zhao, J.; Luo, H.J. Transport and crystallization of NaCl solution in porous silicate materials. J. Cryst. Growth 2019, 519, 25–34. [Google Scholar] [CrossRef]
- Sun, C.; Xue, D. IR Spectral study of mesoscale process during urea crystallization from aqueous solution. Cryst. Growth Des. 2015, 15, 2867–2873. [Google Scholar] [CrossRef]
- Sun, C.; Xue, D. In situ IR spectral identification of NH4H2PO4 structural evolution during crystallization in water–ethanol mixed solvent. CrystEngComm 2015, 17, 2728–2736. [Google Scholar] [CrossRef]
- Sun, C.; Xue, D. Chemical bonding theory of single crystal growth and its application to crystal growth and design. Cryst. Eng. Comm. 2016, 18, 1262–1272. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, H.J.; Huang, X. Preliminary analysis of crystallization of Na2SO4 solution in silicate cultural relics. Stud. Conserv. 2019. [Google Scholar] [CrossRef]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Luo, H.; Huang, X. Migration, Distribution, and Crystallization of NaCl and Na2SO4 Solutions in Three Different Media. Crystals 2020, 10, 444. https://doi.org/10.3390/cryst10060444
Zhao J, Luo H, Huang X. Migration, Distribution, and Crystallization of NaCl and Na2SO4 Solutions in Three Different Media. Crystals. 2020; 10(6):444. https://doi.org/10.3390/cryst10060444
Chicago/Turabian StyleZhao, Jing, Hongjie Luo, and Xiao Huang. 2020. "Migration, Distribution, and Crystallization of NaCl and Na2SO4 Solutions in Three Different Media" Crystals 10, no. 6: 444. https://doi.org/10.3390/cryst10060444
APA StyleZhao, J., Luo, H., & Huang, X. (2020). Migration, Distribution, and Crystallization of NaCl and Na2SO4 Solutions in Three Different Media. Crystals, 10(6), 444. https://doi.org/10.3390/cryst10060444





