Quaternary Misfit Compounds—A Concise Review
Abstract
1. Introduction
2. Synthesis and Structural Elucidation of Nanotubes from Quaternary MLC
2.1. Prelude
2.2. Synthesis
2.3. Sr-Substituted LaS-TaS2 Nanotubes
2.4. Nb-Substituted LaS-TaS2 Nanotubes
2.5. Nanotubes from Mixed Sulfur and Selenium MLC
2.6. Quaternary LnxLa(1−x)S-TaS2 Nanotubes (Ln = Pr, Sm, Ho, and Yb)
2.7. MLC Nanotubes from Alloys of Yttrium and Lanthanum
3. Films and Bulk Quaternary MLC
3.1. Ferecrystals from the Modulated Elemental Reactants Technique
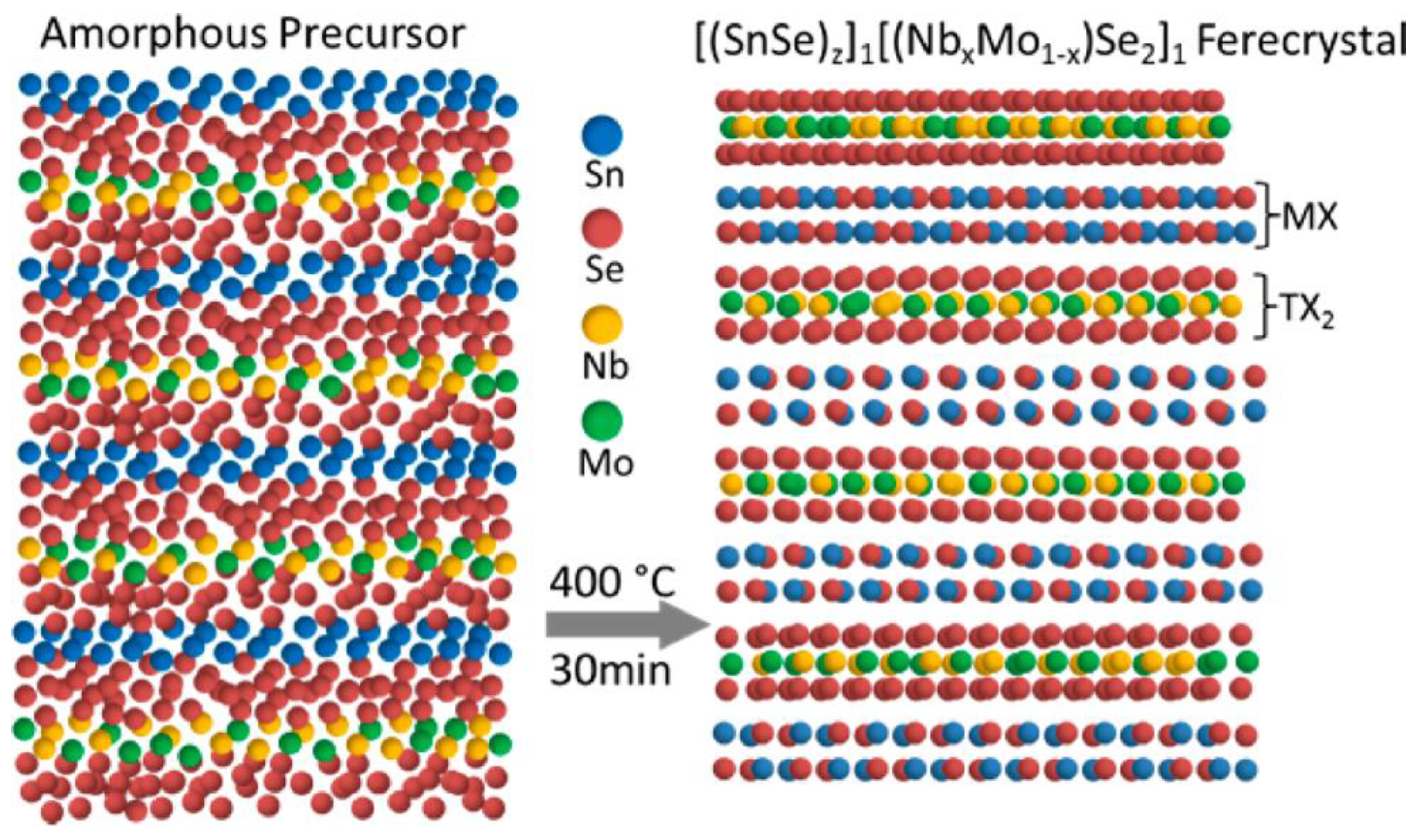
3.2. (SnSe)1.16−1.09(NbxMo1−x)Se2 Ferecrystal Films
3.3. Bulk Quaternary MLC of Chalcogenides
3.4. Quaternary MLC Cobaltites
4. Applications
4.1. Thermoelectric Properties
4.2. Rechargeable Ca-Ion Batteries
4.3. Electronic and Optoelectronics Properties
4.4. Electrochemistry and Catalysis
4.5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Lorenz, T.; Joswig, J.; Seifert, G. Two−dimensional and tubular structures of misfit compounds: Structural and electronic properties. Beilstein J. Nanotechnol. 2014, 5, 2171–2178. [Google Scholar] [CrossRef]
- Panchakarla, L.S.; Radovsky, G.; Houben, L.; Popovitz-Biro, R.; Dunin-Borkowski, R.E.; Tenne, R. Nanotubes from Misfit Layered Compounds: A New Family of Materials with Low Dimensionality. J. Phys. Chem. Lett. 2014, 5, 3724–3736. [Google Scholar] [CrossRef]
- Wiegers, G.A. Misfit Layered Compounds: Structures and Physical Properties. Prog. Solid State Chem. 1996, 24, 1–139. [Google Scholar] [CrossRef]
- Makovicky, E.; Hyde, B.G. Non-Commensurate (Misfit) Layer Structures. In Inorganic Chemistry; Structure and Bonding; Series Springer: Heidelberg, Germany, 1981; Volume 46, p. 101. [Google Scholar]
- Wiegers, G.A.; Meerschaut, A. Incommensurate Sandwiched Layered Compounds. In Materials Science Forum; Meerschaut, A., Ed.; Trans Tech Publications: Pfaffikon, Switzerland, 1992; Volume 100–101, pp. 101–172. [Google Scholar]
- Gómez-Herrero, A.; Landa-Cánovas, A.R.; Hansen, S.; Otero-Díaz, L.C. Electron Microscopy Study of Tubular Crystals (BiS)1+δ(NbS2)n. Micron 2000, 31, 587–595. [Google Scholar] [CrossRef]
- Spiecker, E.; Garbrecht, M.; Jäger, W.; Tillmann, K. Advantages of Aberration Correction for HRTEM Investigation of Complex Layer Compounds. J. Microsc. 2010, 237, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bernaerts, D.; Amelinckx, S.; Van Tendeloo, G.; Van Landuyt, J. Microstructure and Formation Mechanism of Cylindrical and Conical Scrolls of the Misfit Layer Compounds PbNbnS2n+1. J. Cryst. Growth 1997, 172, 433–439. [Google Scholar] [CrossRef]
- Kato, K.; Kawada, I.; Takahashi, T. Die Kristallstruktur von LaCrS3. Acta Cryst. B 1977, 33, 3437–3443. [Google Scholar] [CrossRef]
- Boullay, P.; Domengès, B.; Hervieu, M.; Groult, D.; Raveau, B. Evidence for the First Misfit Layer Oxide Tl0.41(Sr0.9O)1.12CoO2. Chem. Mater. 1996, 8, 1482–1489. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Kudo, K.; Akoshima, M.; Ono, Y.; Koike, Y.; Kajitani, T. Low-Temperature Thermoelectric Properties of the Composite Crystal [Ca2CoO3.34]0.614[CoO2]. Jpn. J. Appl. Phys. 2000, 39, L531–L533. [Google Scholar] [CrossRef]
- Karppinen, M.; Fjellvag, H.; Konno, T.; Morita, Y.; Motohashi, T.; Yamauchi, H. Evidence for Oxygen Vacancies in Misfit-Layered Calcium Cobalt Oxide, [CoCa2O3]qCoO2. Chem. Mater. 2004, 16, 2790–2793. [Google Scholar] [CrossRef]
- Panchakarla, L.S.; Lajaunie, L.; Ramasubramaniam, A.; Arenal, R.; Tenne, R. Nanotubes from Oxide-Based Misfit Family: The Case of Calcium Cobalt Oxide. ACS Nano 2016, 10, 6248–6256. [Google Scholar] [CrossRef]
- Limelette, P.; Hebert, S.; Hardy, V.; Frésard, R.; Simon, C.; Maignan, A. Scaling Behavior in Thermoelectric Misfit Cobalt Oxides. Phys. Rev. Lett. 2006, 97, 046601–046605. [Google Scholar] [CrossRef]
- Butt, S.; Liu, Y.-C.; Lan, J.-L.; Shehzad, K.; Zhan, B.; Lin, Y.; Nan, C.-W. High−Temperature Thermoelectric Properties of La and Fe Co-Doped Ca-Co-O Misfit-Layered Cobaltites Consolidated by Spark Plasma Sintering. J. Alloys Compd. 2014, 588, 277–283. [Google Scholar] [CrossRef]
- Henriksen, R.B.; Makovicky, E.; Stipp, S.L.S.; Nissen, C.; Eggleston, C.M. Atomic-Scale Observations of Franckeite Surface Morphology. Am. Mineral. 2002, 87, 1273–1278. [Google Scholar] [CrossRef]
- Bengel, H.; Jobic, S.; Moëlo, Y.; Lafond, A.; Rouxel, J.; Seo, D.-K.; Whangbo, M.-H. Distribution of the Pb and Sb Atoms in the (Pb,Sb)S Layers of the Franckeite-Type Misfit Compound [(Pb, Sb)S]2.28NbS2 Examined by Scanning Tunneling and Atomic Force Microscopy. J. Solid State Chem. 2000, 149, 370–377. [Google Scholar] [CrossRef]
- Tenne, R.; Margulis, L.; Genut, M.; Hodes, G. Polyhedral and cylindrical structures of tungsten disulphide. Nature 1992, 360, 444–446. [Google Scholar] [CrossRef]
- Margulis, L.; Salitra, G.; Tenne, R.; Talianker, M. Nested Fullerene-Like Structures. Nature 1993, 365, 113–114. [Google Scholar] [CrossRef]
- Feldman, Y.; Wasserman, E.; Srolovitz, D.J.; Tenne, R. High-Rate, Gas-Phase Growth of MoS2 Nested Inorganic Fullerenes and Nanotubes. Science 1995, 267, 222–225. [Google Scholar] [CrossRef]
- Hong, S.Y.; Popovitz-Biro, R.; Prior, Y.; Tenne, R. Synthesis of SnS2/SnS Fullerene-Like Nanoparticles: A Superlattice with Polyhedral Shape. J. Am. Chem. Soc. 2003, 125, 10470–10474. [Google Scholar] [CrossRef]
- Radovsky, G.; Popovitz-Biro, R.; Staiger, M.; Gartsman, K.; Thomsen, C.; Lorenz, T.; Seifert, G.; Tenne, R. Synthesis of Copious Amounts of SnS2 and SnS2/SnS Nanotubes with Ordered Superstructures. Angew. Chem. Int. Ed. 2020, 50, 12316–12320. [Google Scholar] [CrossRef]
- Ohno, Y. Lamellar and Filament-Like Crystals of Misfit-Layer Compounds Containing (Sm, Ta, S) and (Pb, Bi, Nb, S) Elements. J. Solid State Chem. 2005, 178, 1539–1550. [Google Scholar] [CrossRef]
- Radovsky, G.; Popovitz-Biro, R.; Lorenz, T.; Joswig, J.-O.; Seifert, G.; Houben, L.; Dunin-Borkowski, R.E.; Tenne, R. Tubular structures from the LnS-TaS2 (Ln = La, Ce, Nd, Ho, Er) and LaSe-TaSe2 misfit layered compounds. J. Mater. Chem. C 2016, 4, 89–98. [Google Scholar] [CrossRef]
- Cario, L.; Johrendt, D.; Lafond, A.; Felser, C.; Meerschaut, A.; Rouxel, J. Stability and Charge Transfer in the Misfit Compound (LaS)(SrS)0.2CrS2: Ab Initio Band-Structure Calculations. Phys. Rev. B 1997, 55, 9409–9414. [Google Scholar] [CrossRef]
- Nishikawa, T.; Yasui, Y.; Kobayashi, Y.; Sato, M. Studies of the Metal-Insulator Transition of Misfit-Layer Compounds La1.17−xSrxVS3.17. Phys. C Supercond. 1996, 263, 554–557. [Google Scholar] [CrossRef]
- Serra, M.; Anumol, E.A.; Stolovas, D.; Pinkas, I.; Joselevich, E.; Tenne, R.; Enyashin, A.; Deepak, F.L. Synthesis and Characterization of Quaternary La(Sr)STaS2 Misfit-Layered Nanotubes. Beilstein J. Nanotechnol. 2019, 10, 1112–1124. [Google Scholar] [CrossRef]
- Stolovas, D.; Serra, M.; Popovitz-Biro, R.; Pinkas, I.; Houben, L.; Calvino, J.J.; Joselevich, E.; Tenne, R.; Arenal, R.; Lajaunie, L. Nanotubes from the Misfit Compound Alloy LaS-NbxTa(1−x)S2. Chem. Mater. 2018, 30, 8829–8842. [Google Scholar] [CrossRef]
- Lajaunie, L.; Radovsky, G.; Tenne, R.; Arenal, R. Quaternary Chalcogenide-Based Misfit Nanotubes LnS(Se)-TaS(Se)2 (Ln = La, Ce, Nd, and Ho): Synthesis and Atomic Structural Studies. Inorg. Chem. 2017, 57, 747–753. [Google Scholar] [CrossRef]
- Serra, M.; Lajaunie, L.; Sreedhara, M.B.; Miroshnikov, Y.; Pinkas, I.; Calvino, J.J.; Enyashin, A.N.; Tenne, R. Quaternary LnxLa(1−x)S-TaS2 Nanotubes (Ln = Pr, Sm, Ho, and Yb) as a Vehicle for Improving the yield of misfit Nanotubes. Appl. Mater. Today 2020, 19, 100581. [Google Scholar] [CrossRef]
- Hettler, S.; Sreedhara, M.B.; Serra, M.; Popovitz-Biro, R.; Pinkas, I.; Enyashin, A.N.; Tenne, R.; Arenal, R. YS-TaS2 and La1−xYxS-TaS2 (0 ≤ x ≤ 1) Nanotubes: A New Family of Misfit Layered Compounds. ACS Nano 2020. [Google Scholar] [CrossRef] [PubMed]
- Westover, R.D.; Atkins, R.A.; Ditto, J.J.; Johnson, D.C. Synthesis of [(SnSe)1.16−1.09]1[(NbxMo1−x)Se2]1 Ferecrystal Alloys. Chem. Mater. 2014, 26, 3443–3449. [Google Scholar] [CrossRef]
- Varadé-López, R.; Gómez-Herrero, A.; Ávila-Brande, D.; Otero-Díaz, L.C. Synthesis and Electron Microscopy Study of the Quaternary Misfit Layer Chalcogenides (Bi,Nd)S1+δCrS2 and (Pb,Nd)Se1+δ(NbSe2)2. Inorg. Chem. 2020, 59, 4508–4516. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, S.M.; Young, D.P.; Cava, R.J.; Jin, R.; Liu, Y.; Bordet, P.; Qin, Y.; Zandbergen, H.; Godinho, M.; Núñez-Regueiro, M.; et al. Enhancement of metallic behavior in bismuth cobaltates through lead doping. Phys. Rev. B 2001, 63, 094109–0941013. [Google Scholar] [CrossRef]
- Maki, M.; Machida, K.-I.; Mori, T.; Nishizaki, T.; Kobayashi, N. In-plane conduction and c-axis polarization in the misfit-layered oxide [Bi2Ca2O4]qCoO2. Phys. Rev. B 2008, 78, 073101–073106. [Google Scholar] [CrossRef]
- Yin, C.; Hu, Q.; Tang, M.; Liu, H.; Chen, Z.; Wang, Z.; Ang, R. Boosting the thermoelectric performance of misfit−layered (SnS)1.2(TiS2)2 by a Co- and Cu-substituted alloying effect. J. Mater. Chem. A 2018, 6, 22909–22914. [Google Scholar] [CrossRef]
- Hernan, L.; Morales, J.; Sanchez, L.; Tirado, J.L. Electrochemical Intercalation of Sodium into PbNbS3 and PbNb2S5 Misfit Layer Compounds. Solid State Ion. 1992, 58, 179–184. [Google Scholar] [CrossRef]
- Miao, X.; Zhou, S.; Wu, L.; Zhao, J.; Shi, L. Spin-State Transition Enhanced Oxygen Evolving Activity in Misfit-Layered Cobalt Oxide Nanosheets. ACS Sustain. Chem. Eng. 2018, 6, 12337–12342. [Google Scholar] [CrossRef]
- Wiegers, G.A.; Meetsma, A.; van Smaalen, S.; Haange, R.J.; Wulff, J.; Zeinstra, T.; de Boer, J.L.; Kuypers, S.; Van Tendeloo, G.; Van Landuyt, J.; et al. Misfit Layer Compounds (MS)nTS2 (M = Sn, Pb, Bi, Rare Earth Elements; T = Nb, Ta; n = 1.08–1.19), a New Class of Layer Compounds. Solid State Commun. 1989, 70, 409–413. [Google Scholar] [CrossRef]
- Svetin, D.; Vaskivskyi, I.; Brazovskii, S.; Mihailovic, D. Three-dimensional resistivity and switching between correlated electronic states in 1T-TaS2. Sci. Rep. 2017, 7, 46048–46052. [Google Scholar] [CrossRef]
- Kisoda, K.; Hangyo, M.; Nakashima, S.; Suzuki, K.; Enoki, T.; Ohno, Y. Raman scattering from misfit layer compounds (RS)xTaS2(R identical to La,Ce,Sm or Gd; S identical to sulphur; x approximately = 1.2). J. Phys. Condens. Matter. 1995, 7, 5383–5393. [Google Scholar] [CrossRef]
- Karecki, D.R.; Clayman, B.P. Lattice modes and charge density waves in 1T-TaS2. Solid State Commun. 1976, 19, 479–481. [Google Scholar] [CrossRef]
- Radovsky, G.; Popovitz-Biro, R.; Tenne, R. Nanotubes from the Misfit Layered Compounds MS-TaS2, where M= Pb, Sn, Sb and Bi: Synthesis and Study of their Structure. Chem. Mater. 2014, 26, 3757–3770. [Google Scholar] [CrossRef]
- Radovsky, G.; Popovitz-Biro, R.; Stroppa, D.G.; Houben, L.; Tenne, R. Nanotubes from Chalcogenide Misfit Compounds: Sn-S, Nb-Pb-S. Acc. Chem. Res. 2014, 47, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Stolovas, D.; Houben, L.; Popovitz-Biro, R.; Pinkas, I.; Kampmann, F.; Maultzsch, J.; Joselevich, E.; Tenne, R. Synthesis and Characterization of Nanotubes from Misfit (LnS)(1+y)TaS2 (Ln = Pr, Sm, Gd, Yb) Compounds. Chem. Eur. J. 2018, 24, 11354–11363. [Google Scholar] [CrossRef]
- Rabu, P.; Meerschaut, A.; Rouxel, J.; Wiegers, G.A. The Crystal Structure of the Misfit Layer Compound (YS)1.23NbS2. J. Solid State Chem. 1990, 88, 451–458. [Google Scholar] [CrossRef]
- Hangyo, M.; Nishio, T.; Nakashima, S.-I.; Ohno, Y.; Terashima, T.; Kojima, N. Raman and Infrared Spectra of Misfit Layer Compounds MNbS3 (M = Sn, Pb, La, Ce). Jpn. J. Appl. Phys. 1993, 32, 581–583. [Google Scholar] [CrossRef]
- Merrill, D.R.; Sutherland, D.R.; Ditto, J.; Bauers, S.R.; Falmbigl, M.; Medlin, D.L.; Johnson, D.C. Kinetically Controlled Site−Specific Substitutions in Higher-Order Heterostructures. Chem. Mater. 2015, 27, 4066–4072. [Google Scholar] [CrossRef]
- Westover, R.D.; Ditto, J.; Falmbigl, M.; Hay, Z.L.; Johnson, D.C. Synthesis and Characterization of Quaternary Monolayer Thick MoSe2/SnSe/NbSe2/SnSe Heterojunction Superlattices. Chem. Mater. 2015, 27, 6411–6417. [Google Scholar] [CrossRef]
- Falmbigl, M.; Hay, Z.; Ditto, J.; Mitchson, G.; Johnson, D.C. Modifying a Charge Density Wave Transition by Modulation Doping: Ferecrystalline Compounds (Sn1−xBixSe)1.15VSe2 with 0 ≤ x ≤ 0.66. J. Mater. Chem. C 2015, 3, 12308–12315. [Google Scholar] [CrossRef]
- Benjamin, A.; Trump, B.A.; Livi, K.J.T.; McQueen, T.M. The New Misfit Compound (BiSe)1.15(TiSe2)2 and the Role of Dimensionality in the Cux(BiSe)1+δ(TiSe2)n Series. J. Solid State Chem. 2014, 209, 6–12. [Google Scholar]
- Maki, M.; Takakura, S.-I.; Nishizaki, T.; Ichikawa, F. Microscopic adjustment of misfit strain and charge segregation in [Bi2Sr2O4]0.51CoO2. Phys. Rev. B 2015, 92, 165117. [Google Scholar] [CrossRef]
- Hebert, S.; Kobayashi, W.; Muguerra, H.; Brйard, Y.; Raghavendra, N.; Gascoin, F.; Guilmeau, E.; Maignan, A. From oxides to selenides and sulfides: The richness of the CdI2 type crystallographic structure for thermoelectric properties. Phys. Status Solidi B 2012, 210, 69–81. [Google Scholar] [CrossRef]
- Park, H.; Cui, Y.; Kim, S.; Vaughey, J.T.; Zapol, P. Ca Cobaltites as Potential Cathode Materials for Rechargeable Ca-Ion Batteries: Theory and Experiment. J. Phys. Chem. C 2020, 124, 5902–5909. [Google Scholar] [CrossRef]
- Wiegers, G.A.; Meerschaut, A. Misfit Layer Compounds (MS)nTS2 (M = Sn, Pb, Bi, Rare Earth Metals; T = Nb, Ta, Ti, V, Cr; 1.08 < n < 1.23): Structures and Physical Properties, Meerschaut, A. (Ed.). Mater. Sci. Forum 1992, 100–101, 101–172. [Google Scholar]
- Nader, A.; Lafond, A.; Briggs, A.; Meerschaut, A.; Roesky, R. Structural Characterization and Superconductivity in the Misfit Layer Compound (LaSe)1.14NbSe2. Synth. Met. 1998, 97, 147–150. [Google Scholar] [CrossRef]
- Lafond, L.; Nader, A.; Moëlo, Y.; Meerschaut, A.; Briggs, A.; Perrin, S.; Monceau, R.; Rouxel, J. X-Ray Structure Determination and Superconductivity of a New Layered Misfit Compound with a Franckeite-Like Stacking, [(Pb,Sb)S]2.28NbS2. J. Alloys Compd. 1997, 261, 114–122. [Google Scholar] [CrossRef]
- Vaskivskyi, I.; Gospodaric, J.; Brazovskii, S.; Svetin, D.; Sutar, P.; Goreshnik, E.; Mihailovic, I.A.; Mertelj, T.; Mihailovic, D. Controlling the Metal-to-Insulator Relaxation of the Metastable Hidden Quantum State in 1T-TaS2. Sci. Adv. 2015, 1, e1500168. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Sofer, Z.; Jankovsky, O.; Pumera, M. Misfit-Layered Bi1.85Sr2Co1.85O7.7−δ for the Hydrogen Evolution Reaction: Beyond van der Waals Heterostructures. ChemPhysChem 2015, 16, 769–774. [Google Scholar] [CrossRef]









| Compound | Precursor | Growth Technique | Morphology of the Quaternary MLC | Reference |
|---|---|---|---|---|
| Pb4.6Ag0.2Sn2.5Fe0.8Sb2S12.6 | Mineral-Franckeite | Mineral | Flakes | [16] |
| [(Pb, Sb)S]2.28NbS2 | Pb, Sb, Nb, S | Chemical vapor transport | Flakes | [17] |
| SrxLa(1−x)S-TaS2 | La, Sr, Ta, S | Chemical vapor transport | Nanotubes and flakes | [27] |
| LaS-NbxTa(1−x)S2 | La, Ta, Nb, S, TaCl5 | Chemical vapor transport | Nanotubes and flakes | [28] |
| LnS(Se)-TaS2(Se) | Ta, Ln = La, Ce, Nd, Ho; S, Se, and TaCl5 | Chemical vapor transport | Nanotubes and flakes | [29] |
| LnxLa1−xS-TaS2 | Ln = Pr, Sm, Ho, and Yb | Chemical vapor transport | Nanotubes and flakes | [30] |
| YxLa1−xS-TaS2 | La, Y, S, Ta | Chemical vapor transport | Nanotubes and flakes | [31] |
| (SnSe)1.16−1.09NbxMo1−xSe2 | Sn, Nb, Mo, Se | Compositionally modulated elemental reactants synthesis | Films | [32] |
| [(Bi0.4Nd0.6)S]1.25CrS2 and [Pb0.5Nd0.5Se]1.15(NbSe2)2 | Nd, Cr, S, Se, Bi2S3 | Chemical vapor transport | Flakes | [33] |
| Bi2M3Co2Oy with M = Ca, Sr, Ba | Bi2O3,CaCO3, Co3O4 | Flux synthesis | bulk | [34] |
| (Bi2Ca2O4)qCoO2 | Bi2O3,CaCO3, Co3O4 | Flux synthesis | bulk | [35] |
| (SnS)1.2(Co0.02Ti0.98S2)2 | Sn, Ti, S and Co | Chemical vapor transport | bulk | [36] |
| Na: PbNbS3; PbNb2S5 | Na, Pb, Nb, S | Electrochemical intercalation | bulk | [37] |
| Bi2Sr2Co2O8+δ | Bi, Sr, Co, O | Exfoliation of bulk crystal | Exfoliated sheets | [38] |
| Sample | LaTaS320 | (Pr,La)TaS3 | (Sm,La)TaS3 | (Ho,La)TaS3 | (Yb,La)TaS3 |
|---|---|---|---|---|---|
| Yield (%) | 5020,37 | 55 (7)37 | 88 (59)37 | 97 (5)37 | 37 (1)37 |
| Ln (at.%) | - | 11.7 | 13.3 | 4.4 | 10.0 |
| La (at.%) | - | 7.1 | 8.6 | 21.4 | 13.0 |
| Ta (at.%) | - | 18.8 | 22.3 | 22.1 | 23.3 |
| S (at.%) | - | 62.5 | 55.8 | 51.1 | 53.7 |
| Ln/(La + Ln) (%) | - | 62 | 61 | 16 | 43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliev, S.B.; Tenne, R. Quaternary Misfit Compounds—A Concise Review. Crystals 2020, 10, 468. https://doi.org/10.3390/cryst10060468
Aliev SB, Tenne R. Quaternary Misfit Compounds—A Concise Review. Crystals. 2020; 10(6):468. https://doi.org/10.3390/cryst10060468
Chicago/Turabian StyleAliev, Sokhrab B., and Reshef Tenne. 2020. "Quaternary Misfit Compounds—A Concise Review" Crystals 10, no. 6: 468. https://doi.org/10.3390/cryst10060468
APA StyleAliev, S. B., & Tenne, R. (2020). Quaternary Misfit Compounds—A Concise Review. Crystals, 10(6), 468. https://doi.org/10.3390/cryst10060468






