Abstract
The cubane-type structure is a typical representative of tetranuclear coordination compounds. In this work, two anionic Schiff-base ligands, (L1)2− and (L2)2−, each offering an O^N^O coordination pocket, ligate four NiII ions into a [Ni4O4] cubane core. The ligands are H2L1 = 2−[[(3-ethoxy-2−hydroxyphenyl) methylene]amino]benzenemethanol and H2L2 = 2−[[(5-fluoro-2−hydroxyphenyl)methylene]amino]benzenemethanol. In both compounds, [Ni4(L1)4(EtOH)4] (1) and [Ni4(L2)4(MeOH)4] (2), alkoxy oxygens of the ligands act in a bridging μ3-O binding mode. Magnetic susceptibility and magnetization data for compounds 1 and 2 are presented. The Ni–O–Ni bond angles of the cubane core determined from single crystal X-ray diffraction data play a key role for a magneto-structural correlation. Dominant intracube ferromagnetic behavior is observed, and the coupling parameters were determined for both compounds, leading to nonzero spin ground states in accordance with the broadly accepted bond angle guideline.
1. Introduction
In the field of polynuclear coordination chemistry, skillful design strategies involving polytopic ligands often lead to predictable cluster structures, in which bridged transition metal ions exhibit appreciable magnetic spin–spin exchange [1,2,3,4,5,6,7]. Advantageously, such polynucleating ligands comprise coordination pockets, by which the spin centers are bound in adjacent pairs favoring intramolecular spin communication. Amongst the most versatile and widely studied ligand systems for the design of cluster compounds are polydentate Schiff-bases incorporating for the most part O/N donor atoms [8,9,10,11,12,13,14,15,16,17,18,19,20,21]. Due to the ease of access of Schiff-bases and their flexible structures, highly versatile cluster compounds with a broad variety of structure types can be realized. One prominent class of compounds shows a cubane-type structure containing four metal ions and four ligand O-donor atoms at the corners of a cube; each of the subsets forms a tetrahedron [22,23,24,25,26,27,28,29,30]. The compact cubane core with its specific bonding pattern allows for strong magnetic couplings between the spin centers, but also for a rich redox chemistry of the whole unit. For the latter, attention has recently been attracted for testing these tetrametallic molecular systems in applications as water oxidation catalysts [31,32,33] or for electrocatalytic methanol oxidation reactions [34]. In the field of molecular magnetism, cubane-like clusters have for long been in the center of magnetic studies [8,9,10,11,12,13,14,15,16,17,18,19,22,23,24,25,26,27,28,29,30,35,36,37,38,39,40], while deliberately and systematically looking for magnetostructural correlations. Thereby, one can note that even slight structural rearrangements of the cubane core, e.g., caused by exchange of coordinated solvent or loss of lattice solvent can lead to drastic changes of the magnetic properties. As a matter of facts, for NiII cubane structures, a clear correlation between the Ni–O–Ni bond angles formed via triply-bridged oxygen atoms and the magnetic coupling strengths is observed [23,40,41,42,43]. For angles above 99°, the magnetic coupling between the NiII ions is antiferromagnetic, but ferromagnetic for smaller angles. To note, structural distortions may affect this kind of guideline.
In this paper, we report the synthesis, characterization, and magnetic properties of two cubane-type complexes with stoichiometries [Ni4(L1)4(EtOH)4] (1) and [Ni4(L2)4(MeOH)4] (2), both based on anionic Schiff-base ligands (L1)2− and (L2)2−, respectively (Figure 1). The magnetic susceptibility and magnetization data were determined, and the former were fitted based on a Heisenberg Hamiltonian with two different coupling parameters J1 and J2 in the case of 1, and with one J parameter for 2. Both compounds are found to be in a ferromagnetic coupling regime, in agreement with their structural parameters.
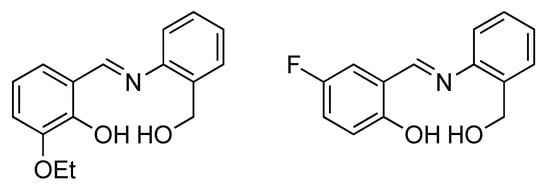
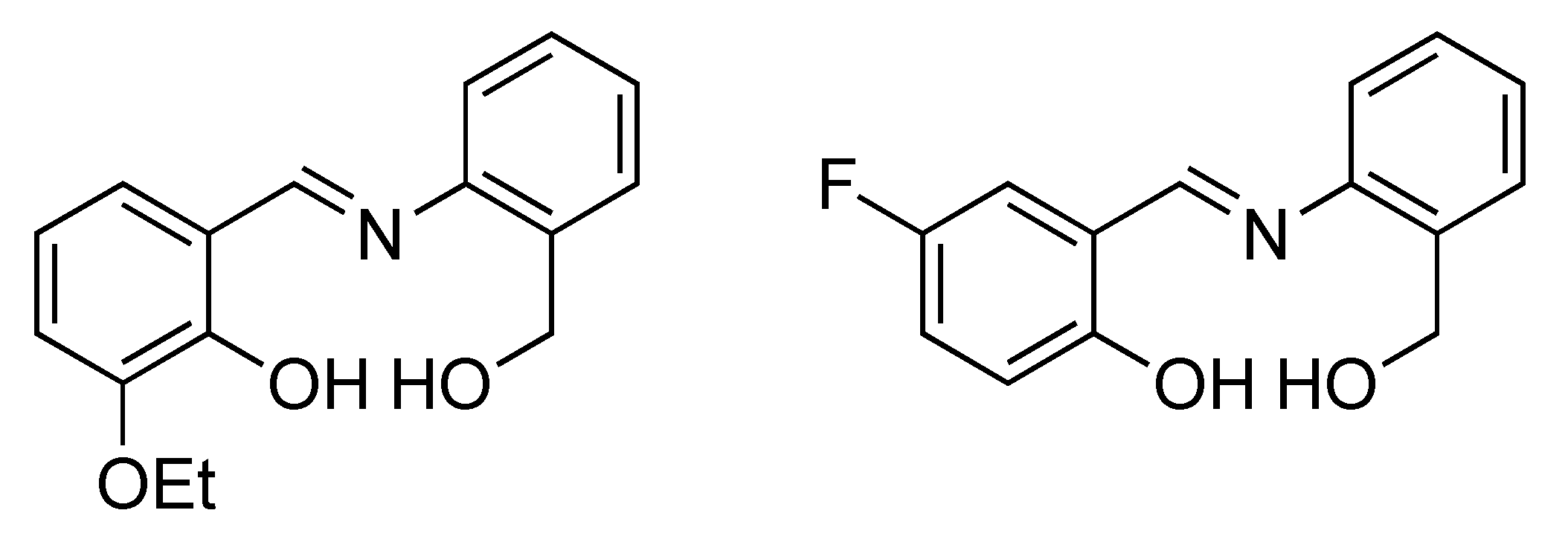
Figure 1.
Chemical Structures of the Ligands H2L1 (left) and H2L2− (right); the doubly deprotonated ligands bind to the NiII ions of the cluster cores.
2. Materials and Methods
2.1. Materials
3-Ethoxysalicylaldehyde, 5-fluorosalicylaldehyde, and 2−aminobenzylalcohol were purchased from Sigma Aldrich, USA. Nickel acetate tetrahydrate and solvents employed for the syntheses were of analytical grade and used as received without further purification.
2.2. General Methods
Elemental analyses were performed on a 240C elemental analyzer (Perkin-Elmer, USA). IR spectra were recorded on an FT/IR-4000 spectrometer (Jasco, Japan) as KBr pellets in 4000–400 cm−1 region. UV-vis spectra were recorded on a Lambda 35 spectrometer (Perkin-Elmer, USA). 1H and 13C NMR spectra were recorded on a 500 MHz spectrometer (Bruker, Germany). Single crystal X-ray diffraction was carried out on an Apex II CCD area diffractometer (Bruker, Germany). Powder X-ray diffraction patterns were measured on a StadiP diffractometer (STOE, Darmstadt, Germany) in Debye Scherrer geometry.
2.3. Synthesis of Ligands and Complexes
2.3.1. Synthesis of H2L1
Similar to the previously reported procedure [44,45], 3-ethoxysalicylaldehyde (1.66 g, 0.01 mol) was rected with 2−aminobenzylalcohol (1.23 g, 0.01 mol) in methanol (30 mL). The mixture was stirred at room temperature for 1 h to give a yellow solution, which was evaporated by distillation to give a yellow solid product. The solid was recrystallized from methanol to give the crystalline product H2L1. Yield: 92%. Elemental analysis (%) calcd for C16H17NO3: C, 70.83; H, 6.32; N, 5.16; found: C, 70.67; H, 6.44; N, 5.0. IR data (KBr, cm−1): 3528 (OH), 1612 (C=N), 1570, 1456, 1384, 1245, 1186, 1105, 1038, 988, 900, 858, 782, 735. UV-vis data (λ, ε): 226 nm, 1.67 × 104 L·mol−1·cm−1; 278 nm, 9.75 × 103 L·mol−1·cm−1; 317 nm, 8.93 × 103 L·mol−1·cm−1. 1H NMR (500 MHz, d6-DMSO): δ 13.26 (s, 1H, OH), 8.87 (s, 1H, CH=N), 7.54 (t, 1H, ArH), 7.36-7.31 (m, 3H, ArH), 7.24 (d, 1H, ArH), 7.12 (d, 1H, ArH), 6.90 (t, 1H, ArH), 5.19 (t, 1H, OH), 4.65 (d, 2H, CH2OH), 4.08 (q, 2H, OCH2CH3), 1.36 (t, 3H, OCH2CH3). 13C NMR (126 MHz, DMSO) δ 163.36, 150.85, 147.03, 145.35, 135.77, 127.81, 127.37, 126.64, 124.08, 119.40, 118.48, 117.73, 116.89, 64.06, 59.39, 14.73.
2.3.2. Synthesis of H2L2
5-Fluorosalicylaldehyde (1.40 g, 0.01 mol) was reacted with 2−aminobenzylalcohol (1.23 g, 0.01 mol) in methanol (30 mL). The mixture was stirred at room temperature for 1 h to give a yellow solution, which was evaporated by distillation to give a yellow solid product. The solid was recrystallized from methanol to give the crystalline product H2L2. Yield: 94%. Elemental analysis (%) calcd for C14H12FNO2: C, 68.56; H, 4.93; N, 5.71; found: C, 68.71; H, 5.02; N, 5.63. IR data (KBr, cm−1): 3338 (OH), 3246, 1620 (C=N), 1565, 1481, 1354, 1255, 1197, 1138, 1028, 957, 867, 778. UV-vis data (λ, ε): 230 nm, 1.81 × 104 L·mol−1·cm−1; 265 nm, 1.30 × 104 L·mol−1·cm−1; 347 nm, 1.17 × 104 L·mol−1·cm−1. 1H NMR (500 MHz, d6-DMSO): δ 12.70 (s, 1H, OH), 8.84 (s, 1H, CH=N), 7.54 (s, 1H, ArH), 7.38-7.28 (m, 3H, ArH), 7.02 (m, 2H, ArH), 6.90 (t, 1H, ArH), 5.19 (t, 1H, OH), 4.78 (d, 2H, CH2OH). 13C NMR (126 MHz, DMSO) δ 162.25, 156.96, 156.35, 151.51, 136.39, 128.37, 127.97, 120.79, 120.53, 120.24, 120.18, 118.27, 115.81, 59.88.
2.3.3. Synthesis of [Ni4(L1)4(EtOH)4] (1)
H2L1 (27.1 mg, 0.1 mmol) was reacted with nickel acetate tetrahydrate (24.9 mg, 0.1 mmol) in ethanol (20 mL). The mixture was stirred at room temperature for 30 min to give a green solution, which was allowed to stand in air for a few days until three quarter of the solvent was evaporated. Green block-shaped single crystals of the complex were formed at the bottom of the vessel. The crystals were isolated by filtration and dried in air. Yield: 27%. Elemental analysis (%) calcd for C72H84N4Ni4O16: C, 57.80; H, 5.66; N, 3.74; found: C, 57.65; H, 5.72; N, 3.67. IR data (KBr, cm−1): 1606 (C=N), 1541, 1443, 1389, 1331, 1230, 1183, 1108, 1045, 740, 620, 565, 525, 461. UV-vis data (λ, ε): 243 nm, 1.56 × 104 L·mol−1·cm−1; 310 nm, 6.91 × 103 L·mol−1·cm−1; 415 nm, 3.79 × 103 L·mol−1·cm−1; 577 nm, 5.10 × 102 L·mol−1·cm−1.
2.3.4. Synthesis of [Ni4(L2)4(MeOH)4] (2)
H2L2 (24.5 mg, 0.1 mmol) was reacted with nickel acetate tetrahydrate (24.9 mg, 0.1 mmol) in methanol (20 mL). The mixture was stirred at room temperature for 30 min to give a green solution, which was allowed to stand in air for a few days until three quarter of the solvent was evaporated. Green block-shaped single crystals of the complex were formed at the bottom of the vessel. The crystals were isolated by filtration and dried in air. Yield: 23%. Elemental analysis (%) calcd for C60H56F4N4Ni4O12: C, 53.95; H, 4.23; N, 4.19; found: C, 54.14; H, 4.30; N, 4.31. IR data (KBr, cm−1): 1608 (C=N), 1538, 1463, 1386, 1313, 1243, 1192, 1135, 1043, 873, 815, 751, 620, 525, 457. UV-vis data (λ, ε): 241 nm, 1.68 × 104 L·mol−1·cm−1; 300 nm, 8.30 × 103 L·mol−1·cm−1; 420 nm, 7.49 × 103 L·mol−1·cm−1; 575 nm, 3.71 × 102 L·mol−1·cm−1.
2.4. X-ray Crystallography
Diffraction intensities for the complexes were collected at 298(2) K using a Bruker Apex II CCD area-detector diffractometer with MoKα radiation (λ = 0.71073 Å). Collected data were reduced with SAINT [46], and multiscan absorption correction was performed using SADABS [47]. Structures of the complexes were solved by direct methods and refined against F2 by full-matrix least-squares method using SHELXTL [48]. All nonhydrogen atoms were refined anisotropically. The hydrogen atoms were placed in calculated positions and constrained to ride on their parent atoms. The ethanol ligand C36-C35-O8 of 1 is disordered over two sites, with occupancies of 0.385(2) and 0.615(2). Crystallographic data for the complexes are summarized in Table 1. Selected Ni-L bond lengths and Ni–O–Ni angles of 1 are given in Table 2. Further L-Ni-L and Ni–O–Ni angles of 2 are reported in Supplementary Materials Table S1.

Table 1.
Details of the data collection and refinement parameters for complexes 1 and 2.

Table 2.
Selected bond lengths (Å) and angles (°) for complexes 1 and 2.
CCDC 1946462 (1) and 1946463 (2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: (+44)1223-336-033; or E-mail: deposit@ccdc.cam.ac.uk.
3. Results and Discussion
3.1. Synthesis and Characterization
The Schiff-bases H2L1 and H2L2 were readily prepared by the condensation reaction of 2−aminobenzylalcohol with 3-ethoxysalicylaldehyde and 5-fluorosalicylaldehyde, respectively, in methanol. The nickel complexes 1 and 2 were prepared by the reaction of nickel acetate tetrahydrate with H2L1 in ethanol, and with H2L2 in methanol, respectively. Consequently, ethanol and methanol molecules were found as terminal ligands in the coordination environment of 1 and 2, respectively (vide infra).
The infrared spectra of the complexes and the free Schiff-bases were recorded in the region 4000–400 cm−1. The intense absorption bands at 1606–1608 cm−1 in the spectra of the complexes are assigned to the imine stretching frequency of the Schiff-base ligands [49]. The shift of the bands to lower frequency, compared to the free Schiff-bases (1612–1620 cm−1), indicates the coordination of the imine nitrogen atom to the NiII ion [49,50]. The phenolic νAr–O in the free Schiff-bases exhibits strong bands at 1245–1255 cm−1, whereas these bands are observed in the lower frequency region at 1183–1192 cm−1 in the complexes, indicating the coordination to the nickel atoms through the phenolate oxygen atoms [51]. The Schiff-base coordination to the nickel atoms is substantiated by prominent bands appearing at low wave numbers of 450-620 cm−1, which can be attributed to ν(Ni–N) and ν(Ni–O) [52].
The UV-vis absorption bands observed around 415–420 nm can be attributed to the transition from the coordinated Schiff-base ligands to the nickel atoms (LMCT) [53]. The bands centered at 241–243 and 300–310 nm may be assigned to the intraligand π-π* and n-π* transitions, respectively [54]. The broad low-intensity absorption bands centered at 570-580 nm are typical d-d bands for the nickel atoms [55].
3.2. Structural Description of the Complexes
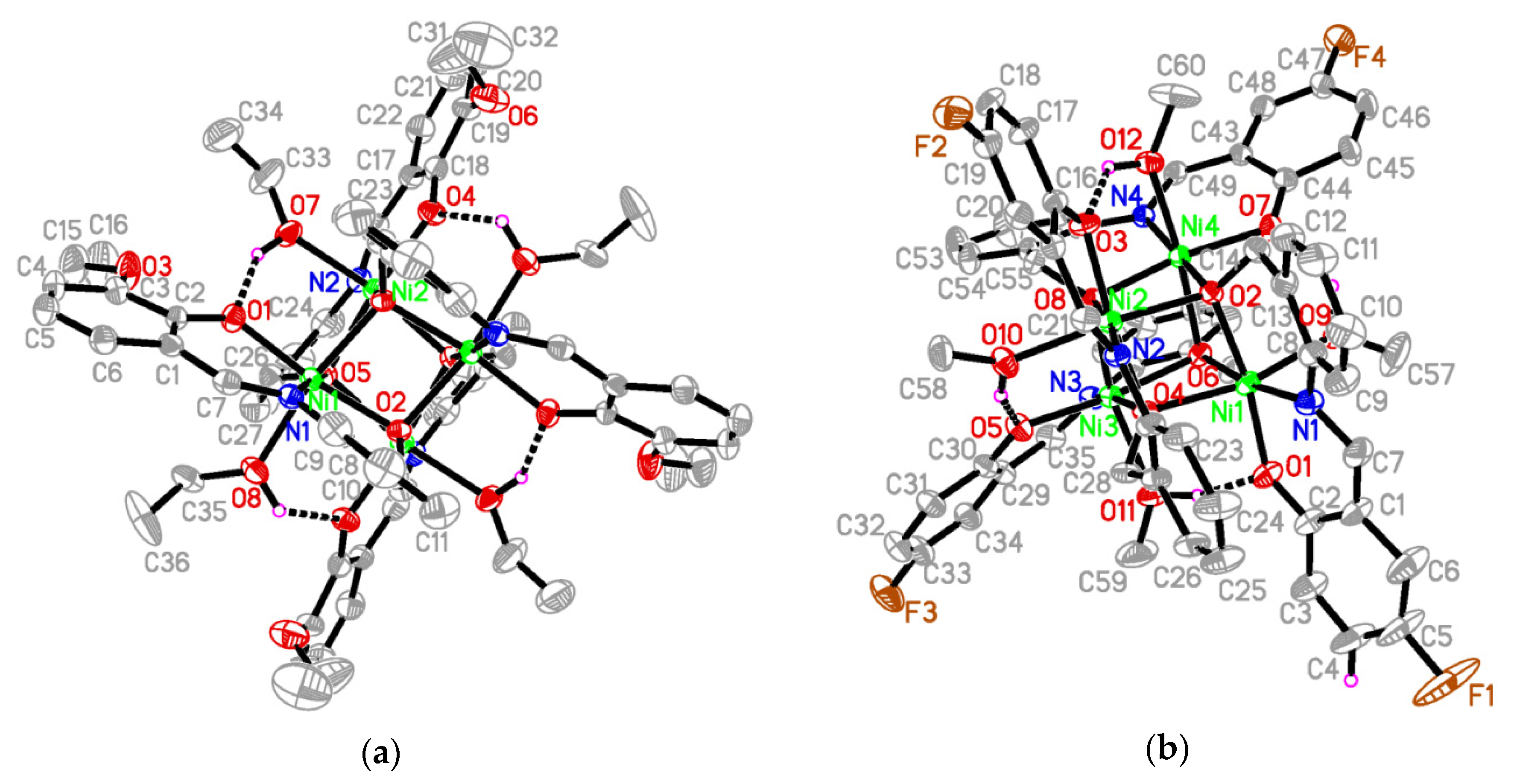
Compounds 1 and 2 crystallize in the monoclinic space groups C2/c and P21/n, respectively, and their molecular structures are shown in Figure 2. Both clusters present analogous stoichiometries, namely, [Ni4(L1)4(EtOH)4] for 1 and [Ni4(L2)4(MeOH)4] for 2, and they reveal the same structural architecture and binding configuration of their ligands. Therefore, the structural description is focused on compound 1. Its main element consists in a [Ni4O4] cubane core, which resides on a crystallographic two-fold rotation axis. The NiII ions are connected by alkoxy oxygens from four anionic Schiff-base ligands (L1)2− exhibiting four μ3-O binding modes. It is worth noting the bridging Ni–O–Ni angles within the cubane core, which amount to 94.6°, 97.8°, 101.6° around O2 and 95.2°, 97.6°, 101.5° around O5, respectively, for complex 1, and amount to 94.5°, 98.6°, 100.6° around O2, 96.0°, 98.3°, 100.3° around O4, 95.3°, 98.7°, 100.3° around O6, and 95.7°, 99.9°, 97.9° around O8, respectively, for complex 2. Figure 3 illustrates the cubane core of 1 and the binding mode of the deprotonated ligand (L1)2− with the NiII ion. The ligand chelates in a nearly coplanar fashion the metal ion in an O^N^O coordination pocket. The alkoxy oxygen connects three adjacent NiII ions in a bridging μ3-O binding mode, whereas the phenolate oxygen binds monodentate to a NiII ion only. In the cluster, the metal ion resides in a slightly distorted octahedral NO5 coordination geometry, comprising oxygens from three alkoxy, one phenolate, and one ethoxy group from a ligated solvent molecule, besides one imino nitrogen. We note also that the hydroxyl hydrogen atoms of the solvent ligands form intracluster hydrogen bonds with adjacent phenolate oxygen atoms (Figure 2 and Table 3).
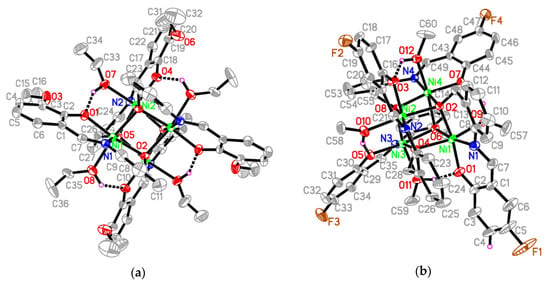
Figure 2.
Crystal structures of (a) complex 1 (unlabeled atoms are related to the symmetry operation 1 − x, y, 3/2 − z.) and of (b) complex 2. Hydrogen atoms except for those related to hydrogen bonds have been omitted for clarity. Intramolecular O–H∙∙∙O hydrogen bonds are shown as dashed lines.
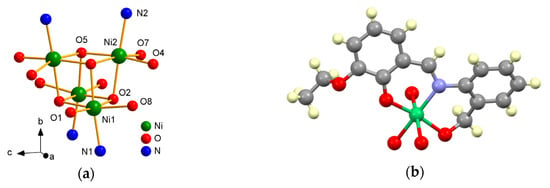
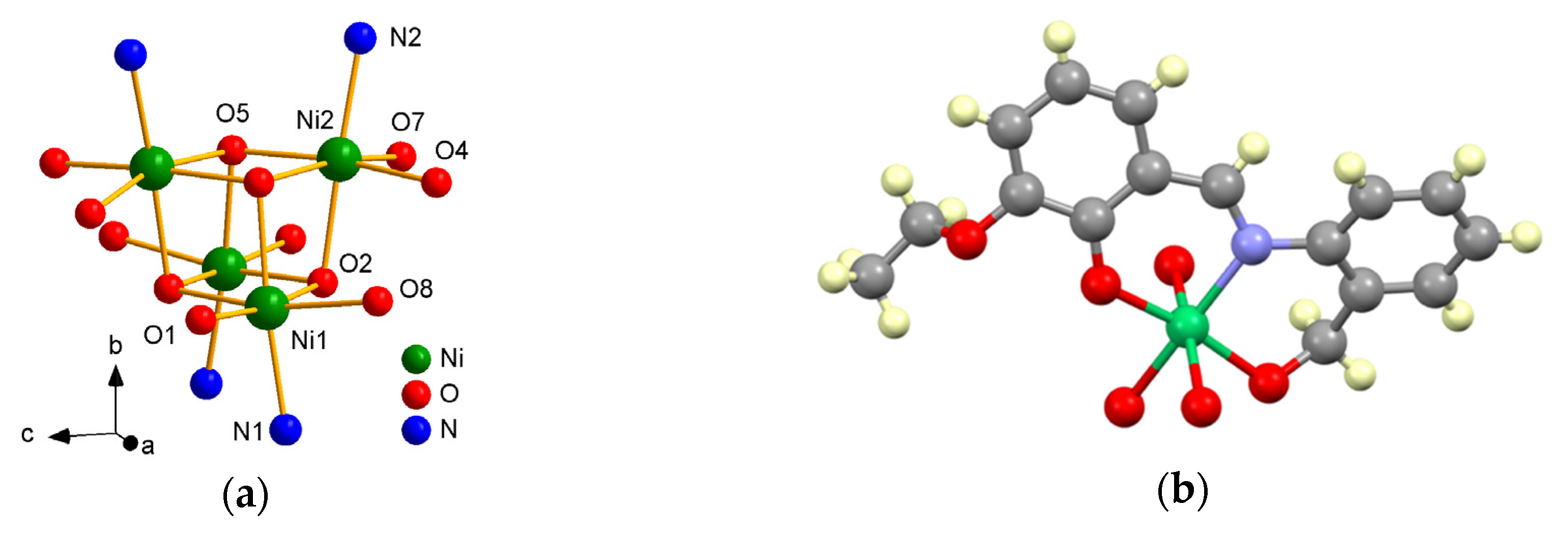
Figure 3.
Cubane core (a) and fragment of structure 1 (b), emphasizing the binding mode of ligand (L1)2− for NiII.

Table 3.
Hydrogen bond distances (Å) and bond angles (°) for complexes 1 and 2.
In contrast to compound 1, there is no crystallographic two-fold rotation axis imposed on cluster 2, which results in an additional scant variation of the structural parameters. The binding mode of (L2)2− with the NiII ion for 2 is shown in Supplementary Materials Figure S1. The mean bridging Ni–O–Ni angles within the cubane core of 2 taken from all four μ3-O atoms amount to 95.4°, 98.3°, and 100.3°, and are thus quite similar to those for 1. The crystal packing of both compounds shows no special feature, and due to the bulky ligand shell around the cubane core, the metal centers on neighbouring molecules are distant from each other (>8.5 Å), which minimizes any intercluster magnetic coupling.
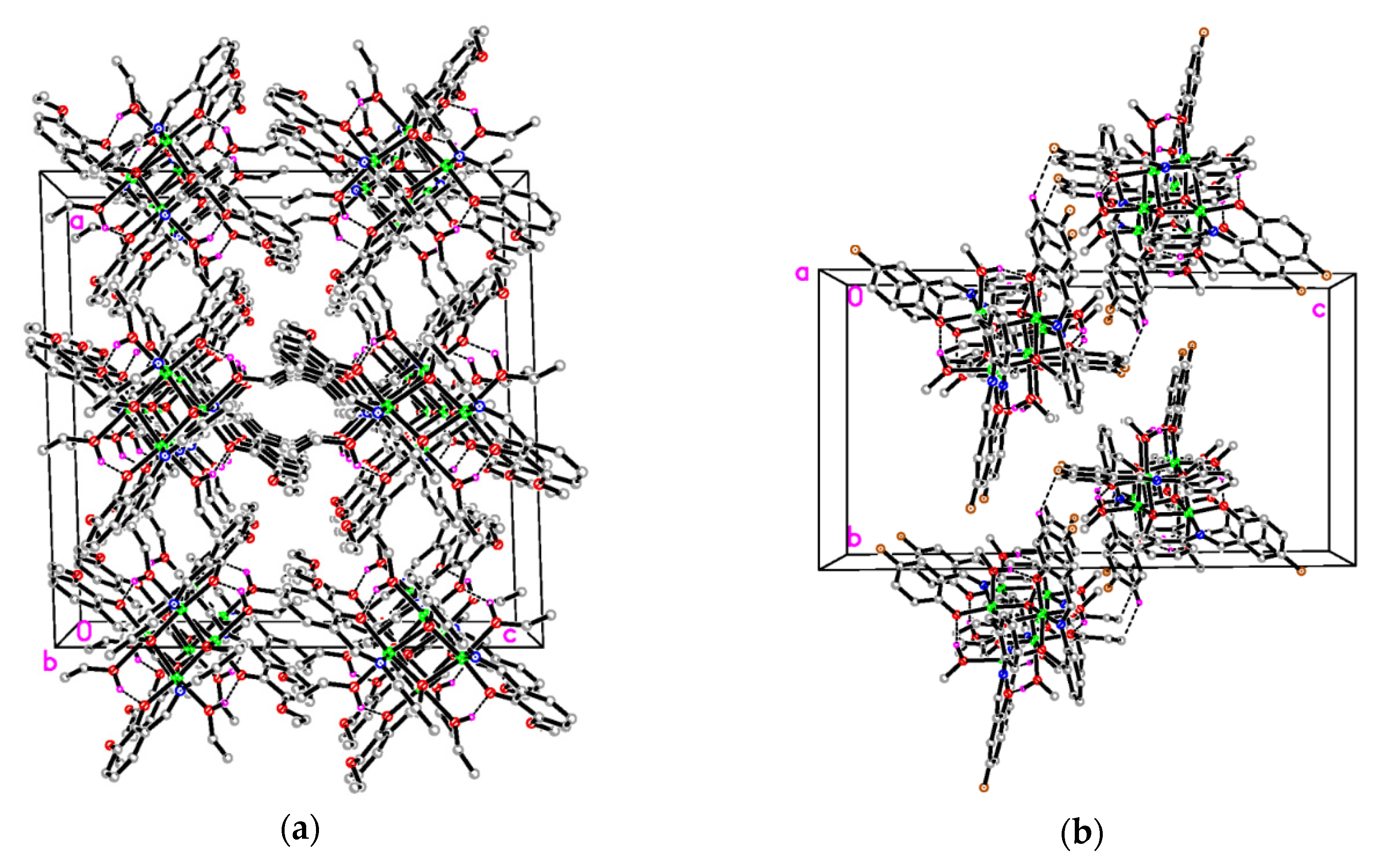
In the crystal structure of 1 (Figure 4a), the molecules are stacked along the b-axis direction. In the crystal structure of 2 (Figure 4b), two adjacent molecules are linked through intermolecular C−H∙∙∙F interactions, forming dimers.
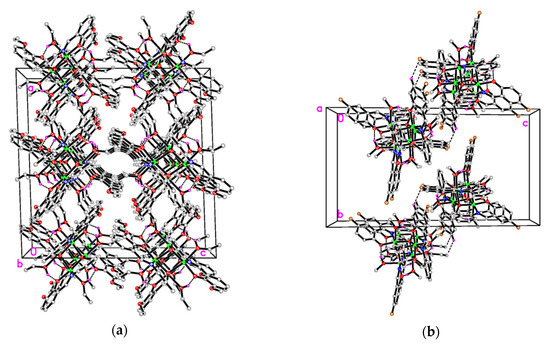
Figure 4.
The molecular packing diagram of complex 1 (a, viewed along the b axis) and 2 (b, viewed along the a axis). Intramolecular O–H∙∙∙O hydrogen and intermolecular C–H∙∙∙F interactions are shown as dashed lines.
3.3. Magnetic Properties
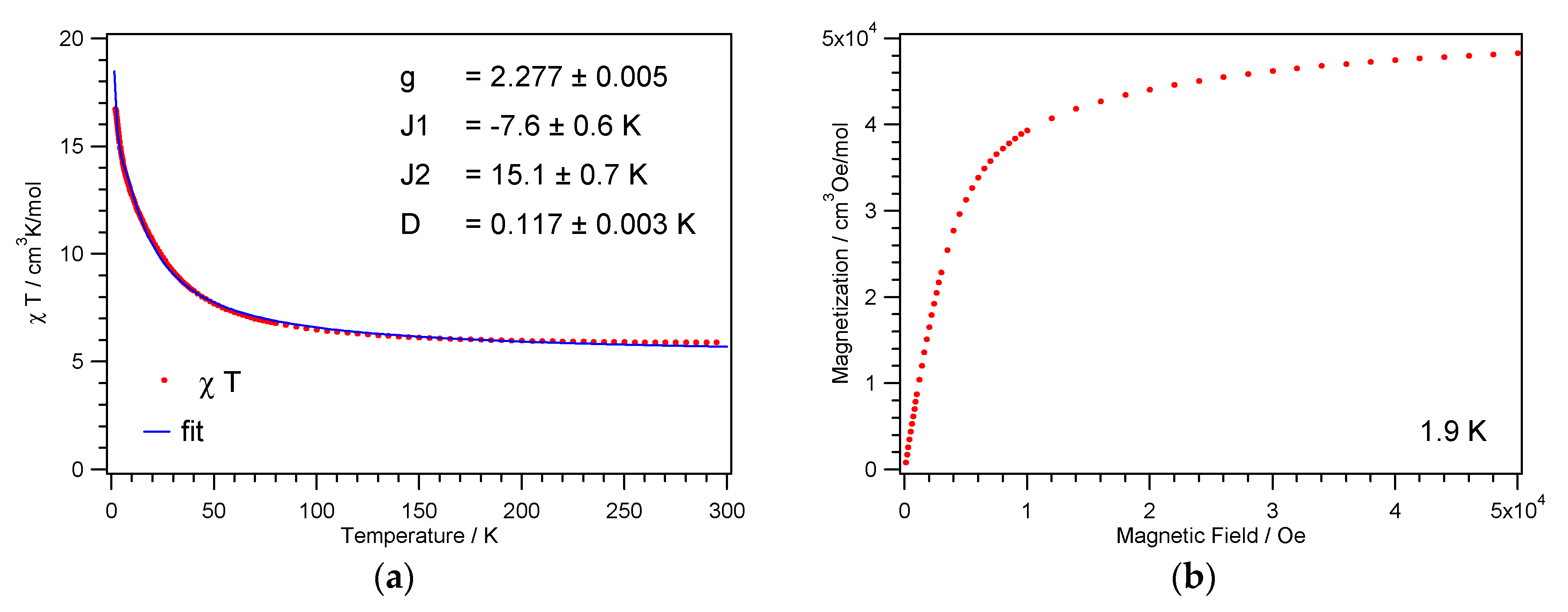
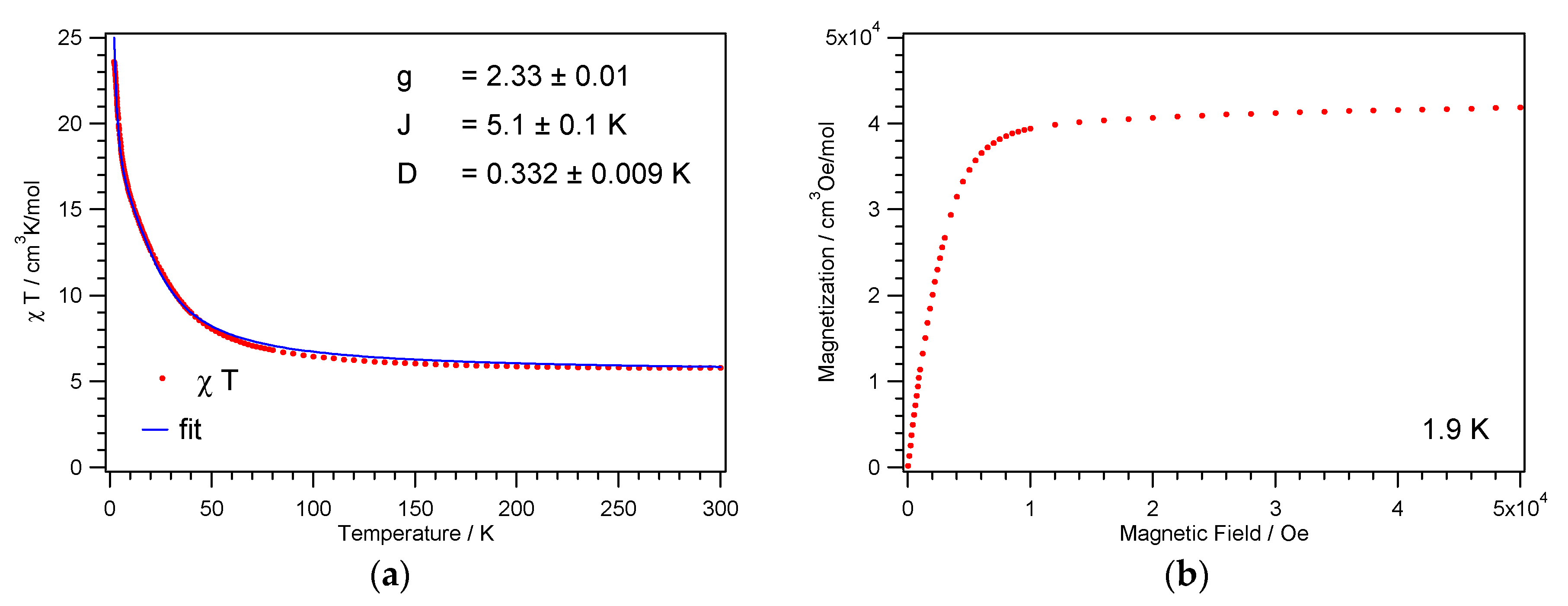
The temperature dependence of the magnetic susceptibilities of the complexes 1 and 2 were each measured on powder samples over the temperature range 1.9–300 K in a 1 kOe magnetic field. Transmission powder X-ray analysis was utilized to ensure that the single-crystal data were representative of the bulk material (Supplementary Materials Figure S2). At room temperature, the χMT product of 1 and 2 amounts to 5.9 and 5.8 cm3 K mol−1, respectively, and is thus greater than the spin-only value of 4.0 cm3 K mol−1 for four noninteracting NiII ions with S = 1 and g = 2. Between 300 and 100 K, the χMT product for 1 and 2 increases slowly; when below 100 K the values increase more rapidly reaching a value of 17 and 24 cm3 K mol−1, respectively (Figure 5a and Figure 6a). This increase in the χMT product is indicative of dominant intracube ferromagnetic interactions between the paramagnetic centers. This goes in line with the 1/χM vs. T plot, where the data above 200 K follow the Curie–Weiss law with a positive Weiss constant θ of 7.0 and 6.8 K, respectively (Figure S3).
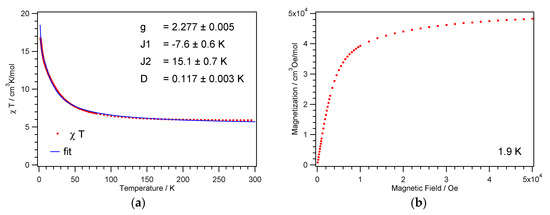
Figure 5.
Temperature dependence of the χMT product (a) and magnetization vs. field (b) for 1.
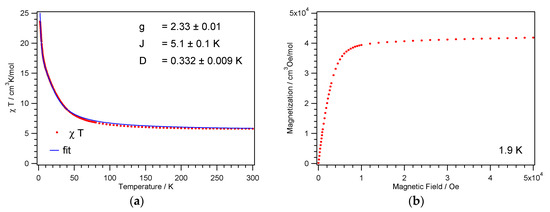
Figure 6.
Temperature dependence of the χMT product (a) and magnetization vs. field (b) for 2.
The field dependence of the magnetization at 1.9 K for 1 and 2 is shown in Figure 5b and Figure 6b. The magnetization shows a rapid increase up to a field of 10 kOe, after which it increases only gradually reaching at 50 kOe values of 8.8 and 7.7 μB, respectively. The initial steep increase of the magnetization points as well to intramolecular ferromagnetic interactions.
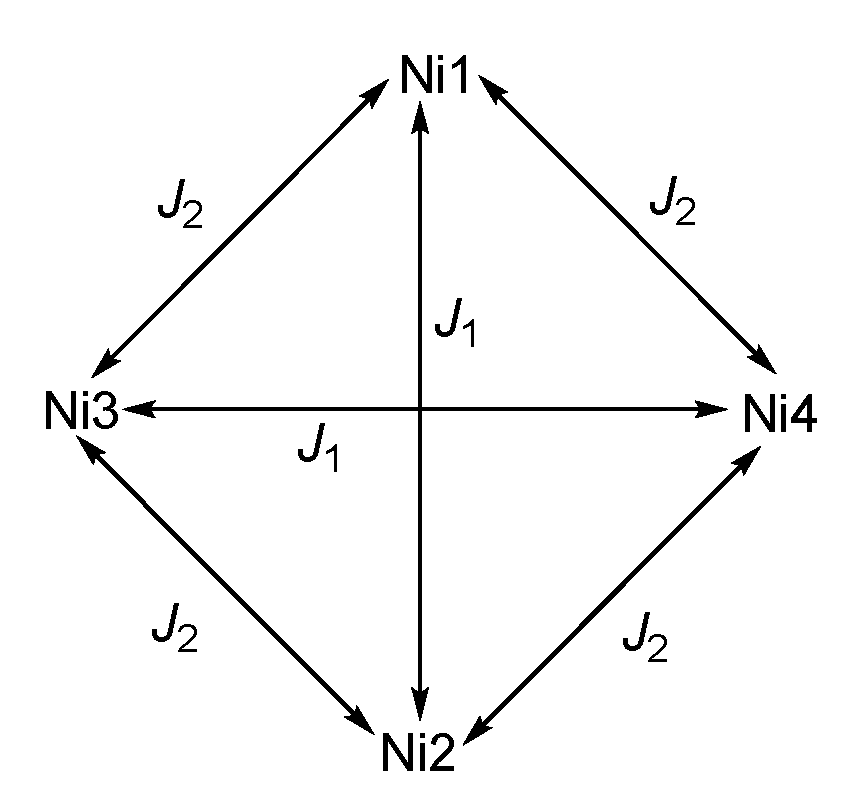
Given that out of the twelve Ni–O–Ni angles per cluster, eight lie in the ferromagnetic and only four in the antiferromagnetic regime, it seems useful to apply a Heisenberg Hamiltonian containing two coupling parameters, J1 and J2,
which is based on the coupling pattern as shown in Scheme 1. The Zero Field Splitting for the ground state is taken into account with the Hamiltonian
H = −2J1(S1S2 + S3S4) − 2J2(S1S3 + S1S4 + S2S3 + S2S4)
H = D[Sz2 − S(S + 1)/3]
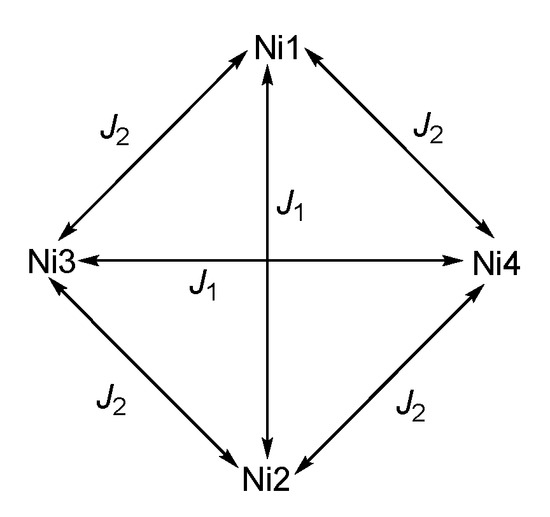
Scheme 1.
Coupling scheme for 2; for 1, the cluster lies on a two-fold rotation axis and thus Ni2 is equivalent to Ni1 and labeled Ni1′; correspondingly, Ni3 and Ni4 are equivalent and labeled as Ni2, with Ni2′ in the structural data, see Figure 3b.
This coupling scheme with the corresponding energy levels, including Van Vlecks’ equation for the magnetic susceptibility and the zero field splitting Hamiltonian, has already been described by Escuer et al. [56]. For 1, the best fit to the χMT product was achieved with parameter values for the g-factor g = 2.28, the antiferromagnetic exchange parameter J1 = −7.6 K, and the ferromagnetic exchange parameter J2 = 15.1 K. The resulting spin ground state, ST = 4, shows a small zero field splitting D = 0.12 K, which is responsible for the continuous increase of the χMT values towards lowest temperatures, see Figure 5. The fit returns well-defined values for g and D; the parameters J1 and J2 are counteracting and exhibit larger error bars. The antiferromagnetic exchange J1 is attributed to the Ni1–O2–Ni1′ and Ni2–O5–Ni2′ paths with Ni–O–Ni angles close to 101.5°, see Figure 3a and Table 2. The ferromagnetic exchange J2 represents an average over the remaining four paths, with Ni–O–Ni angles ranging from 94° to 98°.
For the cubane core of 2, the χMT fit results in g = 2.33, J = 5.1 K, and D = 0.33 K, see Figure 6a. As for 1, the fit yields the spin ground state ST = 4 and returns well-defined g and D parameters. However, an individual fit of J1 and J2 parameters was impossible. These parameters are strongly correlated, and only a single average J could be obtained. The cubane core of 2 exhibits no twofold rotation axis, as present in 1. The reduced symmetry of 2 results in four individual NiII ions, see Scheme 1, and twelve different exchange paths, see Supplementary Materials Table S1. The Ni–O–Ni angles aggregate in three groups around values of 95.4°, 98.3°, and 100.3°, which is similar to 1. Interestingly, a single ferromagnetic J parameter well describes the data. This J parameter matches well the values determined for a NiII cubane cluster with a similar Schiff-base ligand.57 Overall, all the obtained parameters compare well with the range of parameters given in the literature for other NiII cubane clusters [56,57,58,59,60,61,62,63].
4. Conclusions
In summary, two cubane-type compounds comprising a [Ni4O4] core and two different chelating Schiff-base ligands that offer O^N^O coordination pockets have been synthesized and structurally as well as magnetically characterized. Both compounds show dominant intracube ferromagnetic interactions leading to a nonzero spin ground state. It has been demonstrated that even slight structural rearrangements of the cubane core due to a different substitution pattern of the ligands lead to a noticeable variation in strengths of the magnetic coupling between Ni(II) ions. The coupling parameters, however, correlate well with the Ni–O–Ni angles determined from single crystal structure analyses.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/10/7/592/s1, Figure S1: Cubane core (left) and fragment of structure 2 (right), emphasizing the binding mode of ligand (L2)2− for NiII, Table S1: L-Ni-L angles (°) for complexes 1 and 2, Supplementary Materials Figure S2: X-ray powder diffraction pattern with simulation for 1 (top) and 2 (bottom), Figure S3: The 1/χM vs. T plot for 1 (left) and 2 (right).
Author Contributions
Conceptualization, Z.Y.; methodology, Y.L. (Yingying Luo), S.H., and Y.L. (Yanmin Li); investigation, Y.L. (Yingying Luo), S.H., and Y.L. (Yanmin Li); writing—original draft preparation, Z.Y., K.W.K., and S.D.; writing—review and editing, S.-X.L. and S.D.; supervision, Z.Y., K.W.K., and S.D.; project administration, Z.Y. and K.W.K. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful to the Swiss National Science Foundation for financial support under project no. 200020_172659 and the State Key Laboratory of Catalysis of China (Project No. N–19-02).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chaudhuri, P.; Kataev, V.; Büchner, B.; Klauss, H.-H.; Kersting, B.; Meyer, F. Tetranuclear complexes in molecular magnetism: Targeted synthesis, high-field EPR and pulsed-field magnetization. Coord. Chem. Rev. 2009, 253, 2261–2285. [Google Scholar] [CrossRef]
- Thompson, L.K. Polynuclear coordination complexes—From dinuclear to nonanuclear and beyond. Coord. Chem. Rev. 2002, 233–234, 193–206. [Google Scholar] [CrossRef]
- Fielden, J.; Speldrich, M.; Besson, C.; Kögerler, P. Chiral hexanuclear ferric wheels. Inorg. Chem. 2012, 51, 2734–2736. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.; Ram, S. The role of ligands, polytopic ligands and Metal Organic Ligands (Mols) in coordination chemistry. Chem. Sci. Rev. Lett. 2015, 4, 414–428. [Google Scholar]
- Bonanno, M.N.; Lough, J.A.; Poddutoori, K.P.; Lemaire, T.M. Synthesis, characterization and Copper(2+) coordination chemistry of a polytopic paramagnetic ligand. Magnetochemistry 2017, 3, 15. [Google Scholar] [CrossRef]
- Winpenny, R.E.P. Molecular Cluster Magnets; World Scientific: London, UK, 2012. [Google Scholar]
- Gatteschi, D. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Liu, X.; Hamon, J.-R. Recent developments in penta-, hexa- and heptadentate Schiff base ligands and their metal complexes. Coord. Chem. Rev. 2019, 389, 94–118. [Google Scholar] [CrossRef]
- Clarke, R.M.; Herasymchuk, K.; Storr, T. Electronic structure elucidation in oxidized metal–salen complexes. Coord. Chem. Rev. 2017, 352, 67–82. [Google Scholar] [CrossRef]
- Golcu, A.; Tumer, M.; Demirelli, H.; Wheatley, R.A. Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: Synthesis, characterization, properties and biological activity. Inorg. Chim. Acta 2005, 358, 1785–1797. [Google Scholar] [CrossRef]
- Mondal, K.C.; Kostakis, G.E.; Lan, Y.; Wernsdorfer, W.; Anson, C.E.; Powell, A.K. Defect-dicubane Ni2Ln2 (Ln = Dy, Tb) single molecule magnets. Inorg. Chem. 2011, 50, 11604–11611. [Google Scholar] [CrossRef]
- Kühne, I.A.; Griffiths, K.; Hutchings, A.-J.; Townrow, O.P.E.; Eichhöfer, A.; Anson, C.E.; Kostakis, G.E.; Powell, A.K. Stepwise investigation of the influences of steric groups versus counterions to target Cu/Dy complexes. Cryst. Growth Des. 2017, 17, 5178–5190. [Google Scholar] [CrossRef]
- Gheorghe, R.; Andreea Ionita, G.; Maxim, C.; Caneschi, A.; Sorace, L.; Andruh, M. Aggregation of heptanuclear [MII7] (M = Co, Ni, Zn) clusters by a Schiff-base ligand derived from o-vanillin: Synthesis, crystal structures and magnetic properties. Polyhedron 2019, 171, 269–278. [Google Scholar] [CrossRef]
- Wu, J.-C.; Liu, S.-X.; Keene, T.D.; Neels, A.; Mereacre, V.; Powell, A.K.; Decurtins, S. Coordination chemistry of a π-extended, rigid and redox-active tetrathiafulvalene-fused Schiff-base ligand. Inorg. Chem. 2008, 47, 3452–3459. [Google Scholar] [CrossRef] [PubMed]
- Opstal, T.; Verpoort, F. Synthesis of highly active ruthenium indenylidene complexes for atom-transfer radical polymerization and ring-opening-metathesis polymerization. Angew. Chem. Int. Ed. 2003, 42, 2876–2879. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, B.; Verpoort, F. Atom transfer radical polymerization of vinyl monomers mediated by Schiff base ruthenium−alkylidene catalysts and the adventitious effect of water in polymerizations with the analogous cationic complexes. Macromolecules 2002, 35, 8943–8947. [Google Scholar] [CrossRef]
- Hazra, S.; Koner, R.; Lemoine, P.; Sañudo, E.C.; Mohanta, S. Syntheses, structures and magnetic properties of heterobridged dinuclear and cubane-type tetranuclear complexes of Nickel(II) derived from a Schiff base ligand. Eur. J. Inorg. Chem. 2009, 23, 3458–3466. [Google Scholar] [CrossRef]
- Mukherjee, P.; Drew, M.G.B.; Gómez-García, C.J.; Ghosh, A. The crucial role of polyatomic anions in molecular architecture: Structural and magnetic versatility of five Nickel(II) complexes derived from A N,N,O-Donor Schiff base ligand. Inorg. Chem. 2009, 48, 5848–5860. [Google Scholar] [CrossRef]
- Bonadio, F.; Senna, M.-C.; Ensling, J.; Sieber, A.; Neels, A.; Stoeckli-Evans, H.; Decurtins, S. Cyano-bridged structures based on [MnII(N3O2−Macrocycle)]2+: A synthetic, structural, and magnetic study. Inorg. Chem. 2005, 44, 969–978. [Google Scholar] [CrossRef]
- Pioquinto-Mendoza, J.R.; Rosas-Ortiz, J.A.; Reyes-Martínez, R.; Conelly-Espinosa, P.; Toscano, R.A.; Germán-Acacio, J.M.; Avila-Sorrosa, A.; Baldovino-Pantaleón, O.; Morales-Morales, D. Synthesis, characterization and molecular structures of Ni(II) complexes derived from Schiff base pyridylimine ligands. Inorg. Chim. Acta 2015, 438, 146–152. [Google Scholar] [CrossRef]
- Pioquinto-Mendoza, J.R.; Conelly-Espinosa, P.; Reyes-Martínez, R.; Toscano, R.A.; Germán-Acacio, J.M.; Avila-Sorrosa, A.; Baldovino-Pantaleón, O.; Morales-Morales, D. A simple and facile to prepare Pd(II) complex containing the pyridyl imine ligand [C5H4N-2−CH3C=N-(CH2)3NH2]. Structural characterization and catalytic evaluation in Suzuki–Miyaura C–C couplings. J. Organomet. Chem. 2015, 797, 153–158. [Google Scholar] [CrossRef]
- Williams, A.F. A structural analysis of {M4O4} cubanes where M = Mn and Fe. Dalton Trans. 2008, 6, 818–821. [Google Scholar] [CrossRef]
- Isele, K.; Gigon, F.; Williams, A.F.; Bernardinelli, G.; Franz, P.; Decurtins, S. Synthesis, structure and properties of {M4O4} cubanes containing nickel(ii) and cobalt(ii). Dalton Trans. 2007, 3, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Milios, C.J.; Prescimone, A.; Mishra, A.; Parsons, S.; Wernsdorfer, W.; Christou, G.; Perlepes, S.P.; Brechin, E.K. A rare ferromagnetic manganese(iii) ‘cube’. Chem. Commun. 2007, 2, 153–155. [Google Scholar] [CrossRef]
- Aronica, C.; Chumakov, Y.; Jeanneau, E.; Luneau, D.; Neugebauer, P.; Barra, A.-L.; Gillon, B.; Goujon, A.; Cousson, A.; Tercero, J.; et al. Structure, magnetic properties, polarized neutron diffraction, and theoretical study of a Copper(II) Cubane. Chem. Eur. J. 2008, 14, 9540–9548. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Ding, S.; Xu, X.; Wang, R.; Song, Y.; Wang, Y.; Du, C.-f.; Liu, Z.-l. Synthesis, structure and magnetic properties of a series of cubane-like clusters derived from Schiff base ligands. Polyhedron 2014, 83, 36–43. [Google Scholar] [CrossRef]
- Isele, K.; Franz, P.; Ambrus, C.; Bernardinelli, G.; Decurtins, S.; Williams, A.F. Self-assembly and interconversion of tetranuclear Copper(II) complexes. Inorg. Chem. 2005, 44, 3896–3906. [Google Scholar] [CrossRef]
- Papaefstathiou, G.S.; Escuer, A.; Mautner, F.A.; Raptopoulou, C.; Terzis, A.; Perlepes, S.P.; Vicente, R. Use of the Di-2−pyridyl Ketone/Acetate/Dicyanamide “Blend” in Manganese(II), Cobalt(II) and Nickel(II) Chemistry: Neutral Cubane Complexes. Eur. J. Inorg. Chem. 2005, 5, 879–893. [Google Scholar] [CrossRef]
- Kobayashi, F.; Ohtani, R.; Teraoka, S.; Kosaka, W.; Miyasaka, H.; Zhang, Y.; Lindoy, L.F.; Hayami, S.; Nakamura, M. Syntheses, structures and magnetic properties of tetranuclear cubane-type and heptanuclear wheel-type nickel(ii) complexes with 3-methoxysalicylic acid derivatives. Dalton Trans. 2017, 46, 8555–8561. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Oshio, H. Molecular cubes with high-spin ground states. Sci. Technol. Adv. Mater 2005, 6, 565–570. [Google Scholar] [CrossRef]
- Sartorel, A.; Bonchio, M.; Campagna, S.; Scandola, F. Tetrametallic molecular catalysts for photochemical water oxidation. Chem. Soc. Rev. 2013, 4, 2262–2280. [Google Scholar] [CrossRef]
- Song, F.; Al-Ameed, K.; Schilling, M.; Fox, T.; Luber, S.; Patzke, G.R. Mechanistically Driven Control over Cubane Oxo Cluster Catalysts. J. Am. Chem. Soc. 2019, 141, 8846–8857. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Zhong, C.; Li, S.; Shen, Z.; Pu, J.; Liu, J.; Zhou, Y.; Zhang, H.; Ma, H. (Co/Fe)4O4 cubane-containing nanorings fabricated by phosphorylating cobalt ferrite for highly efficient oxygen evolution reaction. ACS Catal. 2019, 9, 3878–3887. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Tian, J.-W.; Liu, S.; Li, B.; Zhao, J.; Ma, L.-F.; Li, D.-S.; Lan, Y.-Q.; Bu, X. Bi-Microporous metal–organic frameworks with cubane [M4(OH)4] (M=Ni, Co) clusters and pore-space partition for electrocatalytic methanol oxidation reaction. Angew. Chem. Int. Ed. 2019, 58, 12185–12189. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.-C.; Wernsdorfer, W.; Zakharov, L.N.; Karaki, Y.; Yamaguchi, A.; Isidro, R.M.; Lu, G.-D.; Wilson, S.A.; Rheingold, A.L.; Ishimoto, H.; et al. Fast magnetization tunneling in Tetranickel(II) single-molecule magnets. Inorg. Chem. 2006, 45, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Iasco, O.; Chumakov, Y.; Guégan, F.; Gillon, B.; Lenertz, M.; Bataille, A.; Jacquot, J.-F.; Luneau, D. Mapping the magnetic anisotropy inside a Ni4 cubane spin cluster using polarized neutron diffraction. Magnetochemistry 2017, 3, 25. [Google Scholar] [CrossRef]
- Ponomaryov, A.N.; Kim, N.; Hwang, J.; Nojiri, H.; van Tol, J.; Ozarowski, A.; Park, J.; Jang, Z.; Suh, B.; Yoon, S.; et al. Structural tailoring effects on the magnetic behavior of symmetric and asymmetric cubane-type Ni complexes. Chem. Asian J. 2013, 8, 1152–1159. [Google Scholar] [CrossRef]
- Rudbari, H.A.; Lloret, F.; Khorshidifard, M.; Bruno, G.; Julve, M. Effects of electron donating/withdrawing groups in the 5-substituted-2−hydroxybenzaldehyde on the synthesis of neutral cubanes with a NiII4O4 core: Synthesis, crystal structures and magnetic properties. RSC Adv. 2016, 6, 7189–7194. [Google Scholar] [CrossRef]
- Petit, S.; Neugebauer, P.; Pilet, G.; Chastanet, G.; Barra, A.-L.; Antunes, A.B.; Wernsdorfer, W.; Luneau, D. Condensation of a nickel tetranuclear cubane into a heptanuclear single-molecule magnet. Inorg. Chem. 2012, 51, 6645–6654. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Weyhermüller, T.; Bothe, E.; Wieghardt, K.; Chaudhuri, P. Single-atom o-bridged urea in a Dinickel(II) complex together with NiII4, CuII2 and CuII4 complexes of a pentadentate phenol-containing Schiff base with (O,N,O,N,O)-Donor atoms. Eur. J. Inorg. Chem. 2003, 5, 863–875. [Google Scholar] [CrossRef]
- Halcrow, M.A.; Sun, J.-S.; Huffman, J.C.; Christou, G. Structural and magnetic properties of [Ni4(μ3-OMe)4(dbm)4(MeOH)4] and [Ni4(η1,μ3-N3)4(dbm)4(EtOH)4]. Magnetostructural correlations for [Ni4X4]4+ cubane complexes. Inorg. Chem. 1995, 34, 4167–4177. [Google Scholar] [CrossRef]
- Das, A.; Klinke, F.J.; Demeshko, S.; Meyer, S.; Dechert, S.; Meyer, F. Reversible solvatomagnetic effect in novel tetranuclear cubane-type Ni4 complexes and magnetostructural correlations for the [Ni4(μ3-O)4] core. Inorg. Chem. 2012, 51, 8141–8149. [Google Scholar] [CrossRef]
- Karmakar, S.; Khanra, S. Polynuclear coordination compounds: A magnetostructural study of ferromagnetically coupled Ni4O4 cubane core motif. CrystEngComm 2014, 16, 2371–2383. [Google Scholar] [CrossRef]
- Syamal, A.; Kumar, D. New oxozirconium(IV) complexes with the Schiff bases derived from salicylaldehyde or substituted salicylaldehydes and o-aminobenzyl alcohol. Indian J. Chem. Sect. A 1980, 19A, 1018–1020. [Google Scholar]
- Syamal, A.; Singhal, O.P. New dioxouranium(VI) complexes with tridentate dibasic Schiff bases containing ONO donor sets. Transit. Met. Chem. 1979, 4, 179–182. [Google Scholar] [CrossRef]
- Bruker. SMART (Version 5.628) and SAINT (Version 6.02); Bruker AXS Inc.: Madison, WI, USA, 1998. [Google Scholar]
- Sheldrick, G.M. SADABS. Program for Empirical Absorption Correction of Area Detector; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Ray, A.; Sadhukhan, D.; Rosair, G.M.; Gómez-García, C.J.; Mitra, S. An unprecedented CuII–Schiff base complex existing as two different trinuclear units with strong antiferromagnetic couplings. Polyhedron 2009, 28, 3542–3550. [Google Scholar] [CrossRef]
- Marinescu, G.; Madalan, A.M.; Shova, S.; Andruh, M. Tetranuclear Zn(II) complexes with compartmental and dicyanamido ligands: Synthesis, structure, and luminescent properties. J. Coord. Chem. 2012, 65, 1539–1547. [Google Scholar] [CrossRef]
- Majumder, A.; Rosair, G.M.; Mallick, A.; Chattopadhyay, N.; Mitra, S. Synthesis, structures and fluorescence of nickel, zinc and cadmium complexes with the N,N,O-tridentate Schiff base N-2−pyridylmethylidene-2−hydroxy-phenylamine. Polyhedron 2006, 25, 1753–1762. [Google Scholar] [CrossRef]
- El-Sherif, A.A.; Fetoh, A.; Abdulhamed, Y.K.; Abu El-Reash, G.M. Synthesis, structural characterization, DFT studies and biological activity of Cu(II) and Ni(II) complexes of novel hydrazone. Inorg. Chim. Acta 2018, 480, 1–15. [Google Scholar] [CrossRef]
- Sadhukhan, D.; Ray, A.; Pilet, G.; Rizzoli, C.; Rosair, G.M.; Gómez-García, C.J.; Signorella, S.; Bellú, S.; Mitra, S. Weak interactions modulating the dimensionality in supramolecular architectures in three new Nickel(II)-hydrazone complexes, magnetostructural correlation, and catalytic potential for epoxidation of alkenes under phase transfer conditions. Inorg. Chem. 2011, 50, 8326–8339. [Google Scholar] [CrossRef]
- Bessy Raj, B.N.; Prathapachandra Kurup, M.R.; Suresh, E. Synthesis, spectral characterization and crystal structure of N-2−hydroxy-4-methoxybenzaldehyde-N′-4-nitrobenzoyl hydrazone and its square planar Cu(II) complex. Spectrochim. Acta A 2008, 71, 1253–1260. [Google Scholar] [CrossRef]
- Zangrando, E.; Islam, M.T.; Islam, M.A.-A.A.A.; Sheikh, M.C.; Tarafder, M.T.H.; Miyatake, R.; Zahan, R.; Hossain, M.A. Synthesis, characterization and bio-activity of nickel(II) and copper(II) complexes of a bidentate NS Schiff base of S-benzyl dithiocarbazate. Inorg. Chim. Acta 2015, 427, 278–284. [Google Scholar] [CrossRef]
- Escuer, A.; Font-Bardıa, M.; Kumar, S.B.; Solans, X.; Vicente, R. Two new nickel(II) cubane compounds derived from pyridine-2−methoxide (Pym): {Ni4(Pym)4Cl4(CH3OH)4} and {Ni4(Pym)4(N3)4(CH3OH)4}. Crystal structures and magnetic properties. Polyhedron 1999, 18, 909–914. [Google Scholar] [CrossRef]
- Yoshitake, M.; Nishihashi, M.; Ogata, Y.; Yoneda, K.; Yamada, Y.; Sakiyama, H.; Mishima, A.; Ohba, M.; Koikawa, M. Syntheses, structures, and magnetic properties of cubane-based cobalt and nickel complexes with ONO-tridentate ligands. Polyhedron 2017, 136, 136–142. [Google Scholar] [CrossRef]
- Lu, Z.; Fan, T.; Guo, W.; Lu, J.; Fan, C. Synthesis, structure and magnetism of three cubane Cu(II) and Ni(II) complexes based on flexible Schiff-base ligands. Inorg. Chim. Acta 2013, 400, 191–196. [Google Scholar] [CrossRef]
- Wikstrom, J.P.; Nazarenko, A.Y.; Reiff, W.M.; Rybak-Akimova, E.V. Synthesis and characterization of tetrakis(μ-hydroxo)tetrakis(2,2′-dipicolylamine)tetranickel perchlorate, a nickel-hydroxy cubane complex. Inorg. Chim. Acta 2007, 360, 3733–3740. [Google Scholar] [CrossRef]
- Wang, J.; Feng, C.; Ge, C.M.; Zhang, S.; Hai, H. Two new cubane-type tetranuclear compounds of Copper(II), Nickel(II) derived from reduced schiff base ligand: Syntheses, structures and magnetic properties. J. Clust. Sci. 2016, 27, 2001–2011. [Google Scholar] [CrossRef]
- Gungor, E.; Kara, H. Ferromagnetic coupling in two tetranuclear Ni(II) complexes with cubane–like Ni4(μ3–O)4 core: Structure, spectroscopic and luminescence properties. J. Mol. Struct. 2020, 1208, 127859. [Google Scholar] [CrossRef]
- Jana, M.S.; Priego, J.L.; Jiménez-Aparicio, R.; Mondal, T.K. Novel tetranuclear Ni(II) Schiff base complex containing Ni4O4 cubane core: Synthesis, X-ray structure, spectra and magnetic properties. Spectrochim. Acta A 2014, 133, 714–719. [Google Scholar] [CrossRef]
- Torić, F.; Pavlović, G.; Pajić, D.; Cindrić, M.; Zadro, K. Tetranuclear Ni4 cubane complexes with high χT maxima: Magneto-structural analysis. CrystEngComm 2018, 20, 3917–3927. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).