Multifunctional Electrochemical Properties of Synthesized Non-Precious Iron Oxide Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Magnetic Iron Oxide Nanoparticles
2.2. Physical Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. X-ray Diffraction Studies
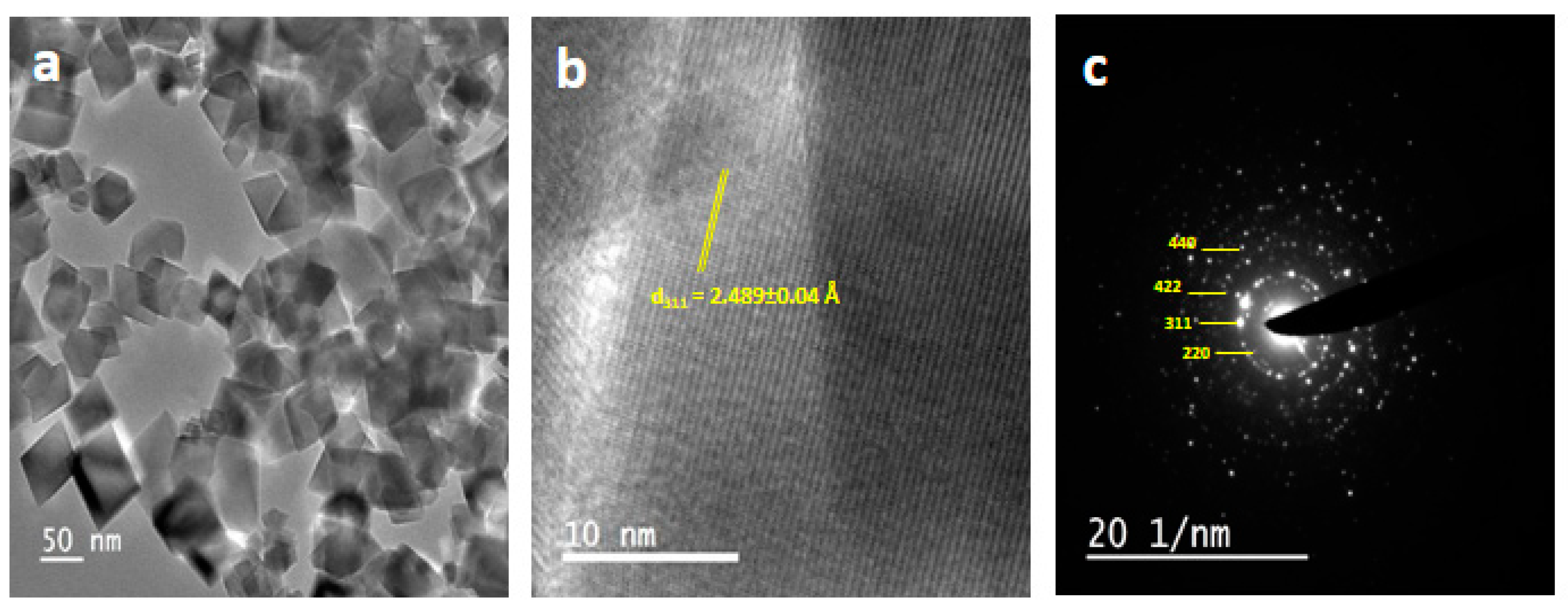
3.2. Electron Microscopic Studies
3.3. Magnetic Properties
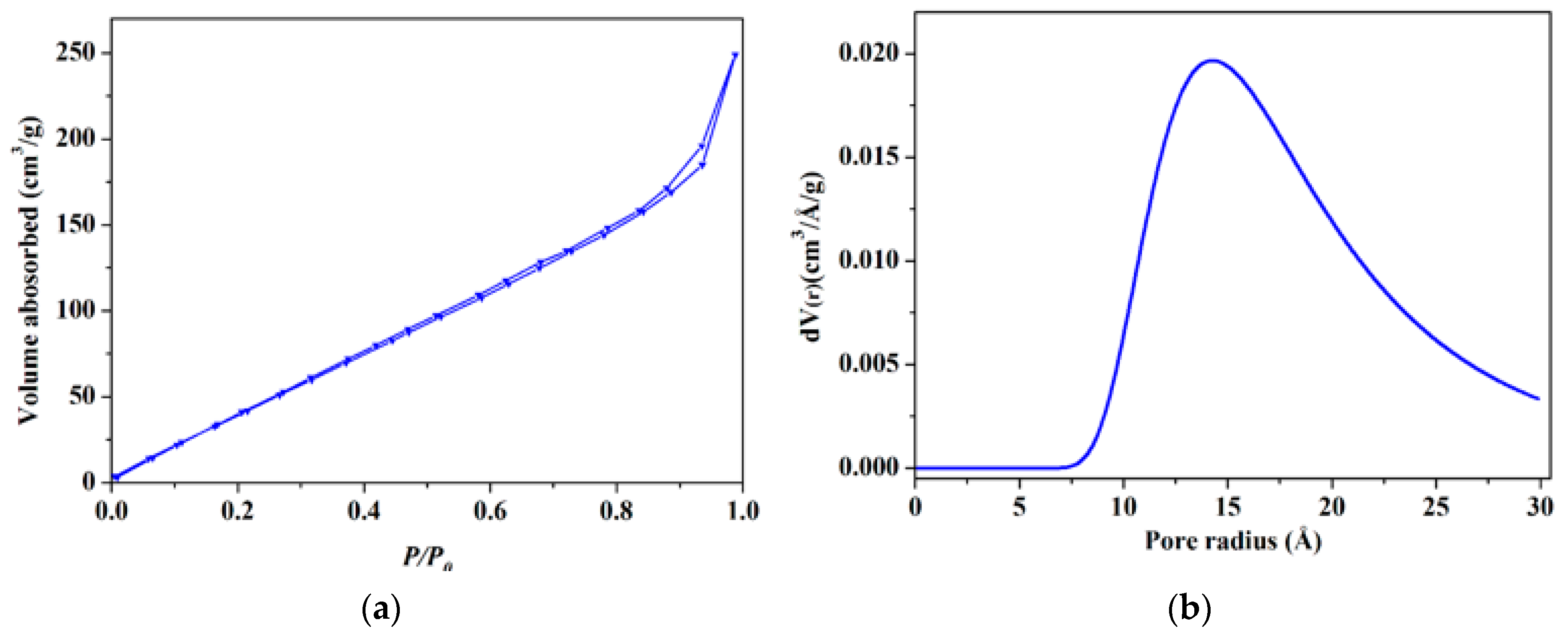
3.4. BET Surface Area Studies
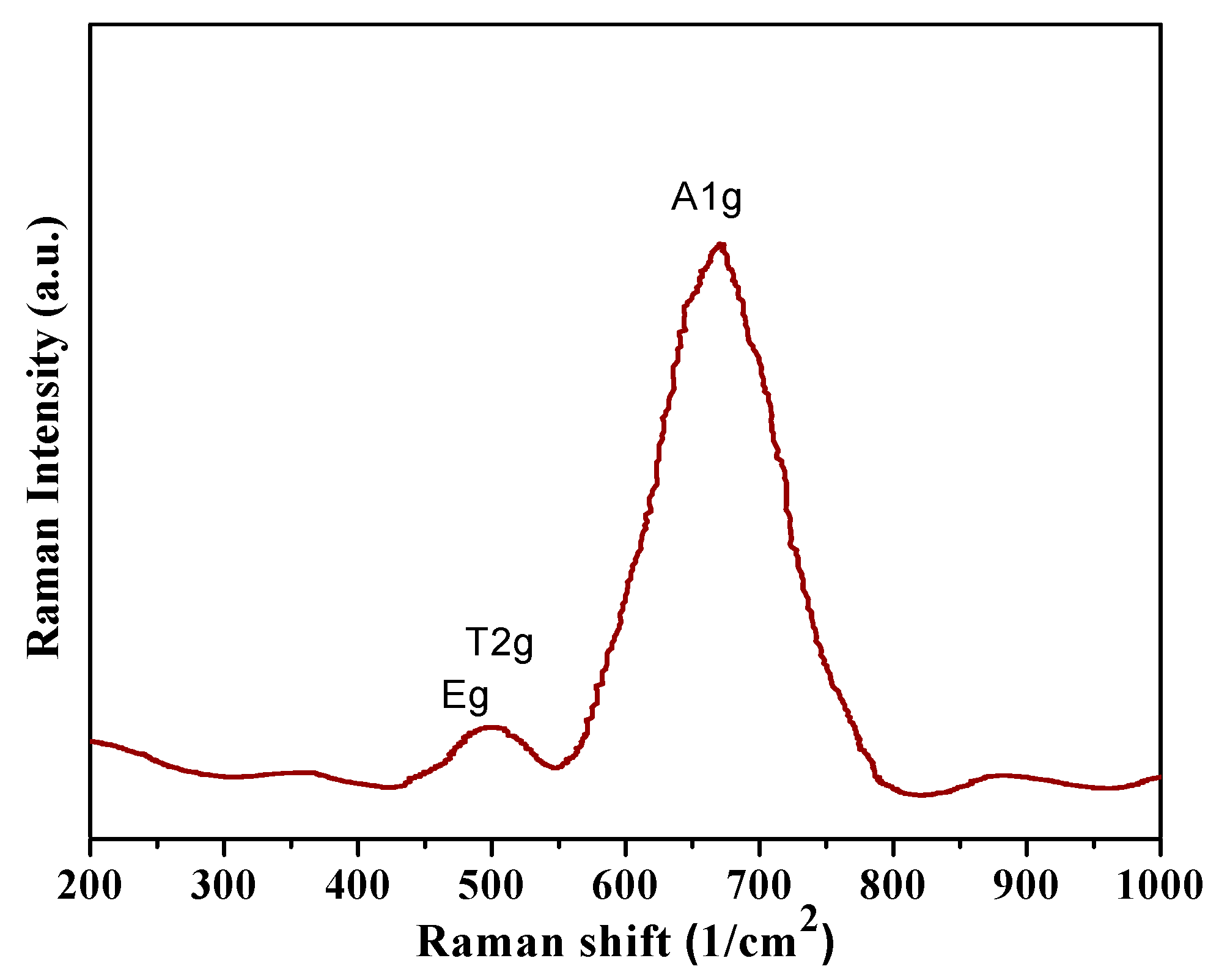
3.5. Raman Studies
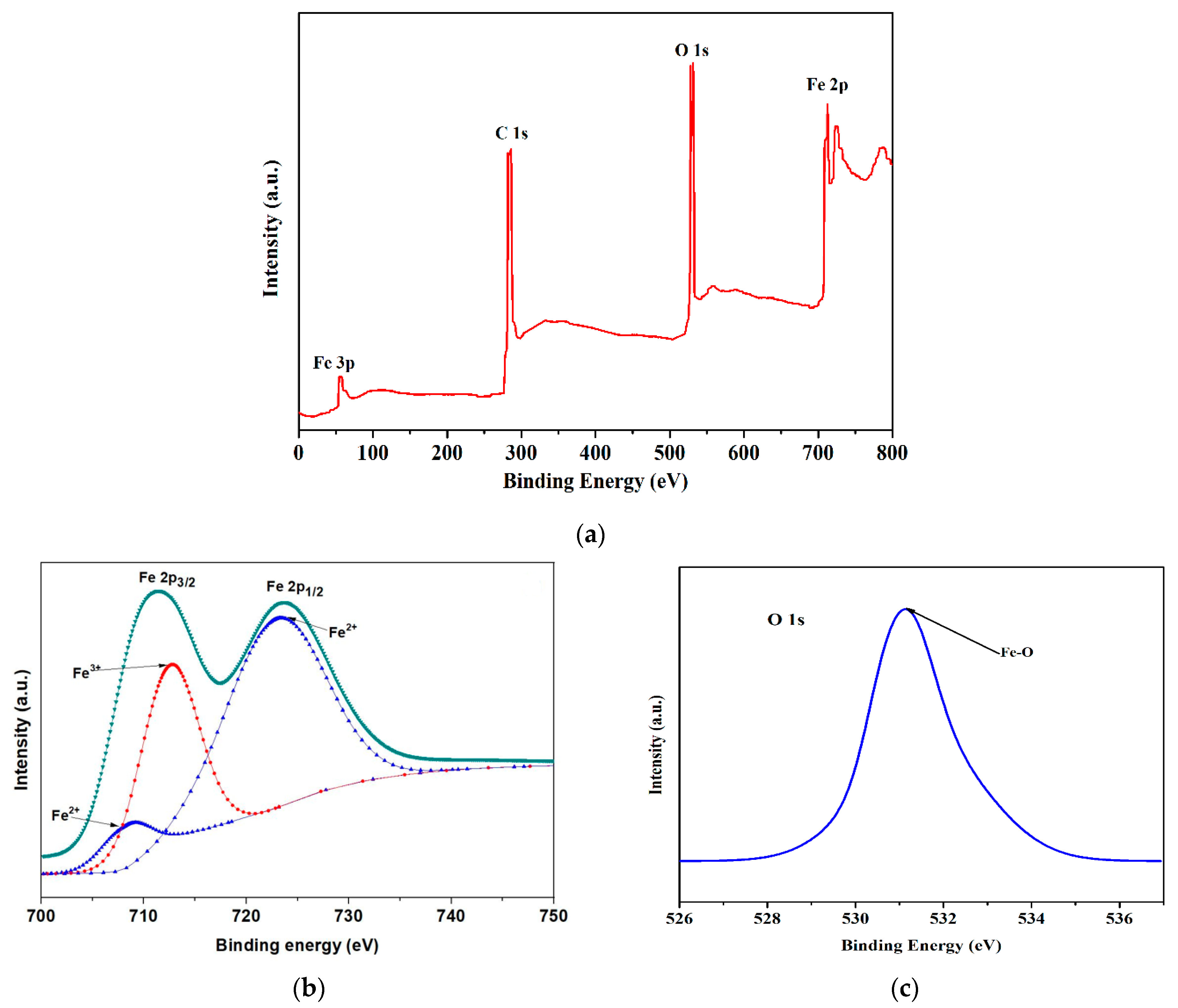
3.6. X-ray Photoelectron Spectroscopic Studies
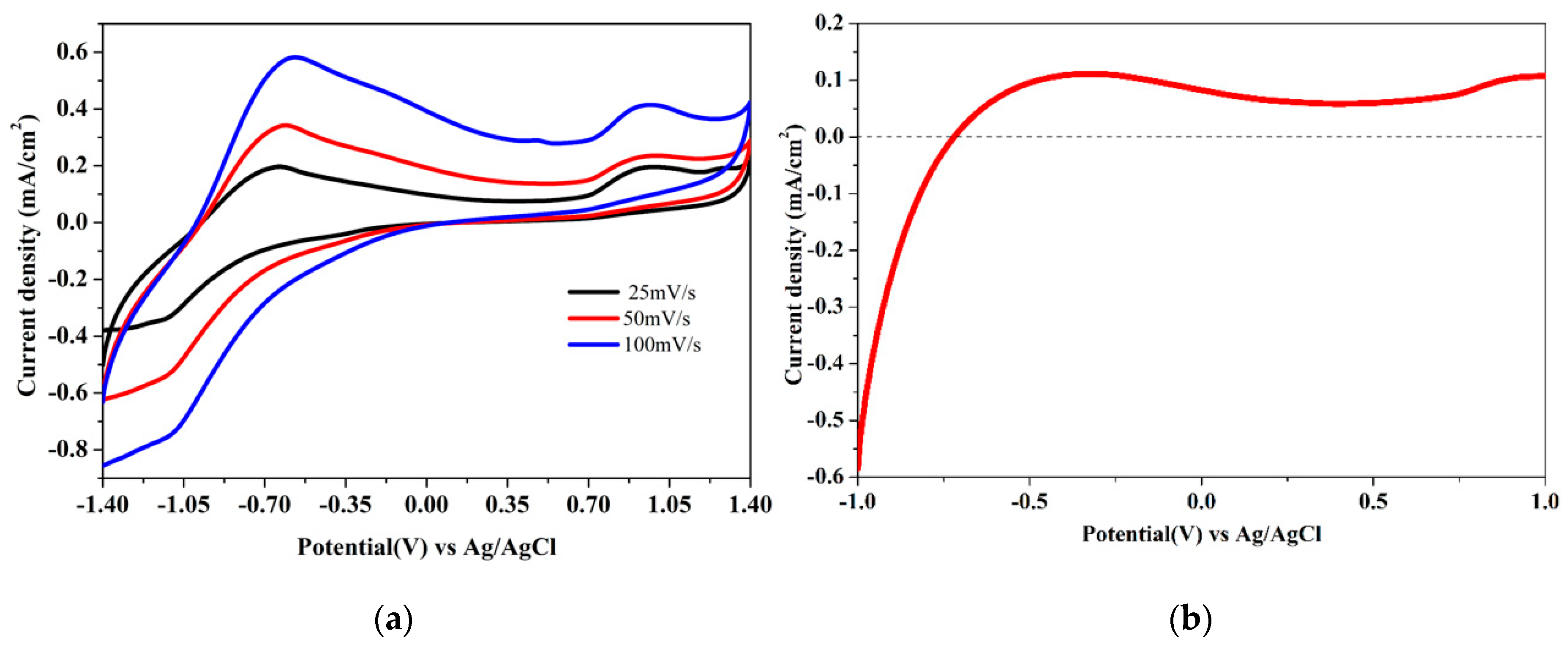
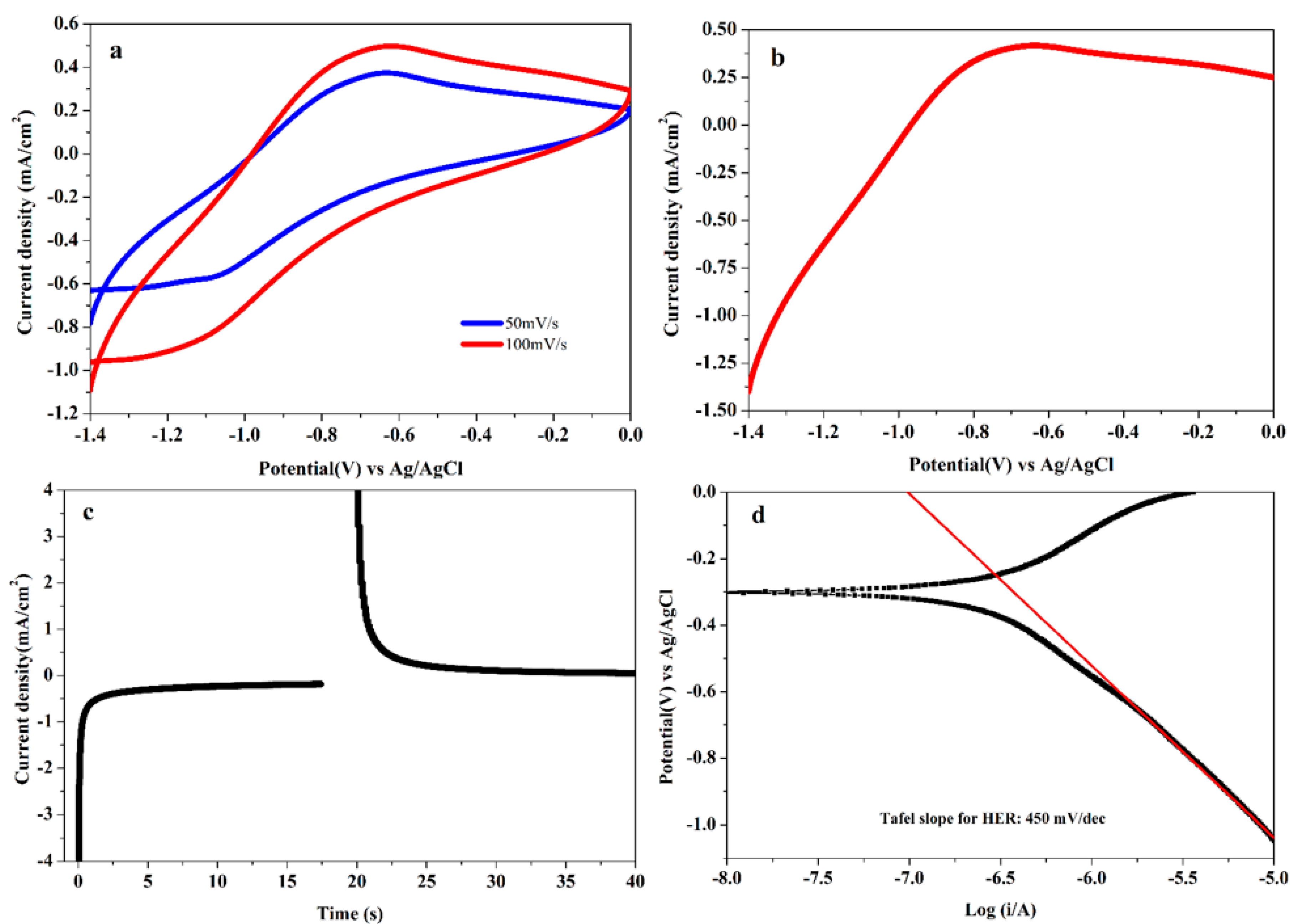
3.7. Electrocatalytic Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Habas, S.E.; Platt, H.A.S.; van Hest, M.F.A.M.; Ginley, D.S. Low-Cost Inorganic Solar Cells: From Ink To Printed Device. Chem. Rev. 2010, 110, 6571–6594. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bard, A.J.; Fox, M.A. Artificial Photosynthesis: Solar Splitting of Water to Hydrogen and Oxygen. Acc. Chem. Res. 1995, 28, 141–145. [Google Scholar] [CrossRef]
- Chow, J.; Kopp, R.J.; Portney, P.R. Energy Resources and Global Development. Science 2003, 302, 1528–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrog. Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Campen, A.; Mondal, K.; Wiltowski, T. Separation of hydrogen from syngas using a regenerative system. Int. J. Hydrog. Energy 2008, 33, 332–339. [Google Scholar] [CrossRef]
- Muradov, N.Z.; Veziroǧlu, T.N. From hydrocarbon to hydrogen–carbon to hydrogen economy. Int. J. Hydrog. Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Fried, T.; Shemer, G.; Markovich, G. Ordered Two-Dimensional Arrays of Ferrite Nanoparticles. Adv. Mater. 2001, 13, 1158–1161. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.E.; Lee, S.H.; Yu, J.H.; Lee, J.H.; Park, T.G.; Hyeon, T. Designed Fabrication of a Multifunctional Polymer Nanomedical Platform for Simultaneous Cancer-Targeted Imaging and Magnetically Guided Drug Delivery. Adv. Mater. 2008, 20, 478–483. [Google Scholar] [CrossRef]
- Lee, H.; Yu, M.K.; Park, S.; Moon, S.; Min, J.J.; Jeong, Y.Y.; Kang, H.-W.; Jon, S. Thermally Cross-Linked Superparamagnetic Iron Oxide Nanoparticles: Synthesis and Application as a Dual Imaging Probe for Cancer in Vivo. J. Am. Chem. Soc. 2007, 129, 12739–12745. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Phul, R. Magnetic Iron Oxide Nanoparticles as Contrast Agents: Hydrothermal Synthesis, Characterization and Properties. Solid State Phenom. 2015, 232, 111–145. [Google Scholar] [CrossRef]
- Nithya, V.D.; Sabari Arul, N. Progress and development of Fe3O4 electrodes for supercapacitors. J. Mater. Chem. A 2016, 4, 10767–10778. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Li, C.; Wang, K.; Sun, X.; Ma, Y. Recent advances in porous graphene materials for supercapacitor applications. RSC Adv. 2014, 4, 45862–45884. [Google Scholar] [CrossRef]
- Divyashree, A.; Hegde, G. Activated carbon nanospheres derived from bio-waste materials for supercapacitor applications—A review. RSC Adv. 2015, 5, 88339–88352. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Feng, Y. Carbon Nanotubes for Supercapacitor. Nanoscale Res. Lett. 2010, 5, 654. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Xu, J.; Yuen, M.-F.; Zhang, J.; Li, Y.; Chen, X.; Zhang, W. Hierarchical Composite Electrodes of Nickel Oxide Nanoflake 3D Graphene for High-Performance Pseudocapacitors. Adv. Funct. Mater. 2014, 24, 6372–6380. [Google Scholar] [CrossRef]
- Mitchell, E.; Gupta, R.K.; Mensah-Darkwa, K.; Kumar, D.; Ramasamy, K.; Gupta, B.K.; Kahol, P. Facile synthesis and morphogenesis of superparamagnetic iron oxide nanoparticles for high-performance supercapacitor applications. New J. Chem. 2014, 38, 4344–4350. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Chaudhari, S.; Yu, J.-S. Cube-like α-Fe2O3 Supported on Ordered Multimodal Porous Carbon as High Performance Electrode Material for Supercapacitors. ChemSusChem 2014, 7, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, K.; Wang, H.; Xue, D. Composition Design upon Iron Element toward Supercapacitor Electrode Materials. Mater. Focus 2015, 4, 78–80. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, M.-Y.; Yan, D.-Y.; Mao, J.; Liu, H.; Hu, W.-B.; Du, X.-W.; Ling, T.; Qiao, S.-Z. Engineering oxygen vacancy on NiO nanorod arrays for alkaline hydrogen evolution. Nano Energy 2018, 43, 103–109. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, H.; Qin, X.; Guo, X.; Cui, G.; Asiri, A.M.; Sun, X. Cathodic electrochemical activation of Co3O4 nanoarrays: A smart strategy to significantly boost the hydrogen evolution activity. Chem. Commun. 2018, 54, 2150–2153. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T. Hydrogen production from water utilizing solar heat at high temperatures. Sol. Energy 1977, 19, 467–475. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Li, J.; Weimer, A.W. A spinel ferrite/hercynite water-splitting redox cycle. Int. J. Hydrog. Energy 2010, 35, 3333–3340. [Google Scholar] [CrossRef]
- Gokon, N.; Kondo, K.; Hatamachi, T.; Sato, M.; Kodama, T. Oxygen-releasing step of nickel ferrite based on Rietveld analysis for thermochemical two-step water-splitting. Int. J. Hydrog. Energy 2013, 38, 4935–4944. [Google Scholar] [CrossRef]
- Bader, R.; Venstrom, L.J.; Davidson, J.H.; Lipiński, W. Thermodynamic Analysis of Isothermal Redox Cycling of Ceria for Solar Fuel Production. Energy Fuels 2013, 27, 5533–5544. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; Steinfeld, A. Solar syngas production from CO2 and H2O in a two-step thermochemical cycle via Zn/ZnO redox reactions: Thermodynamic cycle analysis. Int. J. Hydrog. Energy 2011, 36, 12141–12147. [Google Scholar] [CrossRef]
- Ahmed, J.; Poltavets, V.V.; Prakash, J.; Alshehri, S.M.; Ahamad, T. Sol-Gel synthesis, structural characterization and bifunctional catalytic activity of nanocrystalline delafossite CuGaO2 particles. J. Alloy. Compd. 2016, 688, 1157–1161. [Google Scholar] [CrossRef]
- Ganguli, A.K.; Ahmad, T. Nanorods of Iron Oxalate Synthesized Using Reverse Micelles: Facile Route for α-Fe2O3 and Fe3O4 Nanoparticles. J. Nanosci. Nanotechnol. 2007, 7, 2029–2035. [Google Scholar] [CrossRef]
- Ahmad, T.; Ganguli, A.K. Structural and Dielectric Characterization of Nanocrystalline (Ba,Pb)ZrO3 Developed by Reverse Micellar Synthesis. J. Am. Ceram. Soc. 2006, 89, 3140–3146. [Google Scholar] [CrossRef]
- Ganguly, A.; Ahmad, T.; Ganguli, A.K. Silica Mesostructures: Control of Pore Size and Surface Area Using a Surfactant-Templated Hydrothermal Process. Langmuir 2010, 26, 14901–14908. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Khatoon, S.; Coolahan, K.; Lofland, S.E. Solvothermal synthesis, optical and magnetic properties of nanocrystalline Cd1−xMnxO (0.04 < x = 0.10) solid solutions. J. Alloy. Compd. 2013, 558, 117–124. [Google Scholar] [CrossRef]
- Khatoon, S.; Coolahan, K.; Lofland, S.E.; Ahmad, T. Optical and magnetic properties of solid solutions of In2−xMnxO3 (0.05, 0.10 and 0.15) nanoparticles. J. Alloy. Compd. 2012, 545, 162–167. [Google Scholar] [CrossRef]
- Sui, R.; Charpentier, P. Synthesis of Metal Oxide Nanostructures by Direct Sol–Gel Chemistry in Supercritical Fluids. Chem. Rev. 2012, 112, 3057–3082. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Phul, R.; Khatoon, N.; Sardar, M. Antibacterial efficacy of Ocimum sanctum leaf extract-treated iron oxide nanoparticles. New J. Chem. 2017, 41, 2055–2061. [Google Scholar] [CrossRef]
- Ahmad, T.; Lone, I.H.; Ansari, S.G.; Ahmed, J.; Ahamad, T.; Alshehri, S.M. Multifunctional properties and applications of yttrium ferrite nanoparticles prepared by citrate precursor route. Mater. Des. 2017, 126, 331–338. [Google Scholar] [CrossRef]
- Ahmad, T.; Phul, R.; Alam, P.; Lone, I.H.; Shahazad, M.; Ahmed, J.; Ahamad, T.; Alshehri, S.M. Dielectric, optical and enhanced photocatalytic properties of CuCrO2 nanoparticles. RSC Adv. 2017, 7, 27549–27557. [Google Scholar] [CrossRef] [Green Version]
- Alduhaish, O.; Ubaidullah, M.; Al-Enizi, A.M.; Alhokbany, N.; Alshehri, S.M.; Ahmed, J. Facile Synthesis of Mesoporous α-Fe2O3@g-C3N4-NCs for Efficient Bifunctional Electrocatalytic Activity (OER/ORR). Sci. Rep. 2019, 9, 14139. [Google Scholar] [CrossRef]
- Luo, Z.; Martí-Sànchez, S.; Nafria, R.; Joshua, G.; de la Mata, M.; Guardia, P.; Flox, C.; Martínez-Boubeta, C.; Simeonidis, K.; Llorca, J.; et al. Fe3O4@NiFexOy Nanoparticles with Enhanced Electrocatalytic Properties for Oxygen Evolution in Carbonate Electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 29461–29469. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Shang, X.; Ren, H.; Chi, J.; Fu, H.; Dong, B.; Liu, C.; Chai, Y. Modulation of Inverse Spinel Fe3O4 by Phosphorus Doping as an Industrially Promising Electrocatalyst for Hydrogen Evolution. Adv. Mater. 2019, 31, 1905107. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jian, X.; Liu, J.; Guo, S.; Zhou, C.; Zhang, P.; Yang, Y.; Chen, L. Phosphorus-doped Fe3O4 nanoflowers grown on 3D porous graphene for robust pH-Universal hydrogen evolution reaction. Int. J. Hydrog. Energy 2020, 45, 4435–4443. [Google Scholar] [CrossRef]
- Tancredi, P.; Botasini, S.; Moscoso-Londoño, O.; Méndez, E.; Socolovsky, L. Polymer-assisted size control of water-dispersible iron oxide nanoparticles in range between 15 and 100 nm. Colloids Surf. A Physicochem. Eng. Asp. 2015, 464, 46–51. [Google Scholar] [CrossRef]
- Ahmed, J.; Mao, Y. Delafossite CuAlO2 Nanoparticles with Electrocatalytic Activity toward Oxygen and Hydrogen Evolution Reactions. In Nanomaterials for Sustainable Energy; American Chemical Society: Washington, DC, USA, 2015; Volume 1213, pp. 57–72. [Google Scholar]
- Bernal, J.D.; Dasgupta, D.R.; Mackay, A.L. The oxides and hydroxides of iron and their structural inter-relationships. Clay Miner. Bull. 2018, 4, 15–30. [Google Scholar] [CrossRef]
- Zou, G.; Xiong, K.; Jiang, C.; Li, H.; Li, T.; Du, J.; Qian, Y. Fe3O4 Nanocrystals with Novel Fractal. J. Phys. Chem. B 2005, 109, 18356–18360. [Google Scholar] [CrossRef] [PubMed]
- Linderoth, S.; Hendriksen, P.V.; Bo/dker, F.; Wells, S.; Davies, K.; Charles, S.W.; Mo/rup, S. On spin-canting in maghemite particles. J. Appl. Phys. 1994, 75, 6583–6585. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Wang, L.; Ji, H.; Wang, S.; Kong, L.; Jiang, X.; Yang, G. Preparation of Fe3O4 with high specific surface area and improved capacitance as a supercapacitor. Nanoscale 2013, 5, 3793–3799. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Wang, D.; Wang, M.; Lin, Z.; Tang, H. Sono-assisted preparation of highly-efficient peroxidase-like Fe3O4 magnetic nanoparticles for catalytic removal of organic pollutants with H2O2. Ultrason. Sonochemistry 2010, 17, 526–533. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.; Meng, X.; Li, Y.; Zhao, J. Structural evolution and characteristics of the phase transformations between α-Fe2O3, Fe3O4 and γ-Fe2O3 nanoparticles under reducing and oxidizing atmospheres. CrystEngComm 2013, 15, 8166–8172. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Gao, X.; Mao, L.; Hu, Y.; Lou, X.W. One-Pot Magnetic Field Induced Formation of Fe3O4/C Composite Microrods with Enhanced Lithium Storage Capability. Small 2014, 10, 2815–2819. [Google Scholar] [CrossRef]
- Li, G.; Li, R.; Zhou, W. A Wire-Shaped Supercapacitor in Micrometer Size Based on Fe3O4 Nanosheet Arrays on Fe Wire. Nano Micro Lett. 2017, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Alajmi, M.F.; Ahmed, J.; Hussain, A.; Ahamad, T.; Alhokbany, N.; Amir, S.; Ahmad, T.; Alshehri, S.M. Green synthesis of Fe3O4 nanoparticles using aqueous extracts of Pandanus odoratissimus leaves for efficient bifunctional electrocatalytic activity. Appl. Nanosci. 2018, 8, 1427–1435. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, L.C.C.; Carvalho, W.M.; Criado, D.; Souza, F.L. Vertically Oriented Iron Oxide Films Produced by Hydrothermal Process: Effect of Thermal Treatment on the Physical Chemical Properties. ACS Appl. Mater. Interfaces 2012, 4, 5515–5523. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.J.P.; Westgard, J.A.; Cooper, L.M.; Murray, R.W. Solution Voltammetry of 4 nm Magnetite Iron Oxide Nanoparticles. J. Am. Chem. Soc. 2014, 136, 10783–10789. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; Barclay, J.E.; Butt, J.N.; Lomonossoff, G.P.; Evans, D.J. Redox-active ferrocene-modified Cowpea mosaic virus nanoparticles. Dalton Trans. 2010, 39, 7569–7574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoerzinger, K.A.; Qiao, L.; Biegalski, M.D.; Shao-Horn, Y. Orientation-Dependent Oxygen Evolution Activities of Rutile IrO2 and RuO2. J. Phys. Chem. Lett. 2014, 5, 1636–1641. [Google Scholar] [CrossRef]
- Ahmed, J.; Trinh, P.; Mugweru, A.M.; Ganguli, A.K. Self-assembly of copper nanoparticles (cubes, rods and spherical nanostructures): Significant role of morphology on hydrogen and oxygen evolution efficiencies. Solid State Sci. 2011, 13, 855–861. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Ren, H.; Yu, R.; Qi, J.; Mao, D.; Zhao, H.; Wang, D. pH-Regulated Synthesis of Multi-Shelled Manganese Oxide Hollow Microspheres as Supercapacitor Electrodes Using Carbonaceous Microspheres as Templates. Adv. Sci. 2014, 1, 1400011. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Wang, C.; Chen, M.; Jiao, Y.; Wang, J. Electrochemical Performances of Nanoparticle Fe3O4/Activated Carbon Supercapacitor Using KOH Electrolyte Solution. J. Phys. Chem. C 2009, 113, 2643–2646. [Google Scholar] [CrossRef]
- Guan, D.; Gao, Z.; Yang, W.; Wang, J.; Yuan, Y.; Wang, B.; Zhang, M.; Liu, L. Hydrothermal synthesis of carbon nanotube/cubic Fe3O4 nanocomposite for enhanced performance supercapacitor electrode material. Mater. Sci. Eng. B 2013, 178, 736–743. [Google Scholar] [CrossRef]
- Zhao, X.; Johnston, C.; Crossley, A.; Grant, P.S. Printable magnetite and pyrrole treated magnetite based electrodes for supercapacitors. J. Mater. Chem. 2010, 20, 7637–7644. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Ahmed, J.; Alhabarah, A.N.; Ahamad, T.; Ahmad, T. Nitrogen-Doped Cobalt Ferrite/Carbon Nanocomposites for Supercapacitor Applications. ChemElectroChem 2017, 4, 2952–2958. [Google Scholar] [CrossRef]
- Meng, W.; Chen, W.; Zhao, L.; Huang, Y.; Zhu, M.; Huang, Y.; Fu, Y.; Geng, F.; Yu, J.; Chen, X.; et al. Porous Fe3O4/carbon composite electrode material prepared from metal-organic framework template and effect of temperature on its capacitance. Nano Energy 2014, 8, 133–140. [Google Scholar] [CrossRef]



| Catalyst | Electrolyte | Method | Scan Rate (mV/s) | Cs (F/g) | Ref. |
|---|---|---|---|---|---|
| Fe3O4 | 3 M KOH | Sol-Gel | 20 | 185 | [19] |
| (AC)-Fe3O4 | 6 M KOH | Microwave | 10 | 37.9 | [62] |
| CNT/Fe3O4 | 6 M KOH | Hydrothermal | 10 | 117.2 | [63] |
| Pyrrole treated Fe3O4 film | 0.1 M Na2SO3 | Hydrothermal/spray deposition | 10 | 190 | [64] |
| CoFe2O4 | 5.0 M KOH | Polymeric Route | 5 | 94 | [65] |
| Porous Fe3O4/carbon composite | 1 M KOH | Calcination | 50 | 139 | [66] |
| Fe3O4 | 0.5 M KOH | Co-precipitation | 5 | 135 | Present |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phul, R.; Khan, M.A.M.; Sardar, M.; Ahmed, J.; Ahmad, T. Multifunctional Electrochemical Properties of Synthesized Non-Precious Iron Oxide Nanostructures. Crystals 2020, 10, 751. https://doi.org/10.3390/cryst10090751
Phul R, Khan MAM, Sardar M, Ahmed J, Ahmad T. Multifunctional Electrochemical Properties of Synthesized Non-Precious Iron Oxide Nanostructures. Crystals. 2020; 10(9):751. https://doi.org/10.3390/cryst10090751
Chicago/Turabian StylePhul, Ruby, M. A. Majeed Khan, Meryam Sardar, Jahangeer Ahmed, and Tokeer Ahmad. 2020. "Multifunctional Electrochemical Properties of Synthesized Non-Precious Iron Oxide Nanostructures" Crystals 10, no. 9: 751. https://doi.org/10.3390/cryst10090751








