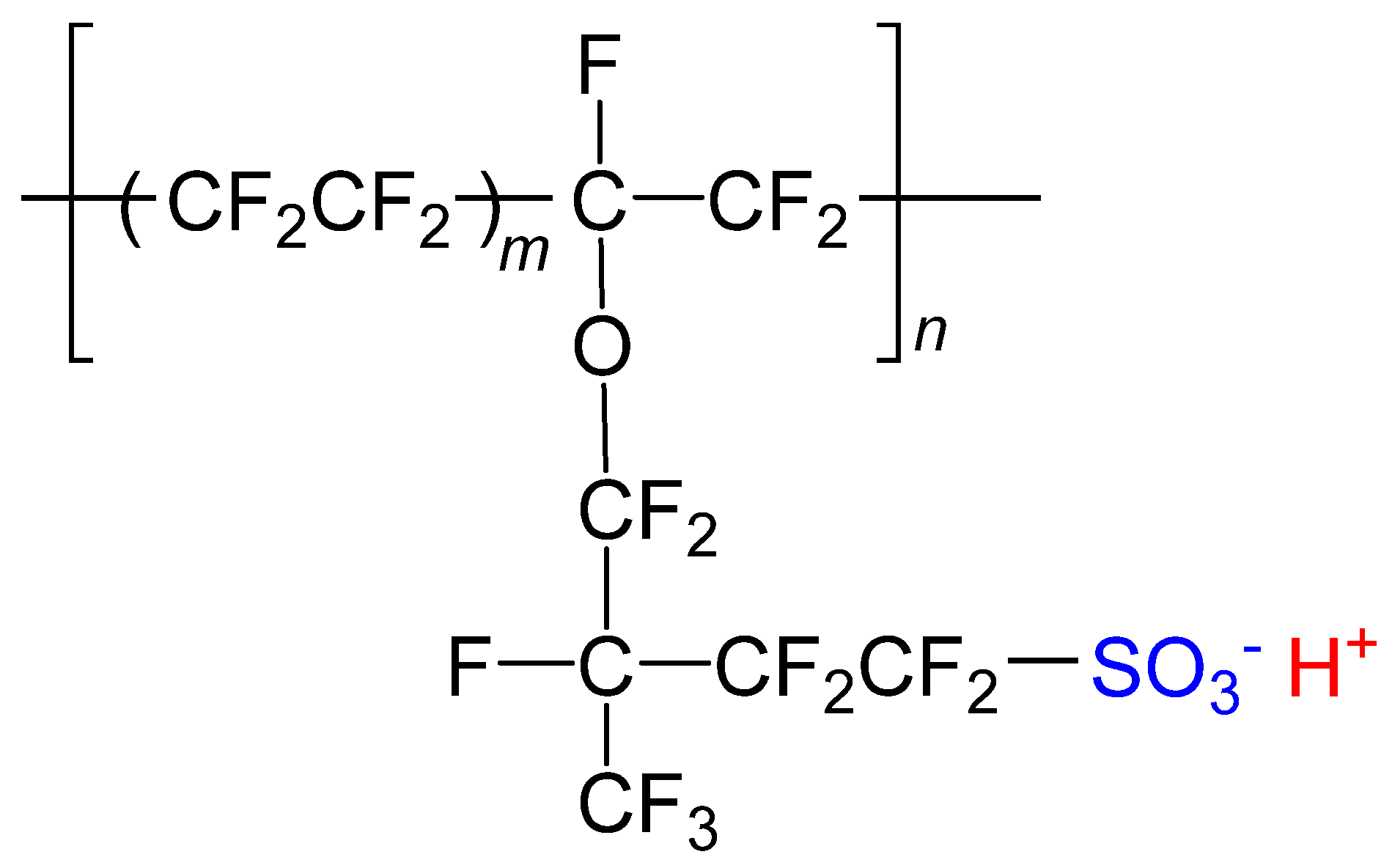Abstract
A transparent film allowing tunable multicolor emission based on a composite of an organometallic compound and a cation-exchange membrane has been developed, in which the cyclometallated iridium(III) complex [IrIII(4Py-ppy)3] (=tris[2-(2-pyridinyl-κN)-4-(4-pyridinyl)phenyl-κC]iridium) (1) with pH-dependent emission wavelengths has been incorporated into Nafion by cation exchange. Soaking Nafion in the solution of 1 for 24 h and exposed to buffers at pH 2, 4, and 10 resulted in maximum emission wavelengths of 587, 560, and 503 nm, respectively. The photophysical properties of 1@Nafion were also enhanced, as its maximum emission wavelength was more blue-shifted than those of 602, 564, and 503 nm in the solutions. The emission quantum yields (Φ) and lifetimes (τ) of 1@Nafion prepared under an acidic condition were up to Φ = 1.8% and τ = 0.11, 0.92 μs, which are considerably higher than the corresponding solutions of Φ = 0.5% and τ = 0.02, 0.18 μs. This is attributed to the fact that 1 is surrounded by the polymer chains of Nafion and immobilized in a relatively rigid medium, which hinders non-radiative deactivation such as thermal relaxation.
1. Introduction
Transparent emitters are expected to be a new energy-saving material because they have less optical loss caused by light scattering by themselves and produce brighter light, and their application to displays and lighting has been actively considered in recent years [1,2]. In addition, durability and convenience of preparation are also important. Transparent emitters with all these features strongly need to be developed.
Nafion is well known as a cation-exchangeable and highly proton-conductive membrane composed of a linear polymer of fluorocarbon and sulfonic acid groups (Figure 1). In the case of Nafion 117, reverse micelles consisting of perfluoroalkyl ether groups and hydrophilic -SO3H groups form clusters of ~4 nm in diameter separated by ~5 nm when they are swollen with water [3,4,5,6,7]. Thanks to the cation exchange properties, simply soaking Nafion in a solution of cationic complexes provides a functional transparent film [8,9,10,11,12,13]. Utilizing this property of Nafion, our group has investigated the synthesis and physical properties of various spin crossover complexes in Nafion [14,15,16,17,18,19,20,21]. We have recently demonstrated transparent films showing pH-dependent spin states accompanied with color changes, where a unique iron(II) complex [FeII(diAMsar)] (diAMsar = 1,8-diaminosarcophagine) was immobilized in the nanospace inside Nafion [14,15]. This scenario can be applied to the development of a transparent and multicolor-tunable luminescence film by adopting complexes showing pH-dependent emission wavelengths. Here, we focus on cyclometallated iridium(III) complexes as a luminophore [22,23].
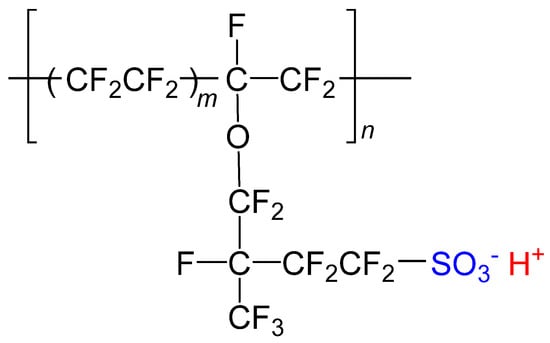
Figure 1.
Structural formula of Nafion (acid form).
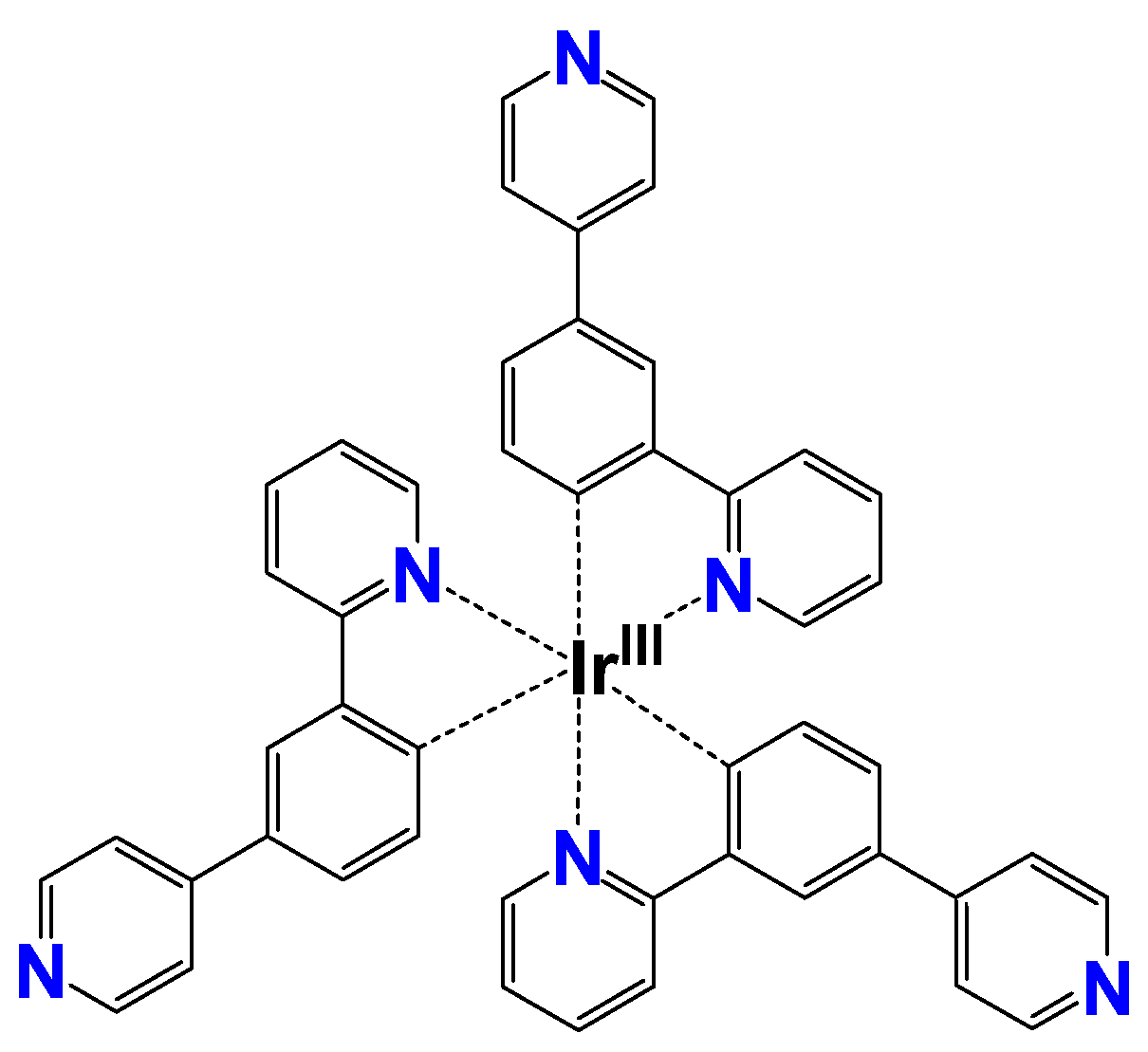
Cyclometallated iridium(III) complexes are known to have higher quantum yields and relatively longer luminescence lifetimes compared to conventional coordination compounds. This is attributed to the strong spin-orbit coupling of the IrIII ion, i.e., the internal heavy atom effect. It facilitates the intersystem crossing from the singlet to the triplet excited states, resulting in strong phosphorescence even at room temperature. Furthermore, it is possible to modulate optical properties such as emission wavelength by chemically modifying the ligands of the complexes. Due to these excellent variable luminescence properties, IrIII complexes are expected to be used for applications such as organic electroluminescence devices [24]. In particular, there is a possibility to achieve changes in luminescence color and intensity according to the protonation on Lewis-base sites. Among them, [IrIII(4Py-ppy)3] (=tris[2-(2-pyridinyl-κN)-4-(4-pyridinyl)phenyl-κC]iridium) (1) is known to emit red light in acidic solutions, yellow in neutral solutions, and green in basic solutions (Figure 2) [25]. In the neutral 1, luminescence derived from metal-to-ligand charge transfer (MLCT) and ligand-centered (LC) transition can be observed. As the pH decreases, the three terminal pyridine groups are protonated in a stepwise manner to become pyridinium species. It has been demonstrated that the emission wavelength is remarkably redshifted for the eventual tricationic [IrIII(4PyH-ppy)3]3+ (1·H3), where the transition mechanism changes to a new low-lying MLCT and an inter-ligand charge transfer (ILCT) [25]. Thus, 1 is considered to be suitable for the development of transparent emitters showing multicolor emission.
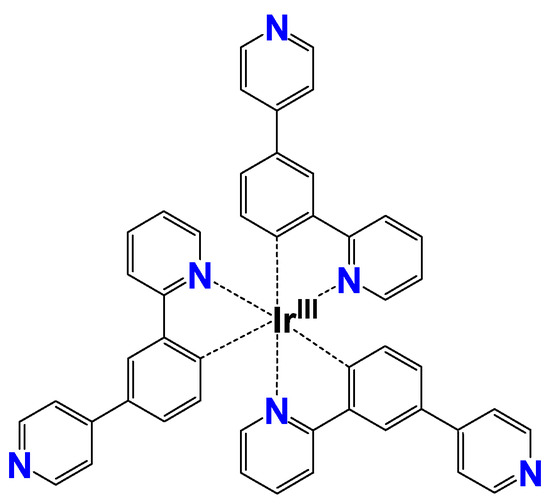
Figure 2.
Molecular structure of [IrIII(4Py-ppy)3] (=tris[2-(2-pyridinyl-κN)-4-(4-pyridinyl)phenyl-κC]iridium) (1).
In this paper, we reported the development of a transparent film 1@Nafion exhibiting multicolor luminescence through the loading of 1 into Nafion film by ion exchange, where variations in proton concentration in the Nafion film are used as an external stimulus. We investigated the pH dependence of the luminescence properties of 1@Nafion by exposure to a buffer solution. In some cases, changes in luminescence properties are dependent on whether the medium surrounding the molecule is a solvent or a polymer. Accordingly, we evaluated how the emission properties are altered by the medium, Nafion, by means of emission spectra, quantum yields, and lifetime measurements for both the solution and the film.
2. Materials and Methods
2.1. Materials and Instruments
All reagents and solvents were obtained from commercially available sources and used without further purification. The Nafion 117, produced by DuPont (Wilmington, DE, USA), was purchased through Furukawa Agency Co., Ltd. (Tokyo, Japan). The iridium(III) complex 1 was synthesized according to the previously reported procedures [25]. The UV-Vis absorption spectra were obtained by using V-530 spectrophotometer (JASCO, Tokyo, Japan). Emission spectra were recorded on RF-6000 Spectrofluorophotometer (SHIMADZU, Tokyo, Japan). The quantum yields were measured using Quantaurus-QY absolute PL quantum yield spectrometer (Hamamatsu Photonics K.K., Shizuoka, Japan) and the luminescence lifetimes were measured using Quantaurus-Tau fluorescence lifetime spectrometer (Hamamatsu Photonics K.K., Shizuoka, Japan).
2.2. Preparation of the Films for Emission Spectroscopy
A Nafion membrane (45W × 15D × 0.187t mm3) was soaked in 2 M NaOH for 24 h to replace the cations within the membrane with Na+ ions (Na form). 1 (1.0 mg, 1.13 μmol) was dissolved in acetonitrile, followed by a nitric acid solution (20 mL) at pH 3. Complexes were loaded into the Nafion film by soaking the Na-formed Nafion film in this mixture for 1 h. The films were divided into three equal pieces (15W × 15D × 0.187t mm3) and the amounts of proton in the films were adjusted using Britton–Robinson buffer (pH 2, 4, and 10) [26].
2.3. Analysis of Ion Exchange
1 (1.02 mg, 1.15 μmol) was dissolved in 60 mL of a mixed solvent as DMSO/H2O = 1/5. Aqueous nitric acid solution (10 mL; 1.0 × 10−3 M) was added to the mixture. This solution (20 mL) was taken in a vial, and H-form Nafion, in which the cations within the film had been replaced with H+ by soaking Nafion in 10% HNO3 aqueous solution for 24 h in advance, was soaked in 1 solution for up to 48 h. To determine the loaded quantity, the UV-Vis absorption spectra of the remaining solution were measured as the membrane was withdrawn from the complex solution and compared to the spectral intensity before soaking the membrane.
3. Results and Discussion
3.1. Emission Spectroscopy
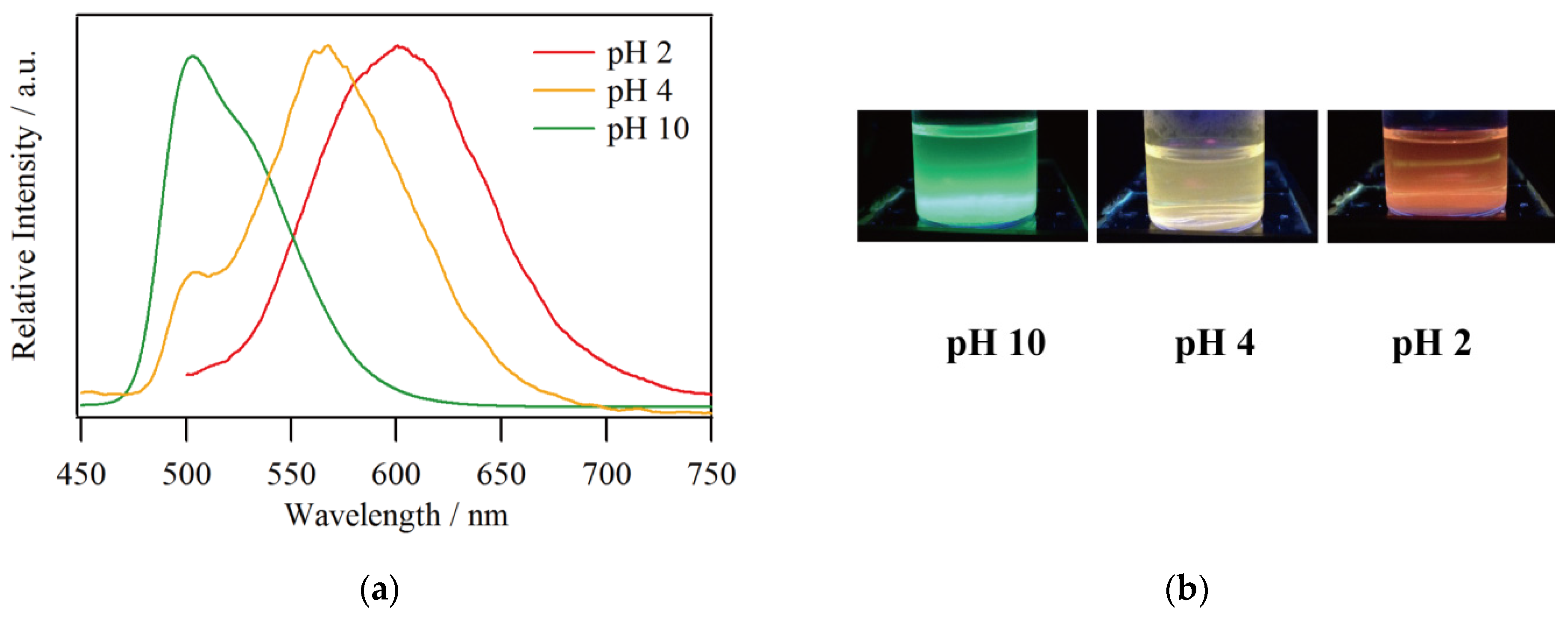
The emission spectra for a solution of 1 in MeOH/CHCl3 = 9/1 with an adjusted proton concentration using Britton–Robinson buffer are shown in Figure 3a. The maximum emission wavelengths were 602, 564, and 503 nm at pH 2, 4, and 10, respectively, demonstrating the remarkable pH dependence of the emission color as can be seen in Figure 3b. Moreover, spectral shoulders were found around 500 and 530 nm at pH 4 and 10, respectively. This behavior is well consistent with the emission spectra in DMSO/buffer reported by S. Aoki et al. in terms of pH sensitivity [25]. This means that the pH sensitivity of compound 1 is not significantly affected by different solvent conditions, indicating the intrinsic luminescence properties of 1 can be reproduced even in MeOH/CHCl3. The pH dependence of the emission wavelength has been supported by density functional theory calculation. The electron density of the HOMO is mainly distributed over IrIII ion and the ppy fragment of the ligand, whether it is 1 or 1·H3 [25]. On the other hand, the distributions with respect to LUMO, LUMO + 1, and LUMO + 2 are present in the ppy fragment for 1 and in the pyridinium fragment for 1·H3. Therefore, protonation reduces the HOMO–LUMO gap and the energy of the new low-lying MLCT states also become lower, leading to the significant red shift in emission wavelength. In fact, the HOMO–LUMO gaps for 1 and 1·H3 are 3.60 and 2.79 eV, respectively.
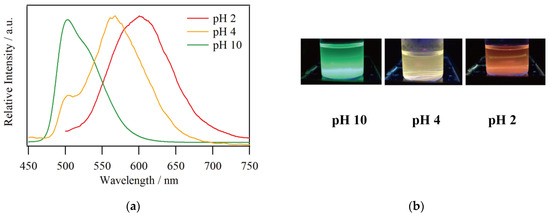
Figure 3.
(a) Emission spectra and (b) photographs of 1 in MeOH/CHCl3 with buffer at pH 2, 4, and 10 under UV irradiation (λex = 365 nm).
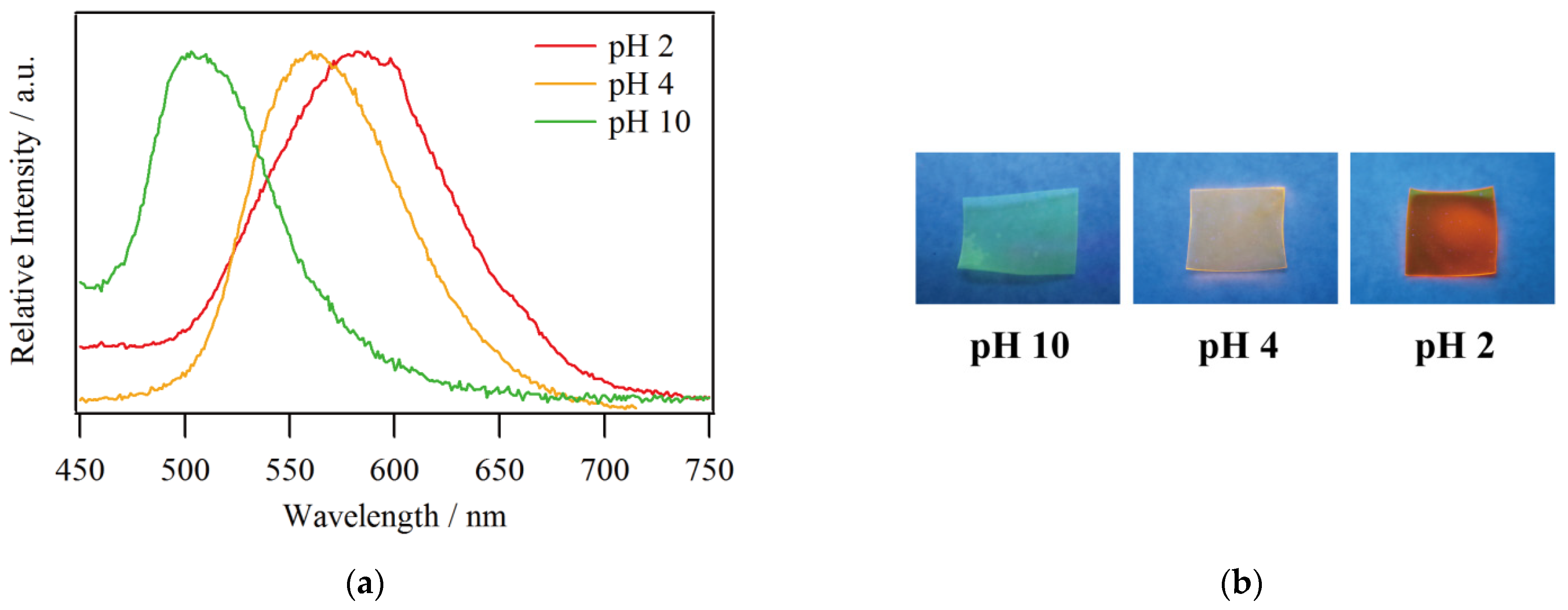
A transparent yellow film was obtained by soaking Na-form Nafion in an acetonitrile solution of compound 1 while ion exchange was enhanced by protons coming from nitric acid. The emission color of 1@Nafion was in close agreement with that of the solution, and the film showed maximum emission wavelengths at 587, 560, and 503 nm at pH 2, 4, and 10, respectively (Figure 4). The emission color change was reversible; the film emitted a greenish light when the as-prepared film was exposed to ammonia vapor and a reddish light after exposing it to nitric acid solution, which was similar to the photographs in Figure 4b. Accordingly, a tunable multicolor-emitting transparent film has been successfully developed. A related reported case is the application of [RuII(bpy)2(dhphen)]@Nafion (bpy = 2,2’-bipyridine, dhphen = 4,7-dihydroxy-1,10-phenanthroline) as a pH sensor [27,28]. The deprotonated dhphen2− works as a π-donor to stabilize the CT-excited state of [RuII(bpy)2(dhphen)], causing a significant red shift of the emission peaks (from 650 to 735 nm) and substantial intensity drop with increasing pH [29]. The [RuII(bpy)2(dhphen)]@Nafion film can serve as a pH sensor over a wide range of pH, from 1 to 8, by monitoring the emission intensity at each change in pH. Regarding the present 1@Nafion, it can be considered to have an advantage in working as a pH sensor which can be recognized with the naked eye, since its emission wavelength varies widely within the visible spectrum in response to a wide range of pH changes.
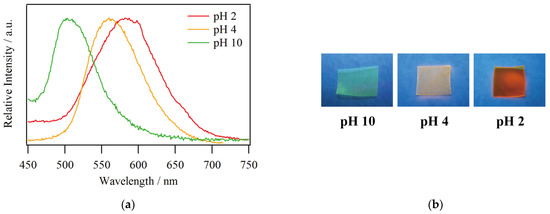
Figure 4.
(a) Emission spectra and (b) photographs of 1@Nafion at pH 2, 4, and 10 under UV irradiation (λex = 365 nm).
Now, the maximum emission wavelength of the film tends to be more blue-shifted than that of the solution, especially at pH 2, where the wavelength shift is 15 nm. This is likely due to the fact that the surrounding environment of the complex molecules is more rigid in the film state than in the solution state. A relevant reported case is that a blueshift of approximately 30 nm was observed in [OsII(bpy)3](PF6)2 in poly(ethylene glycol) dimethacrylate [30]. The effect of the flexibility of the medium surrounding the complex on its photoluminescence is presented here. The mechanism is as follows. In general, the excitation of a molecule by light results in a Frank–Condon excited state, and subsequent relaxation associated with the rearrangement of the surrounding solvent leads to the light emission via a more-stable excited state and a transition back to the ground state. In the film state, the environment around the complex is more rigid than in the solution state, so the thermal relaxation from the Frank–Condon excited state could be hindered and the complex emits light from a higher energy state. Therefore, the maximum emission wavelength is shorter than that of the solution when employing a polymer as a medium.
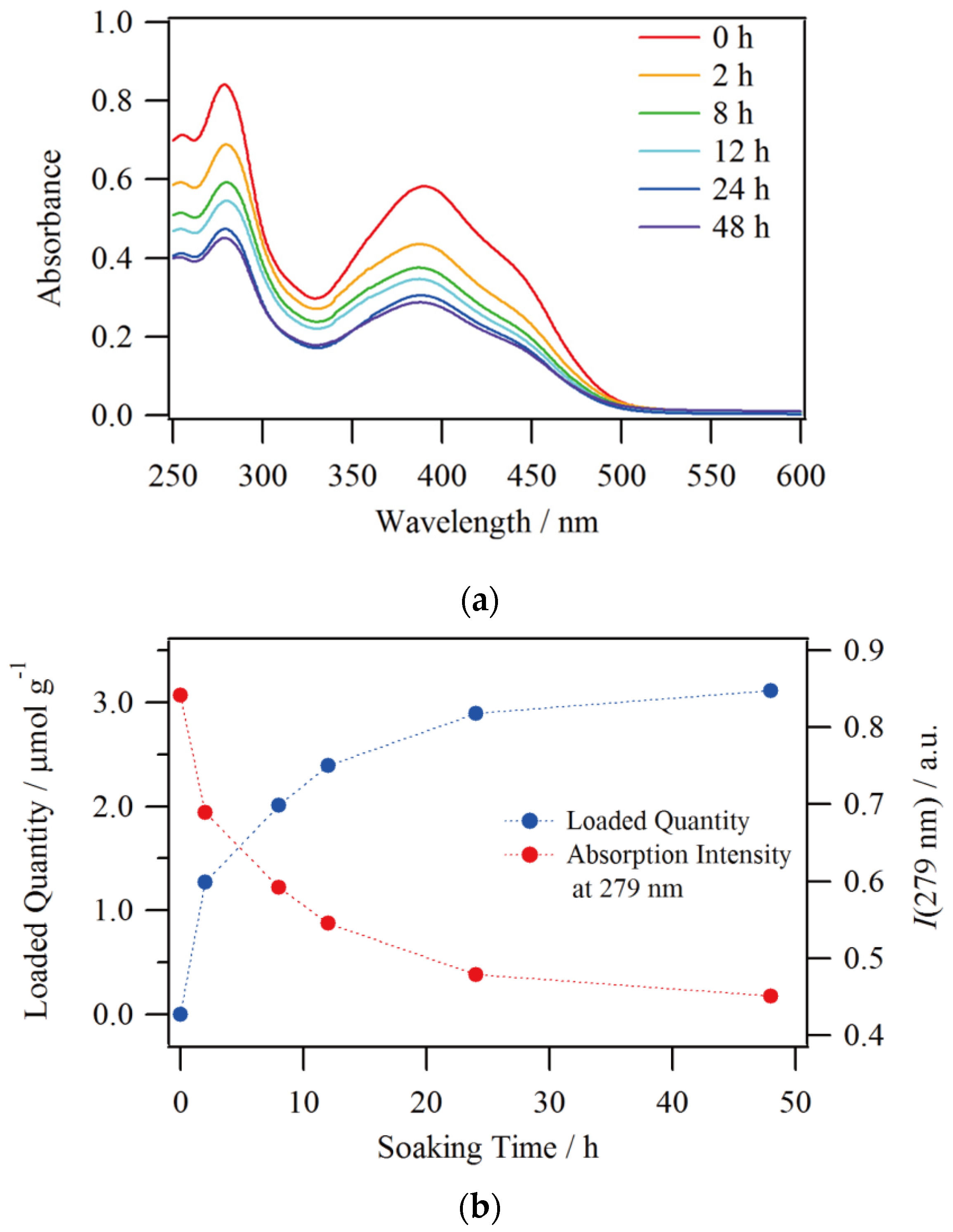
3.2. Analysis of Ion Exchange
Since Nafion has a greater affinity for the more highly charged ions, tricationic [1·H3]3+ was produced by acidifying the DMSO/H2O solution of compound 1 with nitric acid in order to enhance the ion exchange. In addition, it has been reported that DMSO has a much higher degree of swelling to Nafion than other typical organic solvents such as MeOH and CH3CN, which results in higher ion exchange and transport performances [31,32]. These features enabled us to monitor the ion exchange rate of compound 1 by UV-Vis absorption spectroscopy. The absorption spectra of the solutions with respect to Nafion soaking time are shown in Figure 5a. The initial spectrum has peaks at 279 and 390 nm with a shoulder around 440 nm. This spectral shape is in good agreement with the reported 1·H3 [25]. A relatively large decrease in absorbance was observed at 2 h after starting the soaking, and then the decrease became small, and there was almost no difference in spectral intensity between 24 and 48 h of the soaking time. The calculated quantity of complexes loaded into Nafion according to these absorbance ratios at 279 nm are shown in Figure 5b, and it can be seen that the loaded quantity into the film increased with increasing the soaking time. The loaded quantity almost reached saturation at 24 h, and the final amount of complexes loaded at 48 h was 3.11 μmol per 1 gram of Nafion. Besides, a monoexponential fit on the decreasing process of solution concentration ([1]sol) was performed as follows, which is also shown in Figure S1 in Supplementary Materials.
[1]sol = 9.04 + 6.92e−0.136t
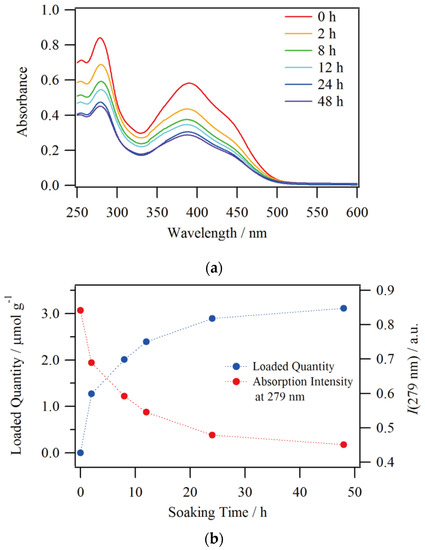
Figure 5.
Soaking time dependence of (a) UV-Vis absorption spectra and (b) loaded quantity of 1@Nafion.
The experimental half-life (t1/2) for the terminal concentration (8.82 μmol L−1) was determined from Equation (1) to be t1/2 = 4.8 h. Nonetheless, it has been reported experimentally and theoretically that ion exchange occurs through two or more steps in common ion exchange resins [33,34]. An informative report on metal complexes with Nafion as an ion-exchange resin includes the investigation concerning the ion-exchange of monometallic [(bpy)2RuII(dpp)]2+ (dpp = 2,3-bis(2-pyridyl)pyrazine) and trimetallic [{(bpy)2RuII(dpp)}2RhIIIBr2]5+ with DMF and CH3CN [35]. In the case of [{(bpy)2RuII(dpp)}2RhIIIBr2]5+, it first undergoes a rapid ion exchange with surface sulfonic acid groups on Nafion and then slowly diffuses towards the interior of the membrane, giving rise to a biexponential uptake behavior. By contrast, a monoexponential process of ion exchange occurs for the smaller [(bpy)2RuII(dpp)]2+ since it has a lower diffusion barrier to exchange ions with the sulfonic acid groups in the interior of the membrane. It is appropriate to assign the compound 1 to have a monoexponential ion exchange process in the present condition since 1·H3 is a tricationic and substantially small mononuclear complex. However, much detailed follow-up should be required to describe the precise ion-exchange behavior. In any case, Equation (1) appears to be satisfactory for estimating the loaded quantity into Nafion and t1/2 of the remaining solution to some extent.
3.3. Emission Quantum Yields and Lifetimes
The films for evaluating the photophysical properties were obtained by soaking Nafion in a mixture of DMSO/H2O solution of 1 followed by an aqueous nitric acid solution. The emission quantum yields (Φ) and lifetimes (τ) of 1 solutions and 1@Nafion with the respective soaking times are summarized in Table 1, and the emission decay curves are shown in Figure S2. For the solution as Φ = 0.5% and τ = 0.25 μs, both of which were poorer than those of the films. The rigidity of the environment surrounding the complex is also reflected in the photophysical properties. Note that the emission intensity and quantum yield in Nafion are also related to the humidity. It has been reported that the emission intensity decreases with increasing humidity in [RuII(bpy)3]@Nafion [36]. The reason for this is that the water content of Nafion increases with increasing humidity, which enhances the molecular motion of [RuII(bpy)3] and the subsequent non-radiative deactivation of the excited states. Considering these facts, it is expected that even higher values of Φ and τ in 1@Nafion would be obtained under sufficiently dry conditions, although we did not obtain experimental data on humidity dependence in this study. In terms of the soaking time dependence of the film, the values of τ were nearly comparable at around 0.1 and 1.0 μs, apart from the very fast decay component of the film at 2 h. Nevertheless, a reduction trend in the values of Φ from 1.8 to 1.1% was observed as the soaking time increased. As noted above, since the increase in the soaking time is associated with the rise in the amount of complexes loaded, it is likely that the decrease in Φ was strongly influenced by self-absorption. It is also known that self-absorption affects the intensity of the emission but not the lifetime, so the results obtained with an almost unchanged value of τ seem to be reasonable. The increase of τ in a film has been reported to be almost negligible for an organofluorescent molecule such as acridine (=Ac) in Nafion [37]. For example, the τ of Ac in aqueous solution and Ac@Nafion are 6.55(4) and 6.86 ns, respectively. Another report on the optical properties of [EuIII(phen)(L)3]@Nafion (L = benzoylacetone, dibenzoylmethane, or thenoyltrifluoroacetone) shows that the more bulky compounds experience a sort of steric hindrance arising from the pores of Nafion more strongly [38]. Considering these relevant previous studies, it can be assumed that Nafion as a medium would affect the molecular structure of the complexes in the ground or excited states to some extent, resulting in photophysical properties not found in solutions. The development and investigation of luminescent complexes immobilized in Nafion films are likely to provide not only a great deal of physicochemical interest, but also an important perspective on the requirements for excellent material performances.

Table 1.
Photophysical data of 1 in solution and Nafion film.
4. Conclusions
The transparent emitter allowing various emission colors on a single film has been successfully developed by employing 1, which changes the emission wavelength in response to protons, as a luminophore. We have also investigated the photophysical properties of the complexes in Nafion. The most beneficial aspect of the presented method is the spontaneous uptake of the metal complexes by simply soaking the film in a cationic complex solution, utilizing the cation exchange properties of Nafion. This allows us to fabricate transparent films that exhibit physical properties of complexes in any size with great ease. The photophysical properties in polymers as well as resins are becoming quite interesting. In particular, Nafion itself behaves as a counter anion for cationic complexes and possesses significant ionic environment. Fundamental physicochemical research and applied research on the development of better-performing transparent emitters such as lighting and pH sensor will be continued in the coming years. In the future, the high proton conductivity of Nafion is expected to lead to the development of transparent materials in which luminescence and solid-state ionics can work in a concerted manner.
Supplementary Materials
The followings are available online at https://www.mdpi.com/2073-4352/10/8/653/s1. Figure S1: Monoexponential fit on the decreasing process of solution concentration. Figure S2: Emission decay curves with fittings for the solution of 1 and 1@Nafion at various soaking times.
Author Contributions
H.K. wrote, checked, and edited the original draft. The film preparation and data collection were done by Y.F. and T.Y., and the data analysis and discussion were made by H.K. and A.O. The original draft was checked and edited by A.O., M.T., and N.K. All authors have read the manuscript and agreed to publish it.
Funding
H.K. is very grateful to the fundings by the Sasakawa Scientific Research Grant from The Japan Science Society (28-341), Tokyo Institute of Technology Foundation Research and Educational Grants (28-036), a research grant from The Mazda Foundation (16KK-354), a research grant from Izumi Science and Technology Foundation (H29-J-109), a research grant from Iketani Science and Technology Foundation (0301067-A).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kitai, A. (Ed.) Luminescent Materials and Applications; WILEY: Chichester, UK, 2008. [Google Scholar]
- Nalwa, H.S.; Rohwer, L.S. (Eds.) Handbook of Luminescence, Display Materials and Devices; American Scientific Publishers: Valencia, Spain, 2003. [Google Scholar]
- Schmidt-Rohr, K.; Chen, Q. Parallel cylindrical water nanochannels in Nafion fuel-cell membranes. Nat. Mater. 2008, 7, 75–83. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Heitner-Wirguin, C. Recent advances in perfluorinated ionomer membranes: Structure, properties and applications. J. Membr. Sci. 1996, 120, 1–33. [Google Scholar] [CrossRef]
- Zawodzinski, T.A., Jr.; Neeman, M.; Sillerud, L.O.; Gottesfeld, S. Determination of water diffusion coefficients in perfluorosulfonate ionomeric membranes. J. Phys. Chem. 1991, 95, 6040–6044. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Gierke, T.D. Ion transport and clustering in nafion perfluorinated membranes. J. Membr. Sci. 1983, 13, 307–326. [Google Scholar] [CrossRef]
- Kosaka, W.; Tozawa, M.; Hashimoto, K.; Ohkoshi, S. Synthesis and superparamagnetic property of a Co–Cr Prussian blue analogue nanoparticles inside Nafion membrane. Inorg. Chem. Commun. 2006, 9, 920–922. [Google Scholar] [CrossRef]
- Tozawa, M.; Ohkoshi, S.; Kojima, N.; Hashimoto, K. Ion-exchange synthesis and magneto-optical spectra of colored magnetic thin films composed of metal(II) hexacyanochromate(III). Chem. Commun. 2003, 10, 1204–1205. [Google Scholar] [CrossRef]
- Funasako, Y.; Takaki, A.; Inokuchi, M.; Mochida, T. Photo-, Thermo-, and Piezochromic Nafion Film Incorporating Cationic Spiropyran. Chem. Lett. 2016, 45, 1397–1399. [Google Scholar] [CrossRef]
- Hosokawa, H.; Mochida, T. Colorimetric Humidity and Solvent Recognition Based on a Cation-Exchange Clay Mineral Incorporating Nickel(II)-Chelate Complexes. Langmuir 2015, 31, 13048–13053. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, H.; Funasako, Y.; Mochida, T. Colorimetric Solvent Indicators Based on Nafion Membranes Incorporating Nickel(II)-Chelate Complexes. Chem. Eur. J. 2014, 20, 15014–15020. [Google Scholar] [CrossRef]
- Funasako, Y.; Mochida, T. Thermochromic and solvatochromic Nafion films incorporating cationic metal-chelate complexes. Chem. Commun. 2013, 49, 4688–4690. [Google Scholar] [CrossRef] [PubMed]
- Kamebuchi, H.; Jo, T.; Shimizu, H.; Okazawa, A.; Enomoto, M.; Kojima, N. Development of pH-Sensitive Spin-crossover Iron(II) Complex Films, [FeII(diAMsar)]–Nafion: Manipulation of the Spin State by Proton Concentration. Chem. Lett. 2011, 40, 888–889. [Google Scholar] [CrossRef]
- Kamebuchi, H.; Enomoto, M.; Kojima, N. Nafion: Properties, Structure and Applications; Sutton, A., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 119–140. [Google Scholar]
- Nakamoto, A.; Kamebuchi, H.; Enomoto, M.; Kojima, N. Study on the spin crossover transition and glass transition for Fe(II) complex film, [Fe(II)(H-triazole)3]@Nafion, by means of Mössbauer spectroscopy. Hyperfine Interact. 2012, 205, 41–45. [Google Scholar] [CrossRef]
- Nakamoto, A.; Kojima, N.; Liu, X.J.; Moritomo, Y.; Nakamura, A. Demonstration of the thermally induced high spin–low spin transition for a transparent spin crossover complex film [Fe(II)(H-trz)3]-Nafion (trz = triazole). Polyhedron 2005, 24, 2909–2912. [Google Scholar] [CrossRef]
- Moritomo, Y.; Isobe, Y.; Liu, X.J.; Kawamoto, T.; Nakamoto, A.; Kojima, N.; Kato, K.; Takata, M. Dynamical phase transition under photo-excitation in a spin-crossover complex. J. Lumin. 2004, 108, 229–232. [Google Scholar] [CrossRef]
- Nakamoto, A.; Ono, Y.; Kojima, N.; Matsumura, D.; Yokoyama, T. Spin Crossover Complex Film, [FeII(H-trz)3]-Nafion, with a Spin Transition around Room Temperature. Chem. Lett. 2003, 32, 336–337. [Google Scholar] [CrossRef]
- Kojima, N.; Toyazaki, S.; Itoi, M.; Ono, Y.; Aoki, W.; Kobayashi, Y.; Seto, M.; Yokoyama, T. Search on Multi-Functional Properties of Spin-Crossover System. Mol. Cryst. Liq. Cryst. 2002, 376, 567–574. [Google Scholar] [CrossRef]
- Liu, X.J.; Moritomo, Y.; Nakamura, A.; Hirao, T.; Toyazaki, S.; Kojima, N. Photoinduced Phase Transition and Relaxation Behavior in a Spin-Crossover Fe(II) Complex Nafion–[Fe(Htrz)3] Film. J. Phys. Soc. Jpn. 2001, 70, 2521–2524. [Google Scholar] [CrossRef]
- Abbas, S.; Din, I.U.; Raheel, A.; Tameez ud Din, A. Cyclometalated Iridium (III) complexes: Recent advances in phosphorescence bioimaging and sensing applications. Appl. Organomet. Chem. 2020, 34, e5413. [Google Scholar] [CrossRef]
- Caporale, C.; Massi, M. Cyclometalated iridium(III) complexes for life science. Coord. Chem. Rev. 2018, 363, 71–91. [Google Scholar] [CrossRef]
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent progress in metal–organic complexes for optoelectronic applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Hisamatsu, Y.; Moromizato, S.; Kohno, M.; Aoki, S. Synthesis and Photochemical Properties of pH Responsive Tris-Cyclometalated Iridium(III) Complexes That Contain a Pyridine Ring on the 2-Phenylpyridine Ligand. Inorg. Chem. 2014, 53, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Britton, H.T.S.; Robinson, R.A. Universal Buffer Solutions and the Dissociation Constant of Veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]
- Chan, C.-M.; Fung, C.-S.; Wong, K.-Y.; Lo, W. Evaluation of a luminescent ruthenium complex immobilized inside Nafion as optical pH sensor. Analyst 1998, 123, 1843–1847. [Google Scholar] [CrossRef]
- Chan, C.-M.; Lo, W.; Wong, K.-Y. Application of a luminescence-based pH optrode to monitoring of fermentation by Klebsiella pneumoniae. Biosens. Bioelectron. 2000, 15, 7–11. [Google Scholar] [CrossRef]
- Giordano, P.J.; Bock, C.R.; Wrighton, M.S. Excited state proton transfer of ruthenium(II) complexes of 4,7-dihydroxy-1,10-phenanthroline. Increased acidity in the excited state. J. Am. Chem. Soc. 1978, 100, 6960–6965. [Google Scholar] [CrossRef]
- Ito, A.; Knight, T.E.; Stewart, D.J.; Brennaman, M.K.; Meyer, T.J. Rigid Medium Effects on Photophysical Properties of MLCT Excited States of Polypyridyl Os(II) Complexes in Polymerized Poly(ethyleneglycol)dimethacrylate Monoliths. J. Phys. Chem. A 2014, 118, 10326–10332. [Google Scholar] [CrossRef]
- Burlatsky, S.; Darling, R.M.; Novikov, D.; Atrazhev, V.V.; Sultanov, V.I.; Astakhova, T.Y.; Su, L.; Brushett, F. Molecular Dynamics Modeling of the Conductivity of Lithiated Nafion Containing Nonaqueous Solvents. J. Electrochem. Soc. 2016, 163, A2232–A2239. [Google Scholar] [CrossRef]
- Doyle, M.; Lewittes, M.E.; Roelofs, M.G.; Perusich, S.A.; Lowrey, R.E. Relationship between ionic conductivity of perfluorinated ionomeric membranes and nonaqueous solvent properties. J. Membr. Sci. 2001, 184, 257–273. [Google Scholar] [CrossRef]
- Turse, R.; Rieman, W., III. Kinetics of ion exchange in a chelating resin. J. Phys. Chem. 1961, 65, 1821–1824. [Google Scholar] [CrossRef]
- Hatanaka, M.; Hoshi, M. Ion-exchange kinetics based on film theory. J. Appl. Polym. Sci. 2014, 131, 39358. [Google Scholar] [CrossRef]
- Naughton, E.M.; Zhang, M.; Troya, D.; Brewer, K.J.; Moore, R.B. Size dependent ion-exchange of large mixed-metal complexes into Nafion® membranes. Polym. Chem. 2015, 6, 6870–6879. [Google Scholar] [CrossRef]
- Kaneko, M.; Hayakawa, S. Application of Polymer-Embedded Tris(2,2’ Bipyridine-Ruthenium(II) to Photodetection of Oxygen. J. Macromol. Sci. Chem. 1988, 25, 1255–1261. [Google Scholar] [CrossRef]
- Ryder, A.G.; Power, S.; Glynn, T.J. Evaluation of Acridine in Nafion as a Fluorescence-Lifetime-Based pH Sensor. Appl. Spectrosc. 2003, 57, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Petushkov, A.A.; Shilov, S.M.; Puzyk, M.V.; Pak, V.N. Luminescence of Europium(III) β-Diketonate Complexes in a Nafion Membrane. Russ. J. Phys. Chem. A 2007, 81, 612–616. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).