Multicolor Emission and Photophysical Properties of Proton-Responsive Cyclometallated Iridium(III) Complex in Transparent Cation-Exchange Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of the Films for Emission Spectroscopy
2.3. Analysis of Ion Exchange
3. Results and Discussion
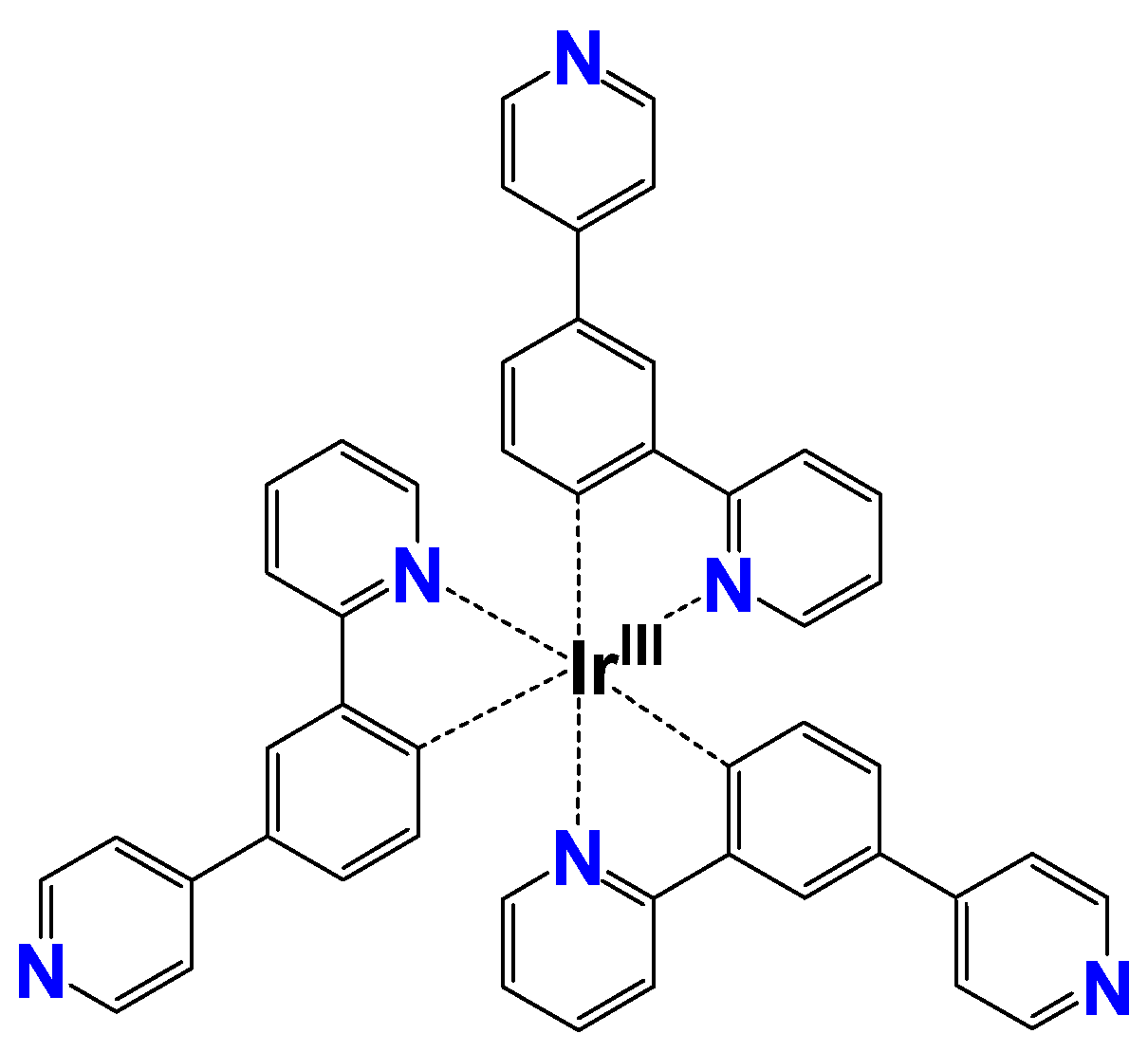
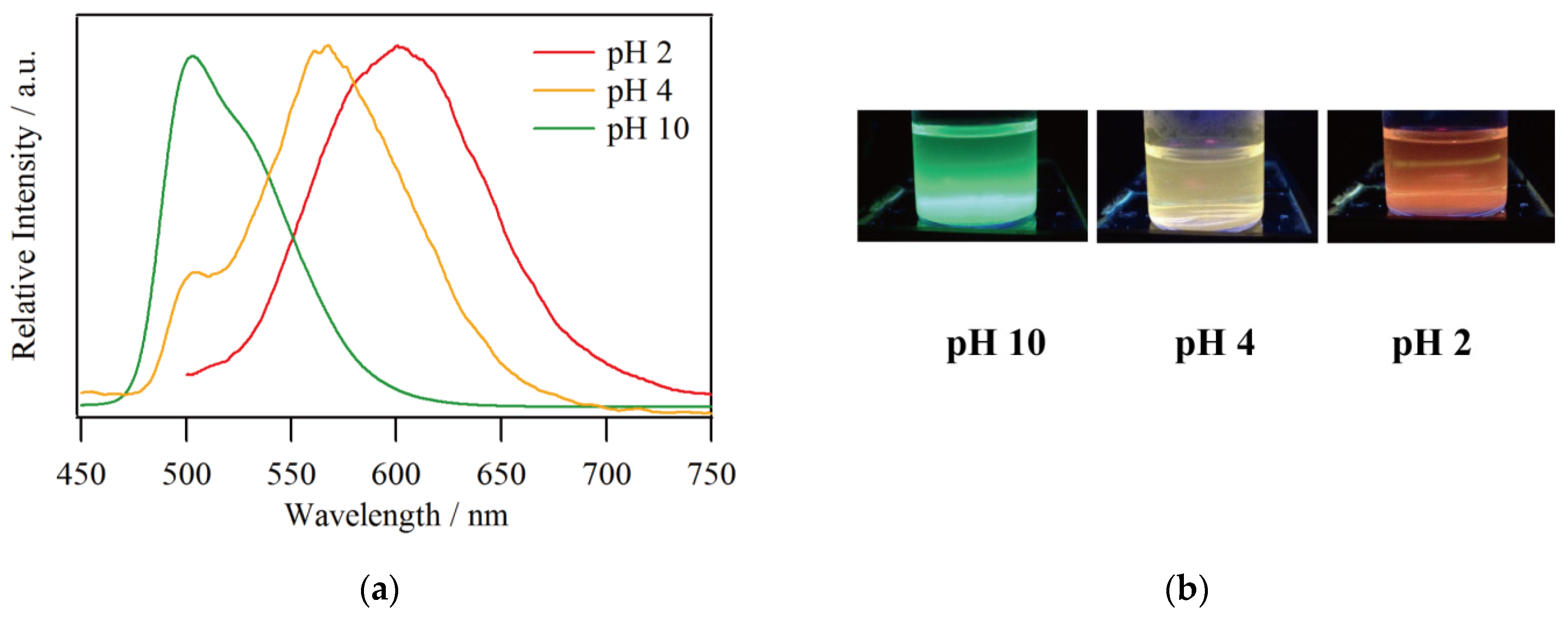
3.1. Emission Spectroscopy
3.2. Analysis of Ion Exchange
3.3. Emission Quantum Yields and Lifetimes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kitai, A. (Ed.) Luminescent Materials and Applications; WILEY: Chichester, UK, 2008. [Google Scholar]
- Nalwa, H.S.; Rohwer, L.S. (Eds.) Handbook of Luminescence, Display Materials and Devices; American Scientific Publishers: Valencia, Spain, 2003. [Google Scholar]
- Schmidt-Rohr, K.; Chen, Q. Parallel cylindrical water nanochannels in Nafion fuel-cell membranes. Nat. Mater. 2008, 7, 75–83. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Heitner-Wirguin, C. Recent advances in perfluorinated ionomer membranes: Structure, properties and applications. J. Membr. Sci. 1996, 120, 1–33. [Google Scholar] [CrossRef]
- Zawodzinski, T.A., Jr.; Neeman, M.; Sillerud, L.O.; Gottesfeld, S. Determination of water diffusion coefficients in perfluorosulfonate ionomeric membranes. J. Phys. Chem. 1991, 95, 6040–6044. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Gierke, T.D. Ion transport and clustering in nafion perfluorinated membranes. J. Membr. Sci. 1983, 13, 307–326. [Google Scholar] [CrossRef]
- Kosaka, W.; Tozawa, M.; Hashimoto, K.; Ohkoshi, S. Synthesis and superparamagnetic property of a Co–Cr Prussian blue analogue nanoparticles inside Nafion membrane. Inorg. Chem. Commun. 2006, 9, 920–922. [Google Scholar] [CrossRef]
- Tozawa, M.; Ohkoshi, S.; Kojima, N.; Hashimoto, K. Ion-exchange synthesis and magneto-optical spectra of colored magnetic thin films composed of metal(II) hexacyanochromate(III). Chem. Commun. 2003, 10, 1204–1205. [Google Scholar] [CrossRef]
- Funasako, Y.; Takaki, A.; Inokuchi, M.; Mochida, T. Photo-, Thermo-, and Piezochromic Nafion Film Incorporating Cationic Spiropyran. Chem. Lett. 2016, 45, 1397–1399. [Google Scholar] [CrossRef]
- Hosokawa, H.; Mochida, T. Colorimetric Humidity and Solvent Recognition Based on a Cation-Exchange Clay Mineral Incorporating Nickel(II)-Chelate Complexes. Langmuir 2015, 31, 13048–13053. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, H.; Funasako, Y.; Mochida, T. Colorimetric Solvent Indicators Based on Nafion Membranes Incorporating Nickel(II)-Chelate Complexes. Chem. Eur. J. 2014, 20, 15014–15020. [Google Scholar] [CrossRef]
- Funasako, Y.; Mochida, T. Thermochromic and solvatochromic Nafion films incorporating cationic metal-chelate complexes. Chem. Commun. 2013, 49, 4688–4690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamebuchi, H.; Jo, T.; Shimizu, H.; Okazawa, A.; Enomoto, M.; Kojima, N. Development of pH-Sensitive Spin-crossover Iron(II) Complex Films, [FeII(diAMsar)]–Nafion: Manipulation of the Spin State by Proton Concentration. Chem. Lett. 2011, 40, 888–889. [Google Scholar] [CrossRef]
- Kamebuchi, H.; Enomoto, M.; Kojima, N. Nafion: Properties, Structure and Applications; Sutton, A., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 119–140. [Google Scholar]
- Nakamoto, A.; Kamebuchi, H.; Enomoto, M.; Kojima, N. Study on the spin crossover transition and glass transition for Fe(II) complex film, [Fe(II)(H-triazole)3]@Nafion, by means of Mössbauer spectroscopy. Hyperfine Interact. 2012, 205, 41–45. [Google Scholar] [CrossRef]
- Nakamoto, A.; Kojima, N.; Liu, X.J.; Moritomo, Y.; Nakamura, A. Demonstration of the thermally induced high spin–low spin transition for a transparent spin crossover complex film [Fe(II)(H-trz)3]-Nafion (trz = triazole). Polyhedron 2005, 24, 2909–2912. [Google Scholar] [CrossRef]
- Moritomo, Y.; Isobe, Y.; Liu, X.J.; Kawamoto, T.; Nakamoto, A.; Kojima, N.; Kato, K.; Takata, M. Dynamical phase transition under photo-excitation in a spin-crossover complex. J. Lumin. 2004, 108, 229–232. [Google Scholar] [CrossRef]
- Nakamoto, A.; Ono, Y.; Kojima, N.; Matsumura, D.; Yokoyama, T. Spin Crossover Complex Film, [FeII(H-trz)3]-Nafion, with a Spin Transition around Room Temperature. Chem. Lett. 2003, 32, 336–337. [Google Scholar] [CrossRef]
- Kojima, N.; Toyazaki, S.; Itoi, M.; Ono, Y.; Aoki, W.; Kobayashi, Y.; Seto, M.; Yokoyama, T. Search on Multi-Functional Properties of Spin-Crossover System. Mol. Cryst. Liq. Cryst. 2002, 376, 567–574. [Google Scholar] [CrossRef]
- Liu, X.J.; Moritomo, Y.; Nakamura, A.; Hirao, T.; Toyazaki, S.; Kojima, N. Photoinduced Phase Transition and Relaxation Behavior in a Spin-Crossover Fe(II) Complex Nafion–[Fe(Htrz)3] Film. J. Phys. Soc. Jpn. 2001, 70, 2521–2524. [Google Scholar] [CrossRef]
- Abbas, S.; Din, I.U.; Raheel, A.; Tameez ud Din, A. Cyclometalated Iridium (III) complexes: Recent advances in phosphorescence bioimaging and sensing applications. Appl. Organomet. Chem. 2020, 34, e5413. [Google Scholar] [CrossRef]
- Caporale, C.; Massi, M. Cyclometalated iridium(III) complexes for life science. Coord. Chem. Rev. 2018, 363, 71–91. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent progress in metal–organic complexes for optoelectronic applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, A.; Hisamatsu, Y.; Moromizato, S.; Kohno, M.; Aoki, S. Synthesis and Photochemical Properties of pH Responsive Tris-Cyclometalated Iridium(III) Complexes That Contain a Pyridine Ring on the 2-Phenylpyridine Ligand. Inorg. Chem. 2014, 53, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Britton, H.T.S.; Robinson, R.A. Universal Buffer Solutions and the Dissociation Constant of Veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]
- Chan, C.-M.; Fung, C.-S.; Wong, K.-Y.; Lo, W. Evaluation of a luminescent ruthenium complex immobilized inside Nafion as optical pH sensor. Analyst 1998, 123, 1843–1847. [Google Scholar] [CrossRef]
- Chan, C.-M.; Lo, W.; Wong, K.-Y. Application of a luminescence-based pH optrode to monitoring of fermentation by Klebsiella pneumoniae. Biosens. Bioelectron. 2000, 15, 7–11. [Google Scholar] [CrossRef]
- Giordano, P.J.; Bock, C.R.; Wrighton, M.S. Excited state proton transfer of ruthenium(II) complexes of 4,7-dihydroxy-1,10-phenanthroline. Increased acidity in the excited state. J. Am. Chem. Soc. 1978, 100, 6960–6965. [Google Scholar] [CrossRef]
- Ito, A.; Knight, T.E.; Stewart, D.J.; Brennaman, M.K.; Meyer, T.J. Rigid Medium Effects on Photophysical Properties of MLCT Excited States of Polypyridyl Os(II) Complexes in Polymerized Poly(ethyleneglycol)dimethacrylate Monoliths. J. Phys. Chem. A 2014, 118, 10326–10332. [Google Scholar] [CrossRef]
- Burlatsky, S.; Darling, R.M.; Novikov, D.; Atrazhev, V.V.; Sultanov, V.I.; Astakhova, T.Y.; Su, L.; Brushett, F. Molecular Dynamics Modeling of the Conductivity of Lithiated Nafion Containing Nonaqueous Solvents. J. Electrochem. Soc. 2016, 163, A2232–A2239. [Google Scholar] [CrossRef] [Green Version]
- Doyle, M.; Lewittes, M.E.; Roelofs, M.G.; Perusich, S.A.; Lowrey, R.E. Relationship between ionic conductivity of perfluorinated ionomeric membranes and nonaqueous solvent properties. J. Membr. Sci. 2001, 184, 257–273. [Google Scholar] [CrossRef]
- Turse, R.; Rieman, W., III. Kinetics of ion exchange in a chelating resin. J. Phys. Chem. 1961, 65, 1821–1824. [Google Scholar] [CrossRef]
- Hatanaka, M.; Hoshi, M. Ion-exchange kinetics based on film theory. J. Appl. Polym. Sci. 2014, 131, 39358. [Google Scholar] [CrossRef]
- Naughton, E.M.; Zhang, M.; Troya, D.; Brewer, K.J.; Moore, R.B. Size dependent ion-exchange of large mixed-metal complexes into Nafion® membranes. Polym. Chem. 2015, 6, 6870–6879. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, M.; Hayakawa, S. Application of Polymer-Embedded Tris(2,2’ Bipyridine-Ruthenium(II) to Photodetection of Oxygen. J. Macromol. Sci. Chem. 1988, 25, 1255–1261. [Google Scholar] [CrossRef]
- Ryder, A.G.; Power, S.; Glynn, T.J. Evaluation of Acridine in Nafion as a Fluorescence-Lifetime-Based pH Sensor. Appl. Spectrosc. 2003, 57, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petushkov, A.A.; Shilov, S.M.; Puzyk, M.V.; Pak, V.N. Luminescence of Europium(III) β-Diketonate Complexes in a Nafion Membrane. Russ. J. Phys. Chem. A 2007, 81, 612–616. [Google Scholar] [CrossRef]
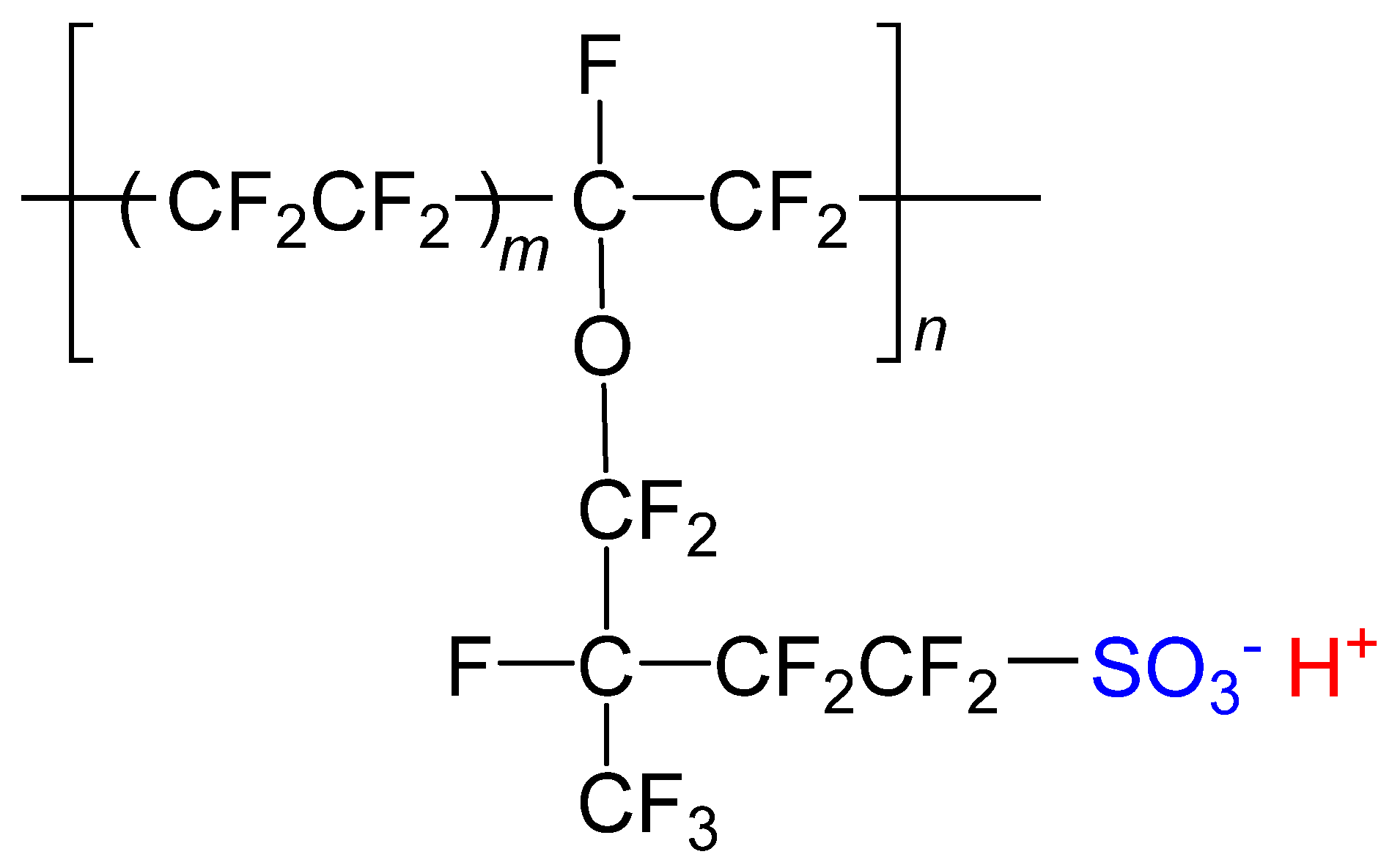


| State | Φ/% | τ/s | Loaded Quantity b/μmol g−1 |
|---|---|---|---|
| Solution a | 0.5 | 0.02, 0.18 | - |
| Film (2 h) | 1.8 | 0.005, 0.11, 0.92 | 1.27 |
| Film (12 h) | 1.7 | 0.11, 1.04 | 2.39 |
| Film (48 h) | 1.1 | 0.18, 1.06 | 3.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamebuchi, H.; Fujimura, Y.; Yoshioka, T.; Okazawa, A.; Tadokoro, M.; Kojima, N. Multicolor Emission and Photophysical Properties of Proton-Responsive Cyclometallated Iridium(III) Complex in Transparent Cation-Exchange Membrane. Crystals 2020, 10, 653. https://doi.org/10.3390/cryst10080653
Kamebuchi H, Fujimura Y, Yoshioka T, Okazawa A, Tadokoro M, Kojima N. Multicolor Emission and Photophysical Properties of Proton-Responsive Cyclometallated Iridium(III) Complex in Transparent Cation-Exchange Membrane. Crystals. 2020; 10(8):653. https://doi.org/10.3390/cryst10080653
Chicago/Turabian StyleKamebuchi, Hajime, Yu Fujimura, Taiho Yoshioka, Atsushi Okazawa, Makoto Tadokoro, and Norimichi Kojima. 2020. "Multicolor Emission and Photophysical Properties of Proton-Responsive Cyclometallated Iridium(III) Complex in Transparent Cation-Exchange Membrane" Crystals 10, no. 8: 653. https://doi.org/10.3390/cryst10080653





