A Survey of Supramolecular Aggregation Based on Main Group Element⋯Selenium Secondary Bonding Interactions—A Survey of the Crystallographic Literature
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Tin Compounds Featuring Sn⋯Se Interactions in their Crystals
3.2. Lead Compounds Featuring Pb⋯Se Interactions in Their Crystals
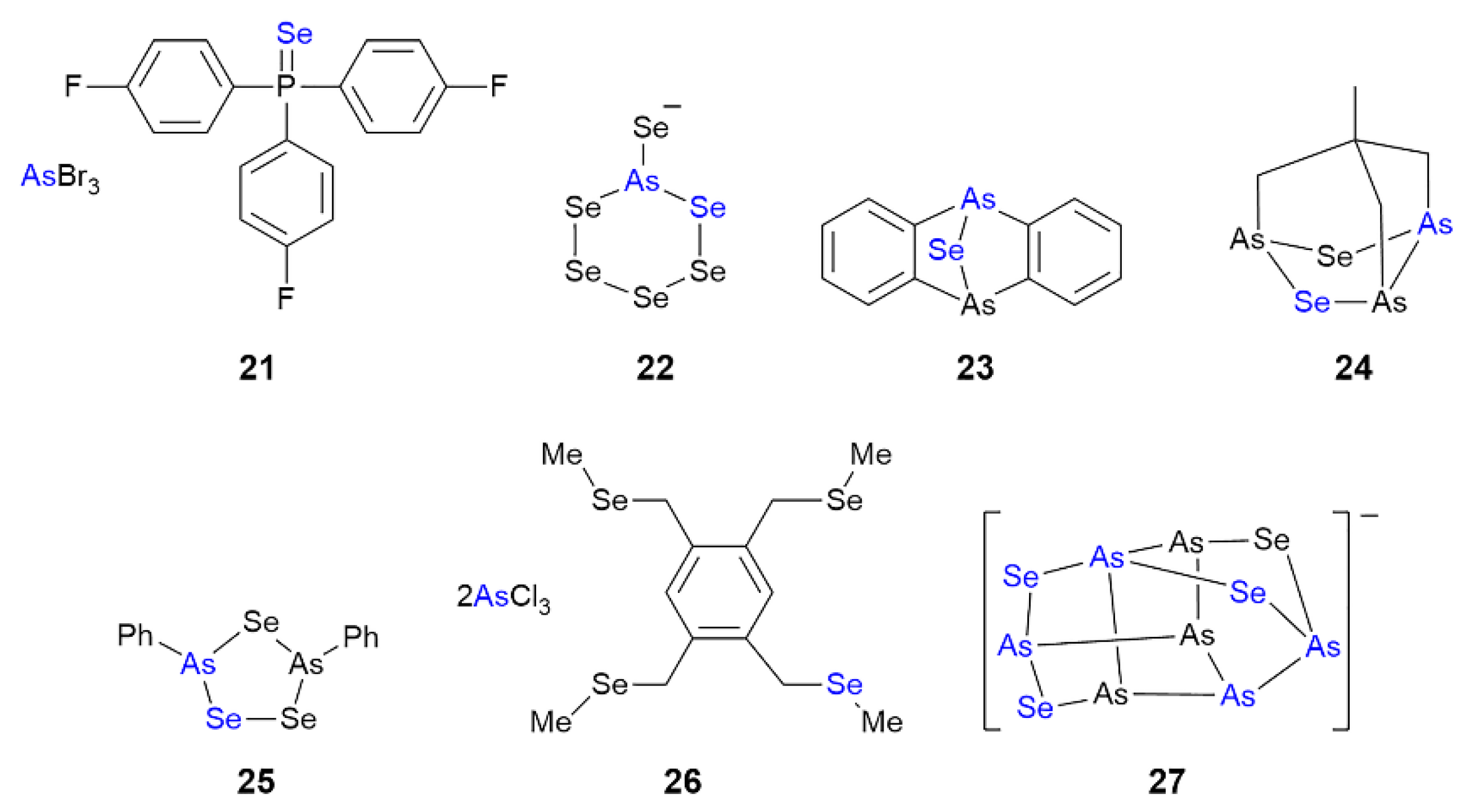
3.3. Arsenic Compounds Featuring As⋯Se Interactions in their Crystals
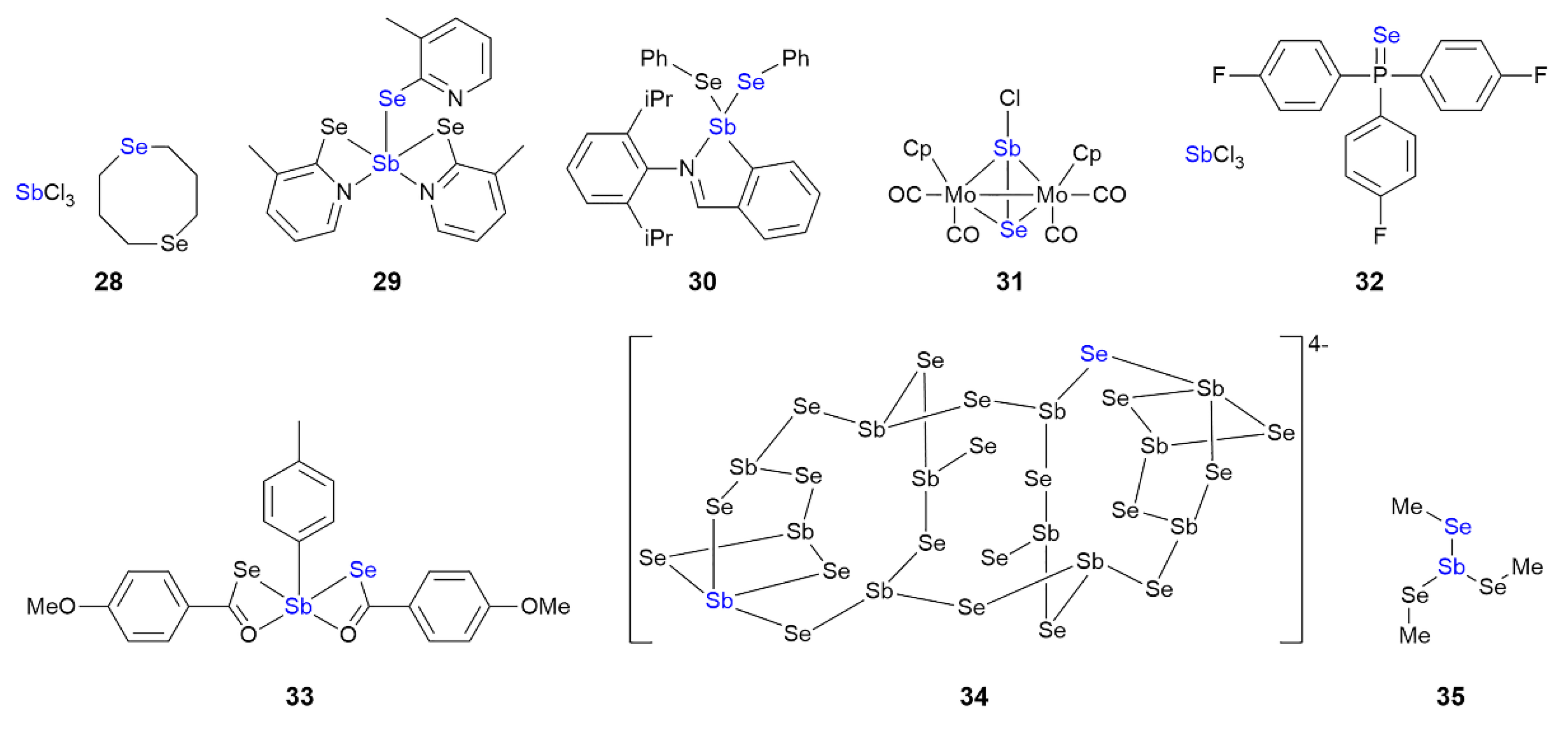
3.4. Antimony Compounds Featuring Sb⋯Se Interactions in their Crystals
3.5. Bismuth Compounds Featuring Bi⋯Se Interactions in their Crystals
3.6. Tellurium Compounds Featuring Te⋯Se Interactions in Their Crystals
4. Discussion and Outlook
Funding
Conflicts of Interest
References
- Minyaev, R.M.; Minkin, V.I. Theoretical study of O - > X (S, Se, Te) coordination in organic compounds. Can. J. Chem. 1998, 76, 776–778. [Google Scholar] [CrossRef]
- Wang, W.; Ji, B.; Zhang, Y. Chalcogen bond: A sister noncovalent bond to halogen bond. J. Phys. Chem. A 2009, 113, 8132–8135. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Frontera, A. Not only hydrogen bonds: Other noncovalent interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Bent, H.A. Structural chemistry of donor-acceptor interactions. Chem. Rev. 1968, 68, 587–648. [Google Scholar] [CrossRef]
- Hassel, O. Structural Aspects of interatomic charge-transfer bonding. Science 1970, 170, 497–502. [Google Scholar] [CrossRef]
- Alcock, N.W. Secondary bonding to nonmetallic elements. Adv. Inorg. Chem. Radiochem. 1972, 15, 1–58. [Google Scholar] [CrossRef]
- Llaguno, E.C.; Paul, I.C. Crystal structure of a [1,2,5]oxaselenazolo[2,3-b][1,2,5]oxaselenazole-7-SeIV: A molecule with ‘short’ intramolecular Se…O distances, or ‘long’ Se–O bonds. J. Chem. Soc. Perkin Trans. 1972, 2, 2001–2006. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Clark, T.; Politzer, P. σ-hole bonding: Molecules containing group VI atoms. J. Mol. Model. 2007, 13, 1033–1038. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. The bright future of unconventional σ/π-hole interactions. ChemPhysChem 2015, 16, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.H.; Hobza, P. Computer modeling of halogen bonds and other σ-hole interactions. Chem. Rev. 2016, 116, 5155–5187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Politzer, P.; Murray, J.S. σ-Hole interactions: Perspectives and misconceptions. Crystals 2017, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Tiekink, E.R.T. Molecular architecture and supramolecular association in the zinc-triad 1,1-dithiolates. Steric control as a design element in crystal engineering? CrystEngComm 2003, 5, 101–113. [Google Scholar] [CrossRef]
- Lai, C.S.; Tiekink, E.R.T. Structural diversity in the mercury(II) bis(N,N-dialkyldithiocarbamate) compounds: An example of the importance of considering crystal structure when rationalising molecular structure. Z. Kristallogr. Cryst. Mater. 2007, 222, 532–538. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Exploring the topological landscape exhibited by binary zinc-triad 1,1-dithiolates. Crystals 2018, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Buntine, M.A.; Kosovel, F.J.; Tiekink, E.R.T. Supramolecular Sn⋯Cl associations in diorganotin dichlorides and their influence on molecular geometry as studied by ab initio molecular orbital calculations. CrystEngComm 2003, 5, 331–336. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Tin dithiocarbamates: Applications and structures. Appl. Organomet. Chem. 2008, 22, 533–550. [Google Scholar] [CrossRef]
- Liu, Y.; Tiekink, E.R.T. Supramolecular associations in binary antimony(III) dithiocarbamates: Influence of ligand steric bulk, influence on coordination geometry, and competition with hydrogenbonding. CrystEngComm 2005, 7, 20–27. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Aggregation patterns in the crystal structures of organometallic Group XV 1,1-dithiolates: The influence of the Lewis acidity of the central atom, metal- and ligand-bound steric bulk, and coordination potential of the 1,1-dithiolate ligands upon supramolecular architecture. CrystEngComm 2006, 8, 104–118. [Google Scholar] [CrossRef]
- Lee, S.M.; Heard, P.J.; Tiekink, E.R.T. Molecular and supramolecular chemistry of mono- and di-selenium analogues of metal dithiocarbamates. Coord. Chem. Rev. 2018, 375, 410–423. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Zukerman-Schpector, J. Stereochemical activity of lone pairs of electrons and supramolecular aggregation patterns based on secondary interactions involving tellurium in its 1,1-dithiolate structures. Coord. Chem. Rev. 2010, 254, 46–76. [Google Scholar] [CrossRef]
- Taylor, R.; Wood, P.A. A million crystal structures: The whole is greater than the sum of its parts. Chem. Rev. 2019, 119, 9427–9477. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2002, 58, 389–397. [Google Scholar] [CrossRef]
- DIAMOND, Version 3.2k; K. Brandenburg & M. Berndt GbR: Bonn, Germany, 2006.
- Rinn, N.; Euβner, J.P.; Kaschuba, W.; Xie, X.; Dehnen, S. Formation and reactivity of organo-functionalized tin selenide clusters. Chem. Eur. J. 2016, 22, 3094–3104. [Google Scholar] [CrossRef]
- Krautscheid, H.; Schmidtke, M. [NaSn12O8Se6]3−—ein chalkogenostannatanion mit schalenförmigem Aufbau. Z. Anorg. Allg. Chem. 2002, 628, 913–914. [Google Scholar] [CrossRef]
- Dehnen, S.; Hanau, K.; Rinn, N.; Argentari, M. Organotin selenide clusters and hybrid capsules. Chem. Eur. J. 2018, 24, 11711–11716. [Google Scholar] [CrossRef]
- Metta-Magaña, A.J.; Lopez-Cardoso, M.; Vargas, G.; Pannell, K.H. Major distinctions in the molecular and supramolecular structures of selenium-containing organotins, (o-MeSe-C6H4CH2)SnPh3–nCln (n = 0, 1, 2). Z. Anorg. Allg. Chem. 2012, 638, 1677–1682. [Google Scholar] [CrossRef]
- Santner, S.; Sprenger, J.A.P.; Finze, M.; Dehnen, S. The role of [BF4]− and [B(CN)4]− anions in the ionothermal synthesis of chalcogenidometalates. Chem. Eur. J. 2018, 24, 3474–3480. [Google Scholar] [CrossRef]
- Kim, K.-W. DMF Solvothermal synthesis and structural characterization of [dabcoH]2[(CH3)2NH2]2[Sn2Se6] DMF. J. Korean Chem. Soc. 2005, 49, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Block, E.; Dikarev, E.V.; Glass, R.S.; Jin, J.; Li, B.; Li, X.; Zhang, S.-Z. Synthesis, structure, and chemistry of new, mixed group 14 and 16 heterocycles: Nucleophile-induced ring contraction of mesocyclic dications. J. Am. Chem. Soc. 2006, 128, 14949–14961. [Google Scholar] [CrossRef] [PubMed]
- Nayek, H.P.; Niedermeyer, H.; Dehnen, S. Preparation and conformation of organo-bridged bis[tris(arylchalcogenolato)tin] compounds–an experimental and quantum chemical study. Z. Anorg. Allg. Chem. 2008, 634, 2805–2810. [Google Scholar] [CrossRef]
- Holligan, K.; Rogler, P.; Rehe, D.; Pamula, M.; Kornienko, A.Y.; Emge, T.J.; Krogh-Jespersen, K.; Brennan, J.G. Copper, indium, tin, and lead complexes with fluorinated selenolate ligands: Precursors to MSex. Inorg. Chem. 2015, 54, 8896–8904. [Google Scholar] [CrossRef] [PubMed]
- Dräger, M.; Mathiasch, B. Kristallstrukturbestimmung und schwingungsanalyse von 2,2,4,4,5,5-hexamethyl-1,3-diselena-2,4,5-tristannolan Se2Sn3(CH3)6. Z. Anorg. Allg. Chem. 1980, 470, 45–58. [Google Scholar] [CrossRef]
- Cheng, Y.; Emge, T.J.; Brennan, J.G. Pyridineselenolate Complexes of tin and lead: Sn(2-SeNC5H4)2, Sn(2-SeNC5H4)4, Pb(2-SeNC5H4)2, and Pb(3-Me3Si-2-SeNC5H3)2. Volatile CVD precursors to Group IV−Group VI semiconductors. Inorg. Chem. 1996, 35, 342–346. [Google Scholar] [CrossRef]
- Herzog, U.; Böhme, U.; Brendler, E.; Rheinwald, G. Group 14 chalcogenides featuring a bicyclo[3.3.0]octane skeleton. J. Organomet. Chem. 2001, 630, 139–148. [Google Scholar] [CrossRef]
- Dräger, M.; Blecher, A.; Jacobsen, H.-J.; Krebs, B. Molekül- und kristallstruktur von hexamethylcyclo-tristannaselenan [(CH3)2SnSe]3. J. Organomet. Chem. 1978, 161, 319–325. [Google Scholar] [CrossRef]
- Leung, W.-P.; Wan, C.-L.; Kan, K.-W.; Mak, T.C.W. Synthesis, structure, and reactivity of Group 14 bis(thiophosphinoyl) metal complexes. Organometallics 2010, 29, 814–820. [Google Scholar] [CrossRef]
- Ritch, J.S.; Chivers, T.; Ahmad, K.; Afzaal, M.; O’Brien, P. Synthesis, structures, and multinuclear NMR spectra of tin(II) and lead(II) complexes of tellurium-containing imidodiphosphinate ligands: Preparation of two morphologies of phase-pure PbTe from a single-source precursor. Inorg. Chem. 2010, 49, 1198–1205. [Google Scholar] [CrossRef]
- Hummel, H.-U.; Fischer, E.; Fischer, T.; Gruβ, D.; Franke, A.; Dietzsch, W. Synthesen, strukturen und thermische abbaureaktionen von TlI-, PbII- und SeII-komplexen mit 2,2-dicyanethylen-1,1-diselenolat. Chem. Ber. 1992, 125, 1565–1570. [Google Scholar] [CrossRef]
- Trindade, T.; Monteiro, O.C.; O’Brien, P.; Motevalli, N. Synthesis of PbSe nanocrystallites using a single-source method. The X-ray crystal structure of lead (II) diethyldiselenocarbamate. Polyhedron 1999, 18, 1171–1175. [Google Scholar] [CrossRef]
- Evans, C.M.; Evans, M.E.; Krauss, T.D. Mysteries of TOPSe revealed: Insights into quantum dot nucleation. J. Am. Chem. Soc. 2010, 132, 10973–10975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, M.; Bensch, W. (Se,O)-Koordinierte komplexe niedervalenter hauptgruppenmetalle: Die kristallstruktur von bis(N,N-diethyl-N’-benzoylselenoureato)blei(II). Z. Naturforsch. B Chem. Sci. 1994, 49, 1615–1619. [Google Scholar] [CrossRef] [Green Version]
- Alhanash, F.B.; Barnes, N.A.; Brisdon, A.K.; Godfrey, S.M.; Pritchard, R.G. Formation of M4Se4 cuboids (M = As, Sb, Bi) via secondary pnictogen–chalcogen interactions in the co-crystals MX3·Se=P(p-FC6H4)3 (M = As, X = Br; M = Sb, X = Cl; M = Bi, X = Cl, Br). Dalton Trans. 2012, 41, 10211–10218. [Google Scholar] [CrossRef]
- Czado, W.; Müller, U. Cyclische polyselenidoarsenate(III) und -antimonate(III): PPh4[Se5AsSe], PPh4[AsSe6–xSx], (PPh4)2[As2Se6]·2CH3CN und (PPh4)2[Se6SbSe]2. Z. Anorg. Allg. Chem. 1998, 624, 239–243. [Google Scholar] [CrossRef]
- Kennard, O.; Wampler, D.L.; Coppola, J.C.; Motherwell, W.D.S.; Mann, F.G.; Watson, D.G.; MacGillavry, C.H.; Stam, C.H.; Benci, P. Crystal and molecular structure of 5,10-epoxy-, 5,10-epithio-, 5,10-episeleno-, and 5,10-epitelluro-5,10-dihydroarsanthren. J. Chem. Soc. C 1971, 1511–1515. [Google Scholar] [CrossRef]
- Thiele, G.; Rotter, H.W.; Lietz, M.; Ellermann, J. Chemistry of polyfunctional molecules, 80 [1] Crystal and molecular structures of the heteronoradamantanes 5-methyl-2.2.8.8-tetra-ethoxycarbonyl-1.3.7-triarsa-tricyclo[3.3.1.03,7]nonane and 5-methyl-1.3.7-triarsa-2.8-diselena-[3.3.1.03,7]nonane. Z. Naturforsch. B Chem. Sci. 1984, 39, 1344–1349. [Google Scholar] [CrossRef]
- Applegate, C.A.; Meyers, E.A.; Zingaro, R.A.; Merijanian, A. reactions of arsinic and arsonic acids with H2S and H2Se: Crystal structure of 1,4-diphenyl-1,4-diarsa-2,3,5-triselenacyclopentane. Phosphorus Sulfur Rel. Elements 1988, 35, 363–370. [Google Scholar] [CrossRef]
- Levason, W.; Maheshwari, S.; Ratnani, R.; Reid, G.; Webster, M.; Zhang, W. Structural diversity in supramolecular complexes of MCl3 (M = As, Sb, Bi) with constrained thio- and seleno-ether ligands. Inorg. Chem. 2010, 49, 9036–9048. [Google Scholar] [CrossRef]
- Angilella, V.; Mercier, H.; Belin, C.J. Heteroatomic polyanions of post-transition elements. Synthesis and structure of a salt containing the novel hybrid hepta-arsenic tetraselenate(1–) anion, As7Se4–. Chem. Soc. Chem. Commun 1989, 1654–1655. [Google Scholar] [CrossRef]
- Hill, N.J.; Levason, W.; Patel, R.; Reid, G.; Webster, M. Unusual structural trends in the [MCl3([8]aneSe2)] (M = As, Sb, Bi) adducts. Dalton Trans. 2004, 980–981. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Kedarnath, G.; Jain, V.K.; Wadawale, A.; Nalliath, M.; Pillai, C.G.S.; Vishwanadh, B. 2-Pyridyl selenolates of antimony and bismuth: Synthesis, characterization, structures and their use as single source molecular precursor for the preparation of metal selenidenanostructures and thin films. Dalton Trans. 2010, 39, 8779–8787. [Google Scholar] [CrossRef] [PubMed]
- Šimon, P.; Jambor, R.; Růžička, A.; Dostál, L. Oxidative addition of diphenyldichalcogenides PhEEPh (E = S, Se, Te) to low-valent CN- and NCN-chelated organoantimony and organobismuth compounds. Organometallics 2013, 32, 239–248. [Google Scholar] [CrossRef]
- Wagner, C.; Merzweiler, K. Neue [{Cp(CO)2Mo}2ESbCl]-cluster mit tetraedrischem Mo2SbE-Gerüst (E = S, Se). Z. Anorg. Allg. Chem. 2011, 637, 651–654. [Google Scholar] [CrossRef]
- Kimura, M.; Iwata, A.; Itoh, M.; Yamada, K.; Kimura, T.; Sugiura, N.; Ishida, M.; Kato, S. Synthesis, structures, and some reactions of [(thioacyl)thio]- and (acylseleno)antimony and -bismuth derivatives ((RCSS)xMR and (RCOSe)xMR with M = Sb, Bi and x = 1–3). Helv. Chim. Acta 2006, 89, 747–783. [Google Scholar] [CrossRef]
- Martin, T.M.; Wood, P.T.; Kolis, J.W. Synthesis and structure of an [Sb12Se20]4- salt: The largest molecular Zintl ion. Inorg. Chem. 1994, 33, 1587–1588. [Google Scholar] [CrossRef]
- Breunig, H.J.; Güleç, S.; Krebs, B.; Dartmann, M. Synthese und struktur von (MeSe)3Sb. Z. Naturforsch. B Chem. Sci. 1989, 44, 1351–1354. [Google Scholar] [CrossRef]
- Sommer, H.; Eichhöfer, A.; Drebov, N.; Ahlrichs, R.; Fenske, D. Preparation, Geometric and electronic structures of [Bi2Cu4(SPh)8(PPh3)4] with a Bi2 dumbbell, [Bi4Ag3(SePh)6Cl3(PPh3)3]2 and [Bi4Ag3(SePh)6X3(PPhiPr2)3]2 (X = Cl, Br) with a Bi4 unit. Eur. J. Inorg. Chem. 2008, 5138–5145. [Google Scholar] [CrossRef]
- Shieh, M.; Liu, Y.-H.; Huang, C.-Y.; Chen, S.-W.; Cheng, W.-K.; Chien, L.T. The first naked bismuth–chalcogen metal carbonyl clusters: Extraordinary nucleophilicity of the Bi atom and semiconducting characteristics. Inorg. Chem. 2019, 58, 6706–6721. [Google Scholar] [CrossRef]
- Calderazzo, F.; Morvillo, A.; Pelizzi, G.; Poli, R.; Ungari, F. Reactivity of molecules containing element-element bonds. 1. Nontransition elements. Inorg. Chem. 1988, 27, 3730–3733. [Google Scholar] [CrossRef]
- Sommer, H.; Eichhofer, A.; Fenske, D. Bismutchalkogenolate Bi(SC6H5)3, Bi(SeC6H5)3 und Bi(S-4-CH3C6H4)3. Z. Anorg. Allg. Chem. 2008, 634, 436–440. [Google Scholar] [CrossRef]
- Morikami, A.; Takimiya, K.; Aso, Y.; Otsubo, T. Novel tellurium containing fulvalene-type electron donors, triselenatellurafulvalene (TSTeF) and diselenaditellurafulvalene (DSDTeF); synthesis, conductivities and crystal structures of their TCNQ complexes. J. Mater. Chem. 2001, 11, 2431–2436. [Google Scholar] [CrossRef]
- Dereu, N.L.M.; Zingaro, R.A.; Meyers, E.A. Bis(4-methoxybenzenetellurenyl)selenide, C14H14O2SeTe2. Cryst. Struct. Commun. 1981, 10, 1345–1352. [Google Scholar]
- Stanford, M.W.; Knight, F.R.; Arachchige, K.S.A.; Camacho, P.S.; Ashbrook, S.E.; Bühl, M.; Slawin, A.M.Z.; Woollins, D.J. Probing interactions through space using spin–spin coupling. Dalton Trans. 2014, 43, 6548–6560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrib, C.G.; Jeske, J.; Jones, P.G.; du Mont, W.-W. Telluroselenophosphonium ions: Their unusual soft–soft interactions with iodotellurate anions. Dalton Trans. 2007, 3483–3485. [Google Scholar] [CrossRef] [PubMed]
- Klapotke, T.M.; Krumm, B.; Mayer, P.; Piotrowski, H.; Schwab, I.; Vogt, M. Synthesis and structures of triorganotelluronium pseudohalides. Eur. J. Inorg. Chem. 2002, 2701–2709. [Google Scholar] [CrossRef]
- Mathur, P.; Payra, P.; Ghose, S.; Hossain, M.M.; Satyanarayana, C.V.V.; Chicote, F.O.; Chadha, R.K. Synthesis and characterisation of [Fe2M3(μ4-E)(μ3-E′)(CO)17] and [Os3(μ3-E)(μ3-E′)(CO)9] (M=Os or Ru; E=S, Se, Te; E′=Se, Te). J. Organomet. Chem. 2000, 606, 176–182. [Google Scholar] [CrossRef]
- Dibrov, S.M.; Ibers, J.A. [TeSe3]2– as a tridentate ligand: Syntheses and crystal structures of [PPh4][(CpM(μ2–Se2))3(μ3–O)(μ3–TeSe3)] (M = Zr, Hf). Comptes Rendus Chim. 2005, 8, 993–997. [Google Scholar] [CrossRef]
- Ogawa, S.; Yoshimura, S.; Nagahora, N.; Kawai, Y.; Mikata, Y.; Sato, R. Novel multi-chalcogen ring systems with three different chalcogen atoms: Synthesis, structure and redox property of five-membered trichalcogenaheterocycles. Chem. Commun. 2002, 1918–1919. [Google Scholar] [CrossRef]
- Åse, K.; Foss, O.; Roti, I. The crystal and molecular structures of trans square-planar complexes of tellurium diselenocyanate with trimethylenethiourea and tetramethylthiourea. Acta Chem. Scand. 1971, 25, 3808–3820. [Google Scholar] [CrossRef]
- Karjalainen, M.M.; Oilunkaniemi, R.; Laitinen, R.S. Chalcogen–chalcogen interactions in trichalcogenaferrocenophanes. Crystal structure of 2-selena-1,3-ditellura[3]ferrocenophane [Fe(C5H4Te)2Se]. Inorg. Chim. Acta 2012, 390, 79–82. [Google Scholar] [CrossRef]
- Karjalainen, M.M.; Sanchez-Perez, C.; Mikko Rautiainen, J.; Oilunkaniemi, R.; Laitinen, R.S. Chalcogen–chalcogen secondary bonding interactions in trichalcogenaferrocenophanes. CrystEngComm 2016, 18, 4538–4545. [Google Scholar] [CrossRef] [Green Version]
- Nordheider, A.; Hüll, K.; Prentis, J.K.D.; Arachchige, K.S.A.; Slawin, A.M.Z.; Woollins, D.J.; Chivers, T. Main group tellurium heterocycles anchored by a P2VN2 scaffold and their sulfur/selenium analogues. Inorg. Chem. 2015, 54, 3043–3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiekink, E.R.T. Perplexing coordination behaviour of potentially bridging bipyridyl-type ligands in the coordination chemistry of zinc and cadmium 1,1-dithiolate compounds. Crystals 2018, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Tiekink, E.R.T. Supramolecular assembly based on “emerging” intermolecular interactions of particular interest to coordination chemists. Coord. Chem. Rev. 2017, 345, 209–228. [Google Scholar] [CrossRef] [Green Version]
- Heywood, V.L.; Alford, T.P.J.; Roeleveld, J.J.; Lekanne Deprez, S.J.; Verhoofstad, A.; van der Vlugt, J.I.; Domingos, S.R.; Melanie Schnell, M.; Davis, A.P.; Mooibroek, T.J. Observations of tetrel bonding between sp3-carbon and THF. Chem. Sci. 2020, 11, 5289–5293. [Google Scholar] [CrossRef]












© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiekink, E.R.T. A Survey of Supramolecular Aggregation Based on Main Group Element⋯Selenium Secondary Bonding Interactions—A Survey of the Crystallographic Literature. Crystals 2020, 10, 503. https://doi.org/10.3390/cryst10060503
Tiekink ERT. A Survey of Supramolecular Aggregation Based on Main Group Element⋯Selenium Secondary Bonding Interactions—A Survey of the Crystallographic Literature. Crystals. 2020; 10(6):503. https://doi.org/10.3390/cryst10060503
Chicago/Turabian StyleTiekink, Edward R. T. 2020. "A Survey of Supramolecular Aggregation Based on Main Group Element⋯Selenium Secondary Bonding Interactions—A Survey of the Crystallographic Literature" Crystals 10, no. 6: 503. https://doi.org/10.3390/cryst10060503
APA StyleTiekink, E. R. T. (2020). A Survey of Supramolecular Aggregation Based on Main Group Element⋯Selenium Secondary Bonding Interactions—A Survey of the Crystallographic Literature. Crystals, 10(6), 503. https://doi.org/10.3390/cryst10060503