Zn/Ni and Zn/Pd Heterobimetallic Coordination Polymers with [SSC-N(CH2COO)2]3− Ligands
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Precoursor Complexes
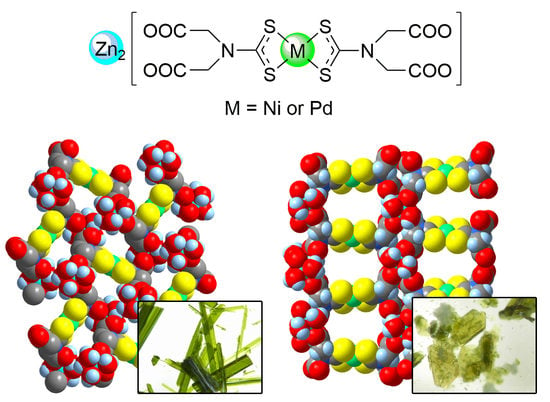
3.2. Heterobimetallic Coordination Polymers
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, S.R.; Du, D.Y.; Tan, K.; Qin, J.S.; Dong, H.Q.; Li, S.L.; He, W.W.; Lan, Y.Q.; Shen, P.; Su, Z.M. Self-assembly versus stepwise synthesis: Heterometal-organic frameworks based on metalloligands with tunable luminescence properties. Chem. Eur. J. 2013, 19, 11279–11286. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro-López, L.; Valverde-Muñoz, F.J.; Seredyuk, M.; Muñoz, M.C.; Haukka, M.; Real, J.A. Guest Induced Strong Cooperative One- and Two-Step Spin Transitions in Highly Porous Iron(II) Hofmann-Type Metal-Organic Frameworks. Inorg. Chem. 2017, 56, 7038–7047. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.Z.; Xu, H.; Cheng, P.; Zhao, B. 3d-4f Heterometal-Organic Frameworks for Efficient Capture and Conversion of CO2. Cryst. Growth Des. 2017, 17, 3128–3133. [Google Scholar] [CrossRef]
- Ma, H.; Wang, L.; Chen, J.; Zhang, X.; Wang, L.; Xu, N.; Yang, G.; Cheng, P. A multi-responsive luminescent sensor for organic small-molecule pollutants and metal ions based on a 4d-4f metal-organic framework. Dalton Trans. 2017, 46, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, G. Transition Metal Dithiocarbamates: 1978–2003. In Progress in Inorganic Chemistry; John Wiley and Sons: Hoboken, NJ, USA, 2005; Volume 53, ISBN 0471463701. [Google Scholar]
- Liebing, P.; Witzorke, J.; Oehler, F.; Schmeide, M. Dithiocarbamatocarboxylate (DTCC) Ligands—Building Blocks for Hard/Soft-Heterobimetallic Coordination Polymers. Inorg. Chem. 2020, 59, 2825–2832. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in Retrospect and Prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. [Google Scholar] [CrossRef]
- Leka, Z.B.; Leovac, V.M.; Lukić, S.; Sabo, T.J.; Trifunović, S.R.; Szécsényi, K.M. Synthesis and physico-chemical characterization of new dithiocarabamato ligand and its complexes with copper(II), nickel(II) and palladium(II). J. Therm. Anal. Calorim. 2006, 83, 687–691. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Stoe & Cie, X-Area, X-Step and X-Shape; Stoe & Cie: Darmstadt, Germany, 2002.
- Kamimura, H.; Koide, S.; Sekiyama, H.; Sugano, S. Magnetic Properties of the Pd and Pt Group Transition Metal Complexes. J. Phys. Soc. Jpn. 1960, 15, 1264–1272. [Google Scholar] [CrossRef]
- Banks, C.V.; Haar, R.W.V.; Wal, R.P.V. Magnetic Studies of Nickel(II) and Palladium(II) Complexes with Some vic-Dioximes. J. Am. Chem. Soc. 1955, 77, 324–325. [Google Scholar] [CrossRef]
- Russell, C.D.; Cooper, G.R.; Vosburgh, W.C. Complex Ions. V. The Magnetic Moments of Some Complex Ions of Nickel and Copper. J. Am. Chem. Soc. 1943, 65, 1301–1306. [Google Scholar] [CrossRef]
- Willis, J.B.; Mellor, D.P. The Magnetic Susceptibility of Some Nickel Complexes in Solution. J. Am. Chem. Soc. 1947, 69, 1237–1240. [Google Scholar] [CrossRef]
- Ojima, I.; Onishi, T.; Iwamoto, T.; Inamoto, N.; Tamaru, K. A new route to metal chelates of dimethyldithiocarbamate and their far infrared spectra. Inorg. Nucl. Chem. Lett. 1970, 6, 65–69. [Google Scholar] [CrossRef]
- Tepavitcharova, S.; Rabadjieva, D.; Havlíček, D.; Němec, I.; Vojtíšek, P.; Plocek, J.; Koleva, Z. Crystallization and characterization of the compounds Gly·MSO 4·mH2O (M = Mg2+, Mn2+, Fe2+, Co2+, Ni2+, Zn2+; M = 0, 3, 5, 6). J. Mol. Struct. 2012, 1018, 113–121. [Google Scholar] [CrossRef]
- Marandi, F.; Hashemi, L.; Morsali, A.; Krautscheid, H. Sonochemical synthesis and characterization of three nano zinc(II) coordination polymers; Precursors for preparation of zinc(II) oxide nanoparticles. Ultrason. Sonochem. 2016, 32, 86–94. [Google Scholar] [CrossRef]
- Okamura, T.A.; Furuya, R.; Onitsuka, K. Regulation of the hydrolytic activity of Mg2+-dependent phosphatase models by intramolecular NH···O hydrogen bonds. J. Am. Chem. Soc. 2014, 136, 14639–14641. [Google Scholar] [CrossRef]
- Larionov, S.V.; Rakhmanova, M.I.; Glinskaya, L.A.; Naumov, D.Y.; Vinogradov, A.S.; Karpov, V.M.; Platonov, V.E.; Fadeeva, V.P. Zinc(II) Complexes with Tetrafluoroterephthalic and Octafluorobiphenyl-4,4′-dicarboxylic Acid Anions and 1,10-Phenanthroline. Russ. J. Gen. Chem. 2019, 89, 261–265. [Google Scholar] [CrossRef]
- Brown, D.A.; Fitzpatrick, N.J.; Muller-Bunz, H.; Ryan, Á.T. Di-, tri-, and tetranuclear zinc hydroxamate complexes as structural models for the inhibition of zinc hydrolases by hydroxamic acids. Inorg. Chem. 2006, 45, 4497–4507. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-P.; Chen, J.; Mu, Y.-H.; Du, M. Exceptional sensitivity to the synthetic approach and halogen substituent for Zn(ii) coordination assemblies with 5-halonicotinic acids. Dalton Trans. 2015, 44, 11109–11118. [Google Scholar] [CrossRef] [PubMed]







| Compound | TDec./°C | χmol/cm3 mol−1 | ν(C-O)/cm−1 | νas(M-S)/cm−1 |
|---|---|---|---|---|
| 2-Pt · 0.5H2O [6] | 241 | 1701, 1682 | 350 | |
| 2-Pd | 248 | 1706 | 363 | |
| 2-Ni | 217 | –9.929 × 10−5 | 1720, 1686 | 399, 392 |
| 4-Pt · 10H2O [6] | 398 | 1573, 1490 | 352 | |
| 4-Pd · 12H2O | 377 | 1588, 1496 | not resolved | |
| 4-Ni · 2H2O | 348 | –8.404 × 10−5 | 1575, 1483 | 388 |
| 5-Ni · 2H2O | 387 | –3.270 × 10−4 | 1642, 1579, 1471 | 398 |
| Compound | M-S | S-M-S | Zn-O 1 | O-Zn-O 1 |
|---|---|---|---|---|
| 4-Pt · 14H2O [6] | 230.0(1), 230.7(1) | 75.30(3)–104.70(3) | 203.9(3)–207.5(2) | 97.5(1) |
| 4-Pd · 14H2O | 232.5(1), 231.1(1) | 75.80(4)–104.20(4) | 204.2(3)–207.1(3) | 97.5(1) |
| 4-Ni · 14H2O | 219.12(5), 220.79(5) | 79.60(2)–100.40(2) | 204.0(2)–207.1(2) | 97.34(6) |
| 5-Ni · 2.5acetone · 8.5H2O | 219.3(2)–220.3(2) | 79.51(7)–79.65(7), 100.35(7)–100.49(7) | 214.2(4)–214.8(4) 2, 205.3(4)–207.1(6) 3 | 88.2(2)–97.1(2), 171.1(2)–173.1(2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebing, P.; Oehler, F.; Witzorke, J. Zn/Ni and Zn/Pd Heterobimetallic Coordination Polymers with [SSC-N(CH2COO)2]3− Ligands. Crystals 2020, 10, 505. https://doi.org/10.3390/cryst10060505
Liebing P, Oehler F, Witzorke J. Zn/Ni and Zn/Pd Heterobimetallic Coordination Polymers with [SSC-N(CH2COO)2]3− Ligands. Crystals. 2020; 10(6):505. https://doi.org/10.3390/cryst10060505
Chicago/Turabian StyleLiebing, Phil, Florian Oehler, and Juliane Witzorke. 2020. "Zn/Ni and Zn/Pd Heterobimetallic Coordination Polymers with [SSC-N(CH2COO)2]3− Ligands" Crystals 10, no. 6: 505. https://doi.org/10.3390/cryst10060505
APA StyleLiebing, P., Oehler, F., & Witzorke, J. (2020). Zn/Ni and Zn/Pd Heterobimetallic Coordination Polymers with [SSC-N(CH2COO)2]3− Ligands. Crystals, 10(6), 505. https://doi.org/10.3390/cryst10060505