Synthesis and Structure of Fluorinated (Benzo[d]imidazol-2-yl)methanols: Bench Compounds for Diverse Applications
Abstract
:1. Introduction
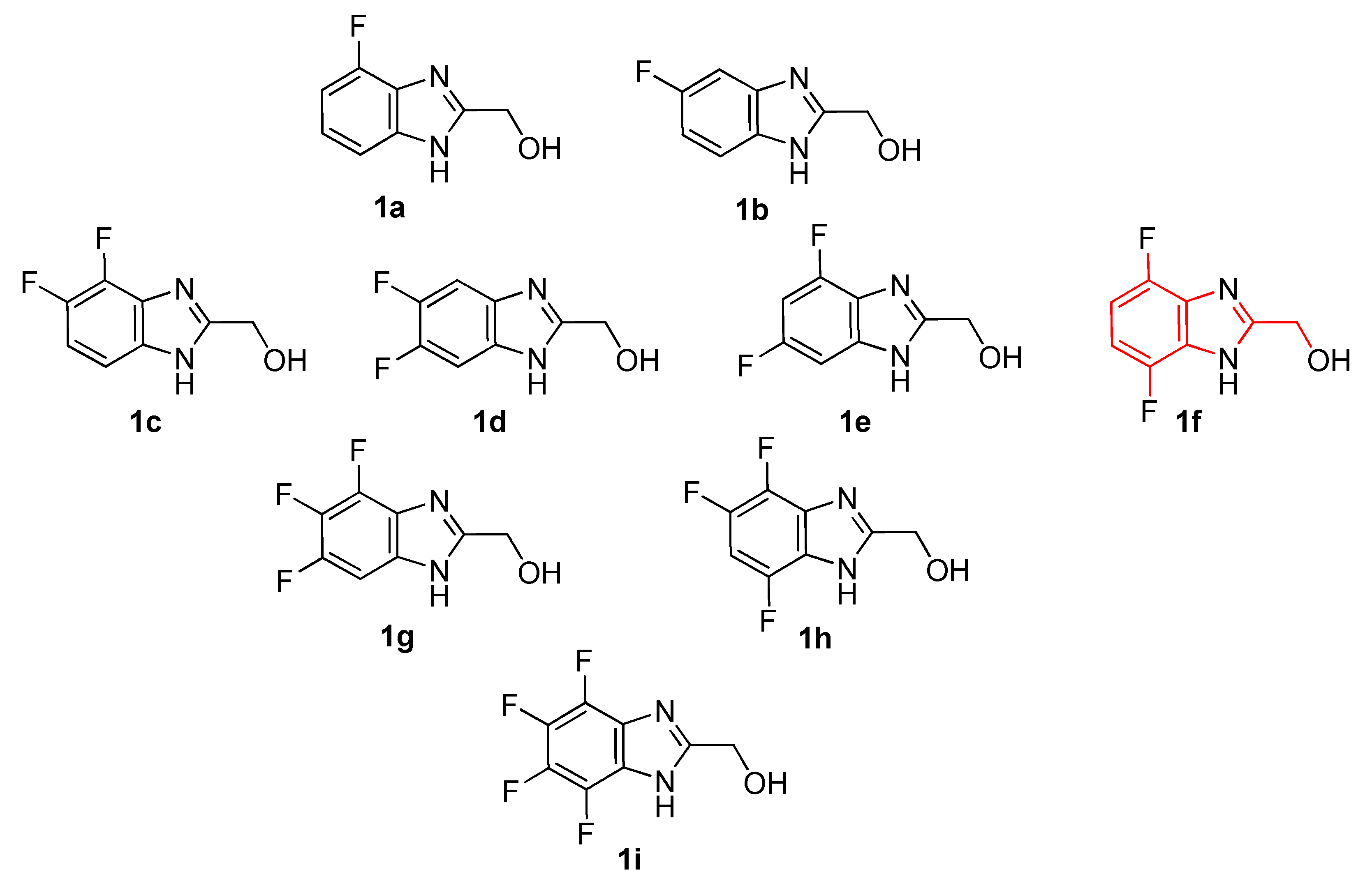
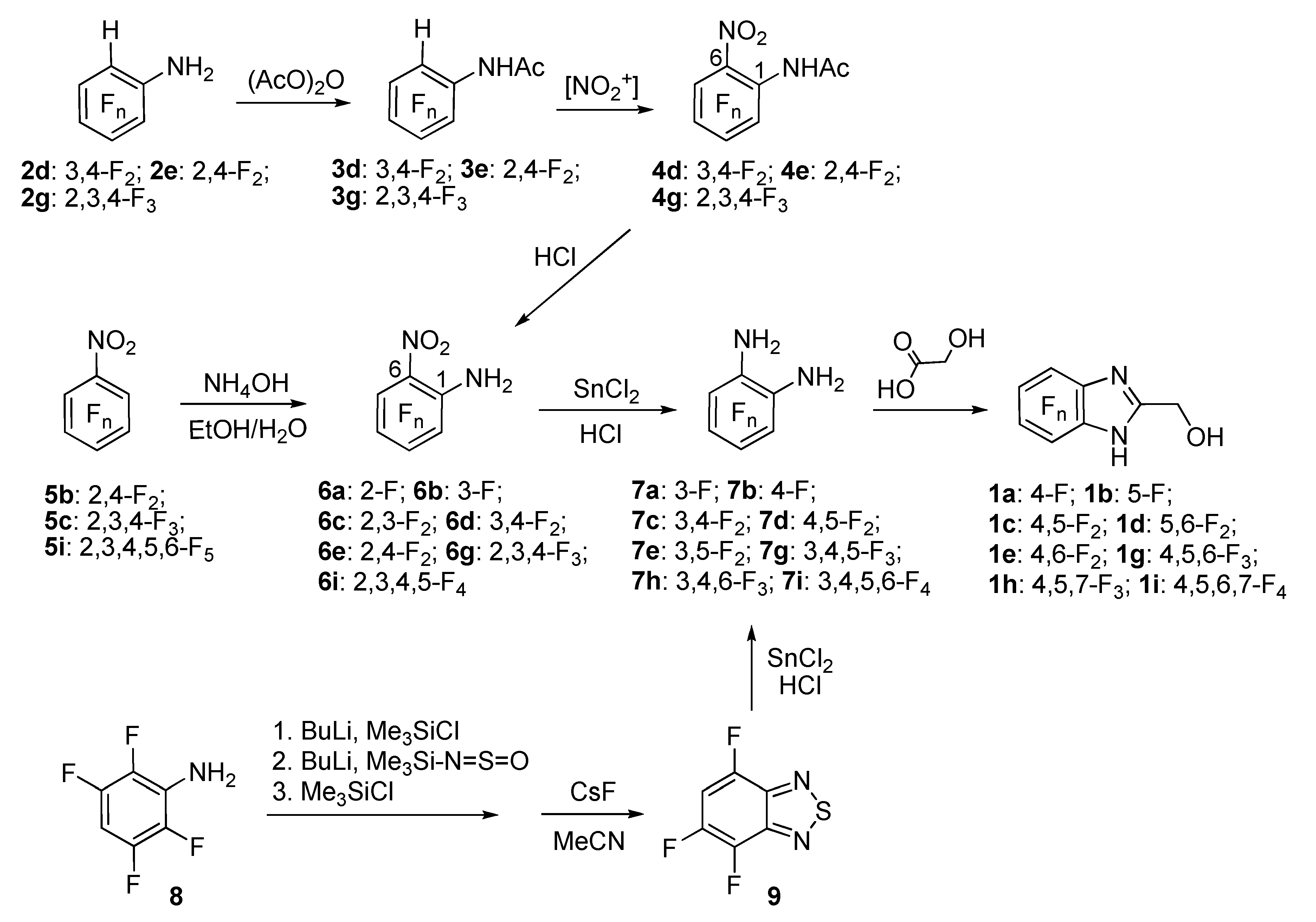
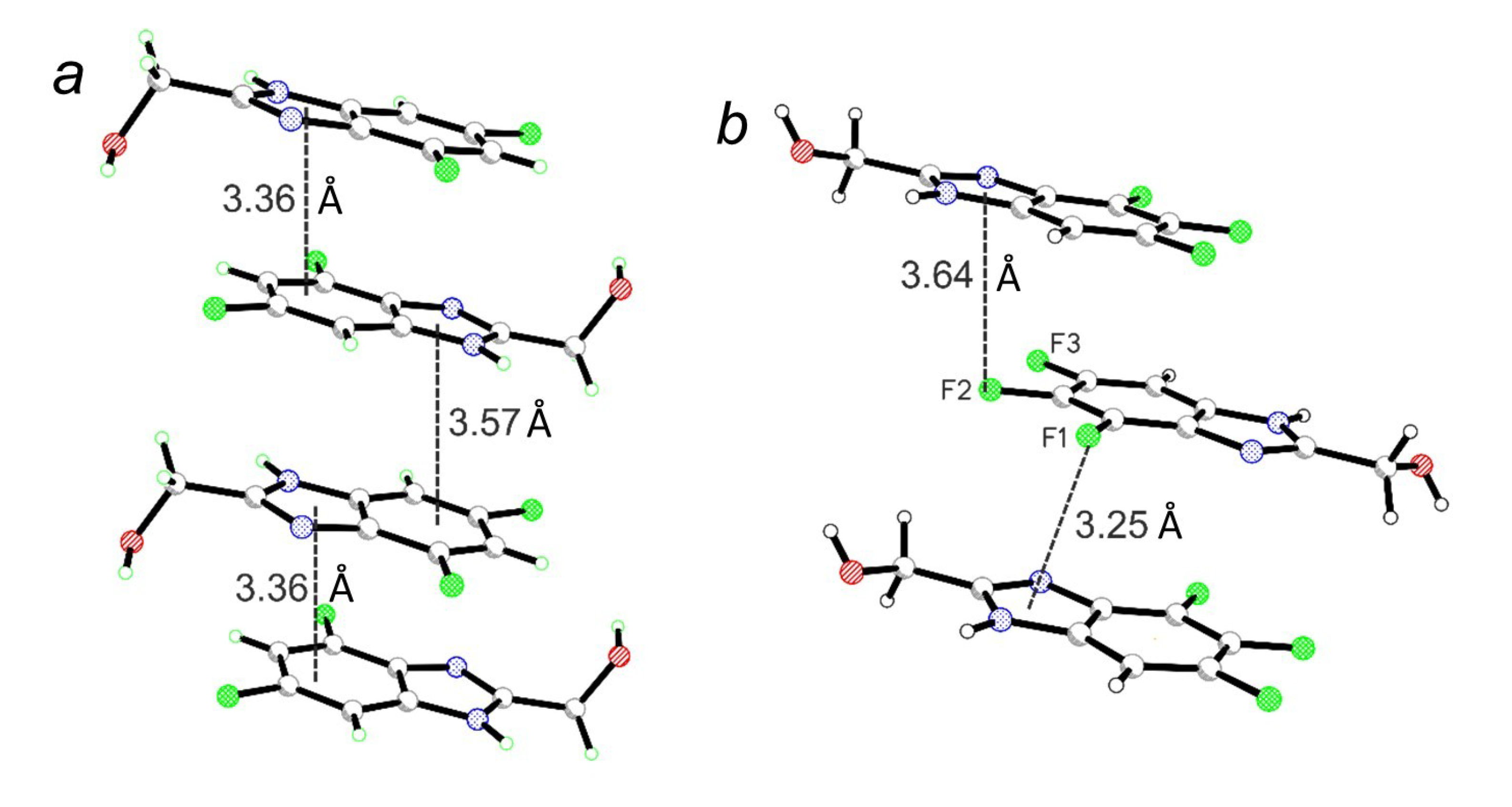
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. General Methods
4.2. Interaction of o-Phenylenediamines with Glycolic Acid: The General Procedure
4.2.1. (4-Fluorobenzo[d]imidazol-2-yl)methanol (1a)
4.2.2. (5-Fluorobenzo[d]imidazol-2-yl)methanol (1b)
4.2.3. (4,5-Difluorobenzo[d]imidazol-2-yl)methanol (1c)
4.2.4. (5,6-Difluorobenzo[d]imidazol-2-yl)methanol (1d)
4.2.5. (4,6-Difluorobenzo[d]imidazol-2-yl)methanol (1e)
4.2.6. (4,5,6-Trifluorobenzo[d]imidazol-2-yl)methanol (1g)
4.2.7. (4,5,7-Trifluorobenzo[d]imidazol-2-yl)methanol (1h)
4.2.8. (4,5,6,7-Tetrafluorobenzo[d]imidazol-2-yl)methanol (1i)
4.3. Single-Crystal X-ray Diffraction Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marinescu, M. Chemistry and Applications of Benzimidazole and Its Derivatives; IntechOpen: London, UK, 2019. [Google Scholar]
- Vestergaard, A.A. Benzimidazole: Preparation and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2020. [Google Scholar]
- Selivanova, G.A.; Tretyakov, E.V. Fluorinated benzimidazoles for medicinal chemistry and new materials. Russ. Chem. Bull. 2020, 69, 838–858. [Google Scholar] [CrossRef]
- Rajasekhar, S.; Maiti, B.; Balamurali, M.M.; Chanda, K. Synthesis and medicinal applications of benzimidazoles: An overview. Curr. Org. Synth. 2017, 14, 40–60. [Google Scholar] [CrossRef]
- Barker, H.A.; Smyth, R.D.; Weissbach, H.; Toohey, J.I.; Ladd, J.N.; Volcani, B.E. Isolation and properties of crystalline cobamide coenzymes containing benzimidazole or 5,6-dimethylbenzimidazole. J. Biol. Chem. 1960, 235, 480–488. [Google Scholar]
- Arjmand, F.; Mohani, B.; Ahmad, S. Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu(II) complex. Eur. J. Med. Chem. 2005, 40, 1103–1110. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef]
- Moniruzzaman, R.S.; Mahmud, T. Quantum chemical and pharmacokinetic studies of some proton pump inhibitor drugs. Am. J. Biomed. Sci. Res. 2019, 2, 259–264. [Google Scholar]
- Njar, V.C.; Brodie, A.M. Discovery and development of Galeterone (TOK-001 or VN/124-1) for the treatment of all stages of prostate cancer. J. Med. Chem. 2015, 58, 2077–2087. [Google Scholar] [CrossRef]
- Tahlan, S.; Kumar, S.; Ramasamy, K.; Lim, S.M.; Shah, S.A.A.; Mani, V.; Pathania, R.; Narasimhan, B. Design, synthesis and biological profile of heterocyclic benzimidazole analogues as prospective antimicrobial and antiproliferative agents. BMC Chem. 2019, 13, 50–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholten, W.K.; Christensen, A.E.; Olesen, A.E.; Drewes, A.M. Quantifying the adequacy of opioid analgesic consumption globally: An updated method and early findings. Am. J. Public Health 2019, 109, 52–57. [Google Scholar] [CrossRef]
- Salahuddin; Shaharyar, M.; Mazumder, A. Benzimidazoles: A biologically active compounds. Arab. J. Chem. 2017, 10, S157–S173. [Google Scholar] [CrossRef] [Green Version]
- Han, X.-F.; He, X.; Wang, M.; Xu, D.; Hao, L.-P.; Liang, A.-H.; Zhang, J.; Zhou, Z.-M. Discovery of novel, potent and low-toxicity angiotensin II receptor type 1 (AT1) blockers: Design, synthesis and biological evaluation of 6-substituted aminocarbonyl benzimidazoles with a chiral center. Eur. J. Med. Chem. 2015, 103, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.-F.; Yan, W.; Cao, L.-L.; Ye, Y.-H. Synthesis and biological evaluation of benzimidazole phenylhydrazone derivatives as antifungal agents against phytopathogenic fungi. Molecules 2016, 21, 1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.C. Toxicity of Fungicides. In Veterinary Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 569–580. [Google Scholar]
- Ermler, S.; Scholze, M.; Kortenkamp, A. Seven benzimidazole pesticides combined at sub-threshold levels induce micronuclei in vitro. Mutagenesis 2013, 28, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, N.R.; Mustakim, M.A.; Sani, M.M.M.; Baharin, S.N.A. Heck reaction using palladium-benzimidazole catalyst: Synthesis, characterisation and catalytic activity. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458, 012019. [Google Scholar] [CrossRef]
- Günnaz, S.; Gökçe, A.G.; Türkmen, H. Synthesis of bimetallic complexes bridged by 2,6-bis(benzimidazol-2-yl)pyridine derivatives and their catalytic properties in transfer hydrogenation. Dalton Trans. 2018, 47, 17317–17328. [Google Scholar] [CrossRef]
- Zhao, Y.; Chao, W.; Qiu, P.; Li, X.; Wang, Q.; Chen, J.; Ma, D. New benzimidazole-based bipolar hosts: Highly efficient phosphorescent and thermally activated delayed fluorescent organic light-emitting diodes employing the same device structure. ACS Appl. Mater. Interfaces 2016, 8, 2635–2643. [Google Scholar] [CrossRef]
- Horak, E.; Kassal, P.; Steinberg, M. Benzimidazole as a structural unit in fluorescent chemical sensors: The hidden properties of a multifunctional heterocyclic scaffold. Supramol. Chem. 2017, 30, 838–857. [Google Scholar] [CrossRef]
- Eldebss, T.M.A.; Farag, A.M.; Shamy, A.Y.M. Synthesis of some benzimidazole-based heterocycles and their application as copper corrosion inhibitors. J. Heterocycl. Chem. 2019, 56, 371–390. [Google Scholar] [CrossRef]
- Yuan, S.; Guo, X.; Aili, D.; Pan, C.; Li, Q.; Fang, J. Poly(imide benzimidazole)s for high temperature polymer electrolyte membrane fuel cells. J. Membr. Sci. 2014, 454, 351–358. [Google Scholar] [CrossRef]
- Igbal, H.M.S.; Bhowmik, S.; Benedictus, R. Performance evaluation of polybenzimidazole coating for aerospace application. Prog. Org. Coat. 2017, 105, 190–199. [Google Scholar] [CrossRef]
- Mandal, S.; Song, G. Characterizing thermal protective fabrics of firefighters’ clothing in hot surface contact. J. Ind. Text. 2016, 47, 622–639. [Google Scholar] [CrossRef]
- Veeraragavan, V.; Kumar, C.R.; Suresh, A.; Jayalakshmi, S.; Mudali, K.M.; Sivaraman, N. Evaluation of polybenzimidazole-based polymers for the removal of uranium, thorium and palladium from aqueous medium. R. Soc. Open Sci. 2018, 5, 171701. [Google Scholar]
- Lahti, P.M. Structure-property relationships for metal-free organic magnetic materials. Adv. Phys. Org. Chem. 2011, 45, 93–169. [Google Scholar]
- Adriano, C.; Freitas, R.S.; Paduan-Filho, A.; Pagliuso, P.G.; Oliveira, N.F.; Lahti, P.M. Magnetic phase diagram of the organic antiferromagnet F4BImNN. Polyhedron 2017, 136, 2–4. [Google Scholar] [CrossRef]
- Lescop, C.; Luneau, D.; Bussiere, G.; Triest, M.; Reber, C. Ligand-Centered Near-Infrared Luminescence from Lanthanide Complexes with Chelating Nitronyl Nitroxide Free Radicals. Inorg. Chem. 2000, 39, 3740–3741. [Google Scholar] [CrossRef]
- Seber, G.; Freitas, R.S.; Mague, J.T.; Paduan-Filho, A.; Gratens, X.; Bindilatti, V.; Oliveira, N.F.; Yoshioka, N.; Lahti, P.M. Magnetic tuning of all-organic binary alloys between two stable radicals. J. Am. Chem. Soc. 2012, 134, 3825–3833. [Google Scholar] [CrossRef] [PubMed]
- Sugano, T.; Blundell, S.J.; Lancaster, T.; Pratt, F.L.; Mori, H. Magnetic order in the purely organic quasi-one-dimensional ferromagnet 2-benzimidazolyl nitronyl nitroxide. Phys. Rev. B 2010, 82, 180401. [Google Scholar] [CrossRef]
- Naoki, Y.; Munetoshi, I.; Yuichiro, M.; Takanari, K.; Hidenari, I.; Shigeru, O. Unusually large magnetic interactions observed in hydrogen-bonded nitronyl nitroxides. Chem. Lett. 1997, 26, 251–252. [Google Scholar]
- Luneau, D. Coordination chemistry of nitronyl nitroxide radicals has memory. Eur. J. Inorg. Chem. 2020, 7, 597–604. [Google Scholar] [CrossRef]
- Luneau, D.; Borta, A.; Chumakov, Y.; Jacquot, J.-F.; Jeanneau, E.; Lescop, C.; Rey, P. Molecular magnets based on two-dimensional Mn(II)-nitronyl nitroxide frameworks in layered structures. Inorg. Chim. Acta 2008, 361, 3669–3676. [Google Scholar] [CrossRef]
- Luneau, D.; Rey, P. Magnetism of metal-nitroxide compounds involving bis-chelating imidazole and benzimidazole substituted nitronyl nitroxide free radicals. Coord. Chem. Rev. 2005, 249, 2591–2611. [Google Scholar] [CrossRef]
- Lescop, C.; Bussiere, G.; Beaulac, R.; Belisle, H.; Belorizky, E.; Rey, P.; Reber, C.; Luneau, D. Magnetic and optical properties of nitroxide radicals and their lanthanide complex. J. Phys. Chem. Solids 2004, 65, 773–779. [Google Scholar] [CrossRef]
- Lescop, C.; Luneau, D.; Rey, P.; Bussiere, G.; Reber, C. Synthesis, structures, and magnetic properties of a series of lanthanum(III) and gadolinium(III) complexes with chelating benzimidazole-substituted nitronyl nitroxide free radicals. Evidence for antiferromagnetic Gd(III)-radical interactions. Inorg. Chem. 2002, 41, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Fegy, K.; Luneau, D.; Belorizky, E.; Novac, M.; Tholence, J.L.; Paulsen, C.; Ohm, T.; Rey, P. 1D Manganese(II) Derivatives of an Imidazole-Substituted Nitronyl Nitroxide. An Approach toward Molecular Magnetic Materials of High Dimensionality. Inorg. Chem. 1998, 37, 4524–4532. [Google Scholar] [CrossRef] [PubMed]
- Fegy, K.; Luneau, D.; Ohm, T.; Paulsen, C.; Rey, P. Two-dimensional nitroxide-based molecular magnetic materials. Angew. Chem. Int. Ed. 1998, 37, 1270–1273. [Google Scholar] [CrossRef]
- Hu, P.; Zhu, M.; Mei, X.; Tian, H.; Ma, Y.; Li, L.; Liao, D. Single-molecule magnets based on rare earth complexes with chelating benzimidazole-substituted nitronyl nitroxide radicals. Dalton Trans. 2012, 41, 14651–14656. [Google Scholar] [CrossRef]
- Ma, X.-F.; Wang, Z.; Chen, X.-L.; Kurmoo, M.; Zeng, M.-H. Ligand effect on the single-molecule magnetism of tetranuclear Co(II) cubane. Inorg. Chem. 2017, 56, 15178–15186. [Google Scholar] [CrossRef]
- Chen, B.-T.; Morlanes, N.; Adogla, E.; Takanabe, K.; Rodionov, V.O. An efficient and stable hydrophobic molecular cobalt catalyst for water electro-oxidation at neutral pH. ACS Catal. 2016, 6, 4647–4652. [Google Scholar] [CrossRef]
- Patalag, L.J.; Jones, P.G.; Werz, D.B. BOIMPYs: Rapid access to a family of red-emissive fluorophores and NIR dyes. Angew. Chem. Int. Ed. 2016, 55, 13340–13344. [Google Scholar] [CrossRef]
- Sharma, P.; Srinivasa Reddy, T.; Thummuri, D.; Senwar, K.R.; Kumar, N.P.; Naidu, V.G.M.; Bhargava, S.K.; Shankaraiah, N. Synthesis and biological evaluation of new benzimidazole-thiazolidinedione hybrids as potential cytotoxic and apoptosis inducing agents. Eur. J. Med. Chem. 2016, 124, 608–621. [Google Scholar] [CrossRef]
- Alasmary, F.A.S.; Snelling, A.M.; Zain, M.E.; Alafeefy, A.M.; Awaad, A.S.; Karodia, N. Synthesis and evaluation of selected benzimidazole derivatives as potential antimicrobial agents. Molecules 2015, 20, 15206–15223. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Hao, Q.; Jiang, K.; He, M.; Wang, J. Discovery of heterocyclic carbohydrazide derivatives as novel selective fatty acid amide hydrolase inhibitors: Design, synthesis and anti-neuroinflammatory evaluation. Bioorg. Med. Chem. Lett. 2020, 30, 127118. [Google Scholar] [CrossRef] [PubMed]
- Ryabukhin, D.S.; Turdakov, A.N.; Soldatova, N.S.; Kompanets, M.O.; Ivanov, A.Y.; Boyarskaya, I.A.; Vasilyev, A.V. Reactions of 2-carbonyl- and 2-hydroxy(or methoxy)alkyl-substituted benzimidazoles with arenes in the superacid CF3SO3H. NMR and DFT studies of dicationic electrophilic species. Beilstein J. Org. Chem. 2019, 15, 1962–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patalag, L.J.; Loch, M.; Jones, P.G.; Werz, D.B. Exploring the π-system of the (aza-)BOIMPY scaffold: Electron-rich pyrrole moieties working in concert with electron-depleted meso-positions. J. Org. Chem. 2019, 84, 7804–7814. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guan, L.; Zang, J.; Xing, K.; Zhang, J.; Liu, D.; Zhao, L. Structure-based design of novel benzimidazole derivatives as Pin1 inhibitors. Molecules 2019, 24, 1198. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Miao, Y.; Xu, J.; Bi, Q.; Qu, W.; Liu, W.; Feng, F.; Sun, H. A facile and efficient [4+2] annulation reaction of sulfur ylides: Access to N-fused benzimidazoles. Org. Chem. Front. 2019, 6, 205–208. [Google Scholar] [CrossRef]
- Zheng, H.-L.; Chen, X.-L.; Li, T.; Yin, Z.; Zhang, Y.; Kurmoo, M.; Zeng, M.-H. Manipulating clusters by use of competing N,O-chelating ligands: A combined crystallographic, mass spectrometric, and DFT study. Chem. Eur. J. 2018, 24, 7906–7912. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Sun, Q.; Sun, W. Efficient aliphatic C-H bond oxidation catalyzed by manganese complexes with hydrogen peroxide. Chem. Asian J. 2018, 13, 2458–2464. [Google Scholar] [CrossRef]
- Politanskaya, L.; Selivanova, G.A.; Panteleeva, E.V.; Tretyakov, E.V.; Platonov, V.E.; Nikul’Shin, P.V.; Vinogradov, A.S.; Zonov, Y.V.; Karpov, V.M.; Mezhenkova, T.V.; et al. Organofluorine chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2019, 88, 425–569. [Google Scholar] [CrossRef]
- Makarov, A.G.; Selikhova, N.Y.; Makarov, A.Y.; Malkov, V.S.; Bagryanskaya, I.Y.; Gatilov, Y.V.; Knyazev, A.S.; Slizhov, Y.G.; Zibarev, A.V. New fluorinated 1,2-diaminoarenes, quinoxalines, 2,1,3-arenothia(selena)diazoles and related Compd. J. Fluor. Chem. 2014, 165, 123–131. [Google Scholar] [CrossRef]
- Finger, G.C.; Reed, F.H.; Burness, D.M.; Fort, D.M.; Blough, R.R. Aromatic fluorine compounds. II. 1,2,4,5-Tetrafluorobenzene and related compounds. J. Am. Chem. Soc. 1951, 73, 145–149. [Google Scholar] [CrossRef]
- Campbell, J.E.; Hewitt, M.C.; Jones, P.; Xie, L. Heteroaryl Compounds and Methods of Use Thereof. U.S. Patent No. WO 2011/150156 A2, 1 December 2011. [Google Scholar]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. II 1987, S1–S19. [Google Scholar] [CrossRef]
- DcOria, E.; Novoa, J.J. On the hydrogen bond nature of the C–H···F interactions in molecular crystals. An exhaustive investigation combining a crystallographic database search and ab initio theoretical calculations. Cryst. Eng. Comm. 2008, 10, 423–436. [Google Scholar]
- Rybalova, T.V.; Bagryanskaya, I.Y. C.-F…π, F…H., and F…F intermolecular interactions and F-aggregation: Role in crystal engineering of fluoroorganic compounds. J. Struct. Chem. 2009, 50, 741–753. [Google Scholar] [CrossRef]
- Lannes, A.; Suffren, Y.; Tommasino, J.B.; Chiriac, R.; Toche, F.; Khrouz, L.; Molton, F.; Duboc, C.; Kieffer, I.; Hazemann, J.-L.; et al. Room temperature magnetic switchability assisted by hysteretic valence tautomerism in a layered two-dimensional manganese-radical coordination framework. J. Am. Chem. Soc. 2016, 138, 16493–16501. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, M.D.; Guru Row, T.N. C–halogen···π interactions and their influence on molecular conformation and crystal packing: A database study. Cryst. Eng. 2000, 3, 135–154. [Google Scholar] [CrossRef]
- Lorenzo, S.; Lewis, G.R.; Dance, I. Supramolecular potentials and embraces for fluorous aromatic molecules. New J. Chem. 2000, 24, 295–304. [Google Scholar] [CrossRef]
- Ooi, H.C.; Suschitzky, H. Practicable syntheses of 2-hydroxymethyl-substituted benzimidazoles and 2-formylbenzimidazole. J. Chem. Soc. Perkin Trans. 1 1982, 12, 2871–2875. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX-97. Programs for Crystal Structure Analysis (Release 97-2); University of Göttingen: Göttingen, Germany, 1998. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- SADABS, v. 2008-1; Bruker AXS: Madison, WI, USA, 2008.
- Spek, A.L. PLATON. In A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2003. [Google Scholar]
- Spek, A. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
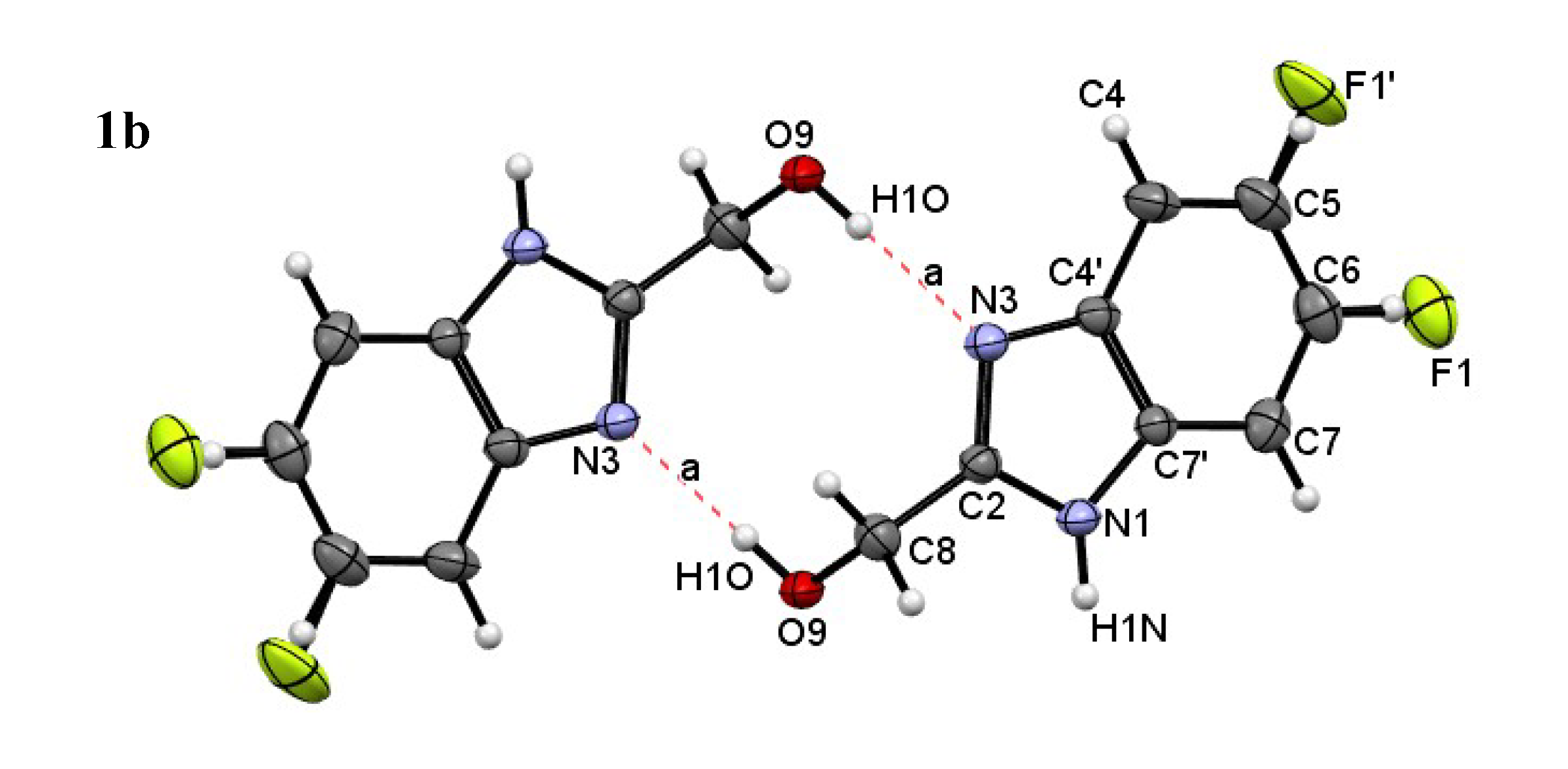
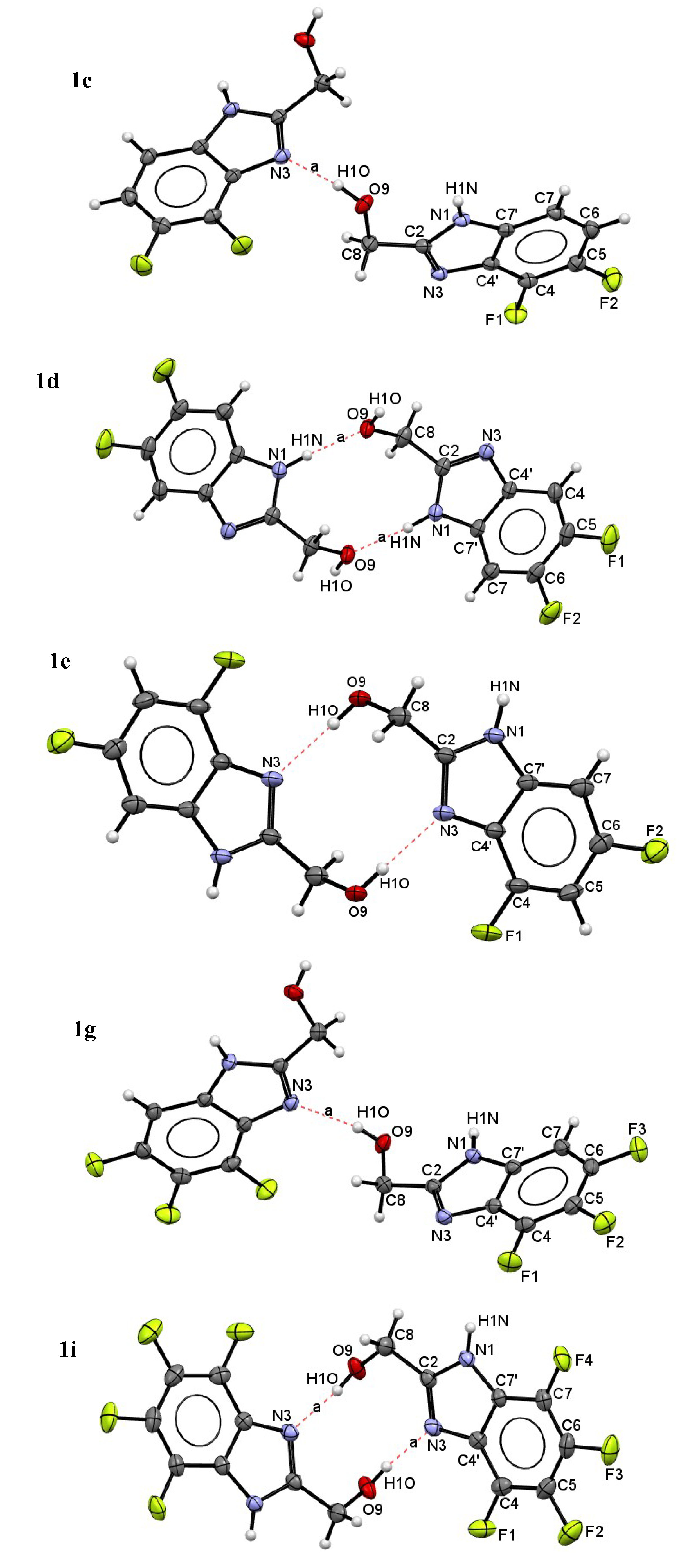
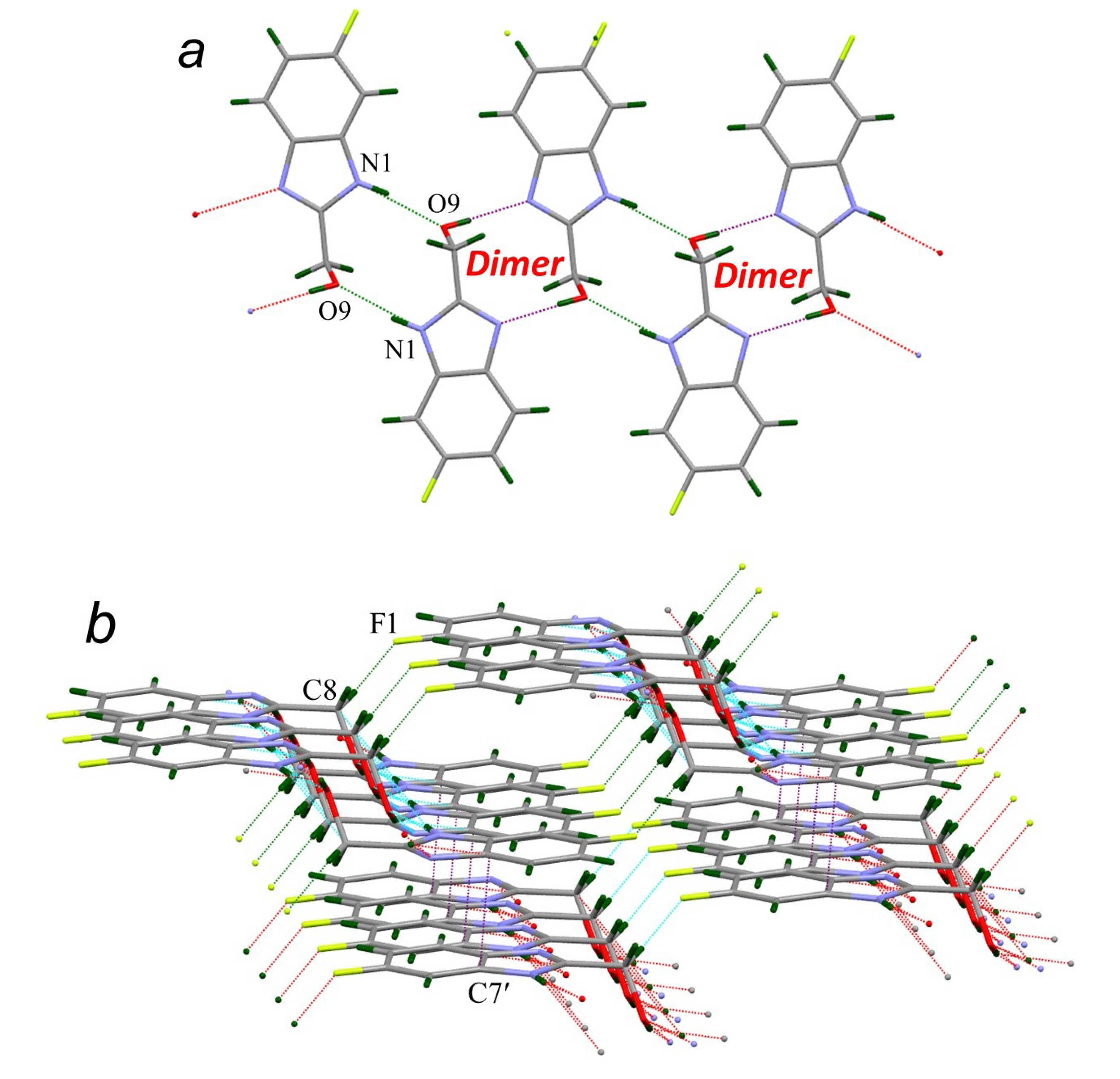
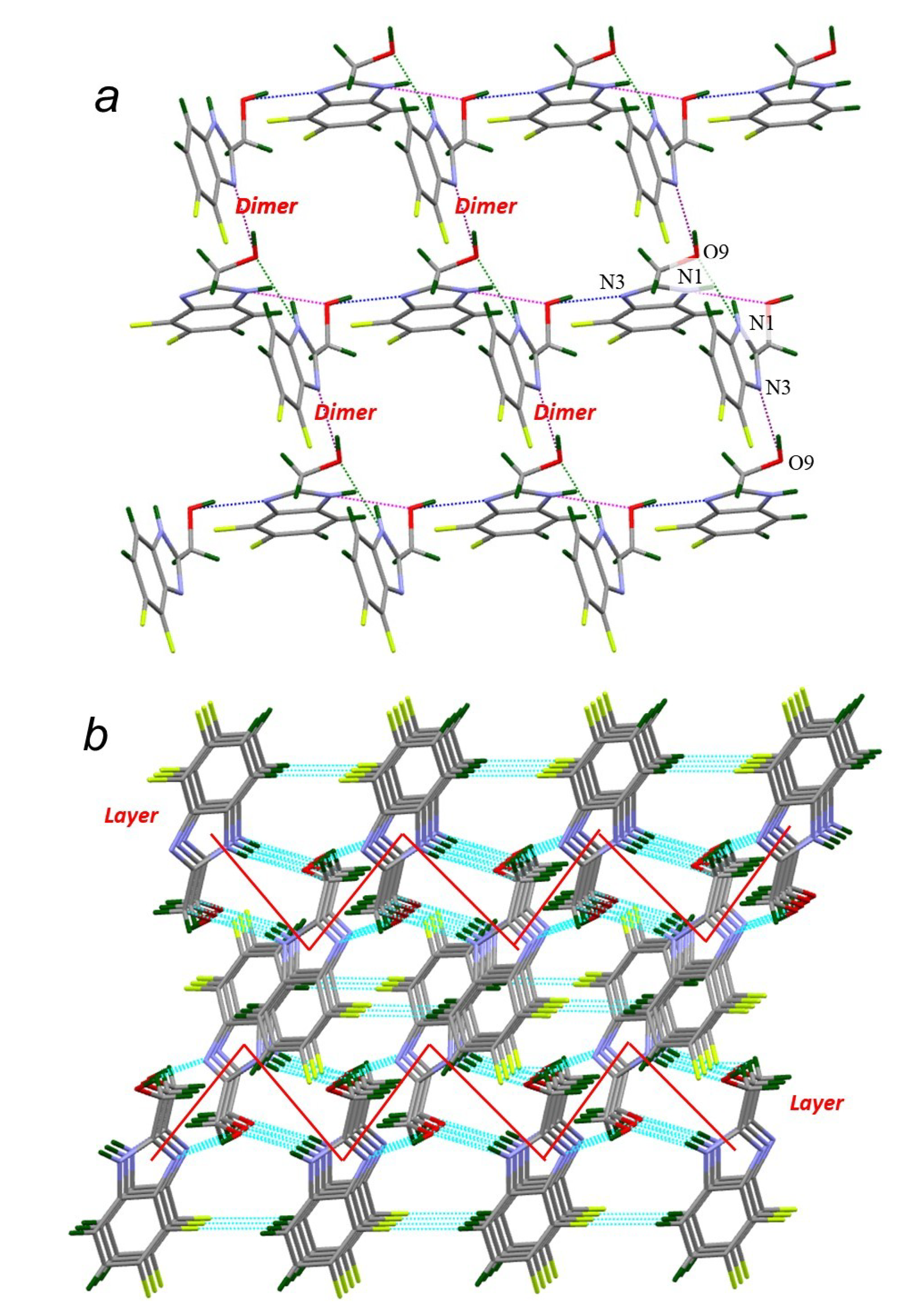
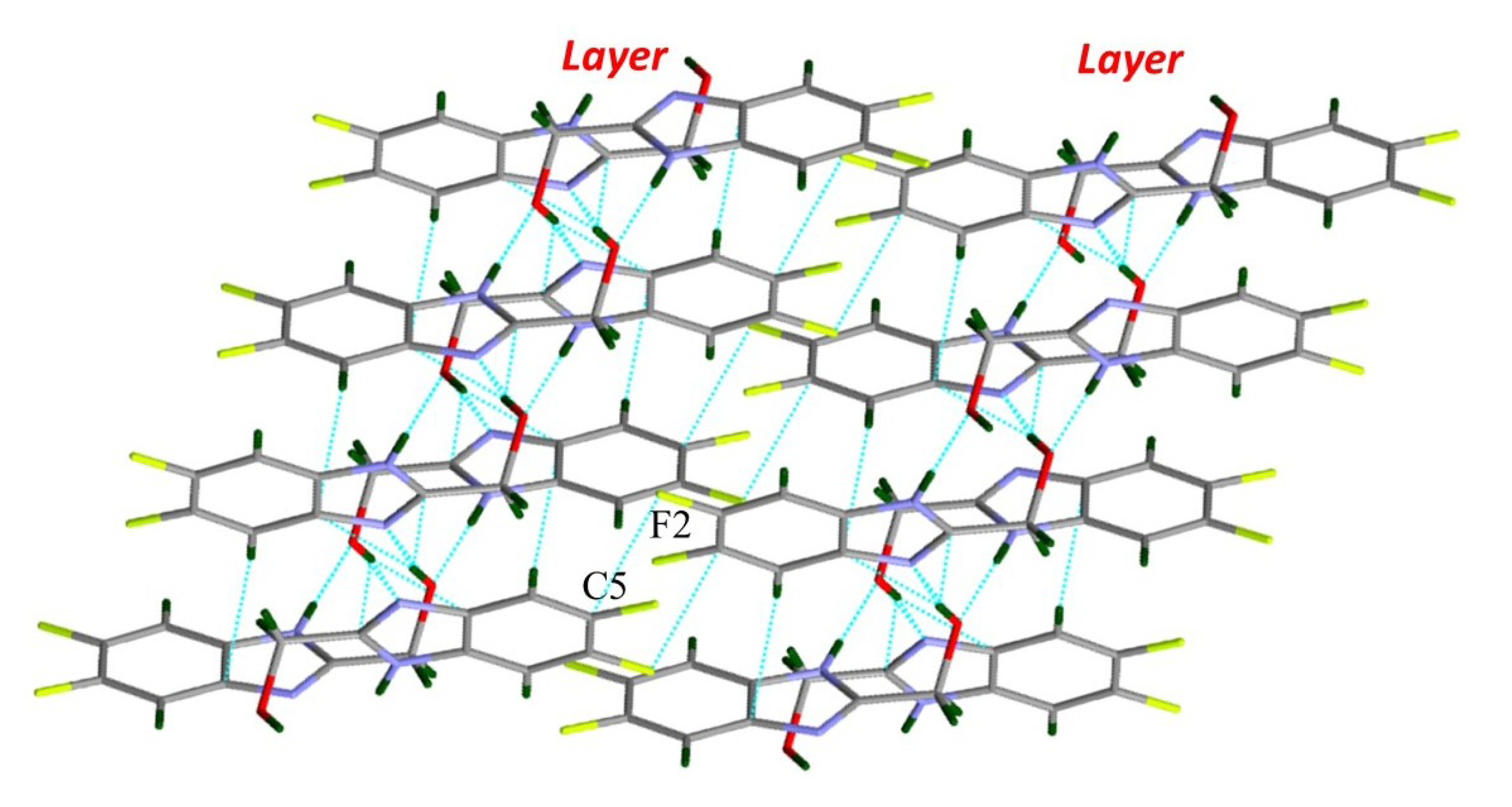
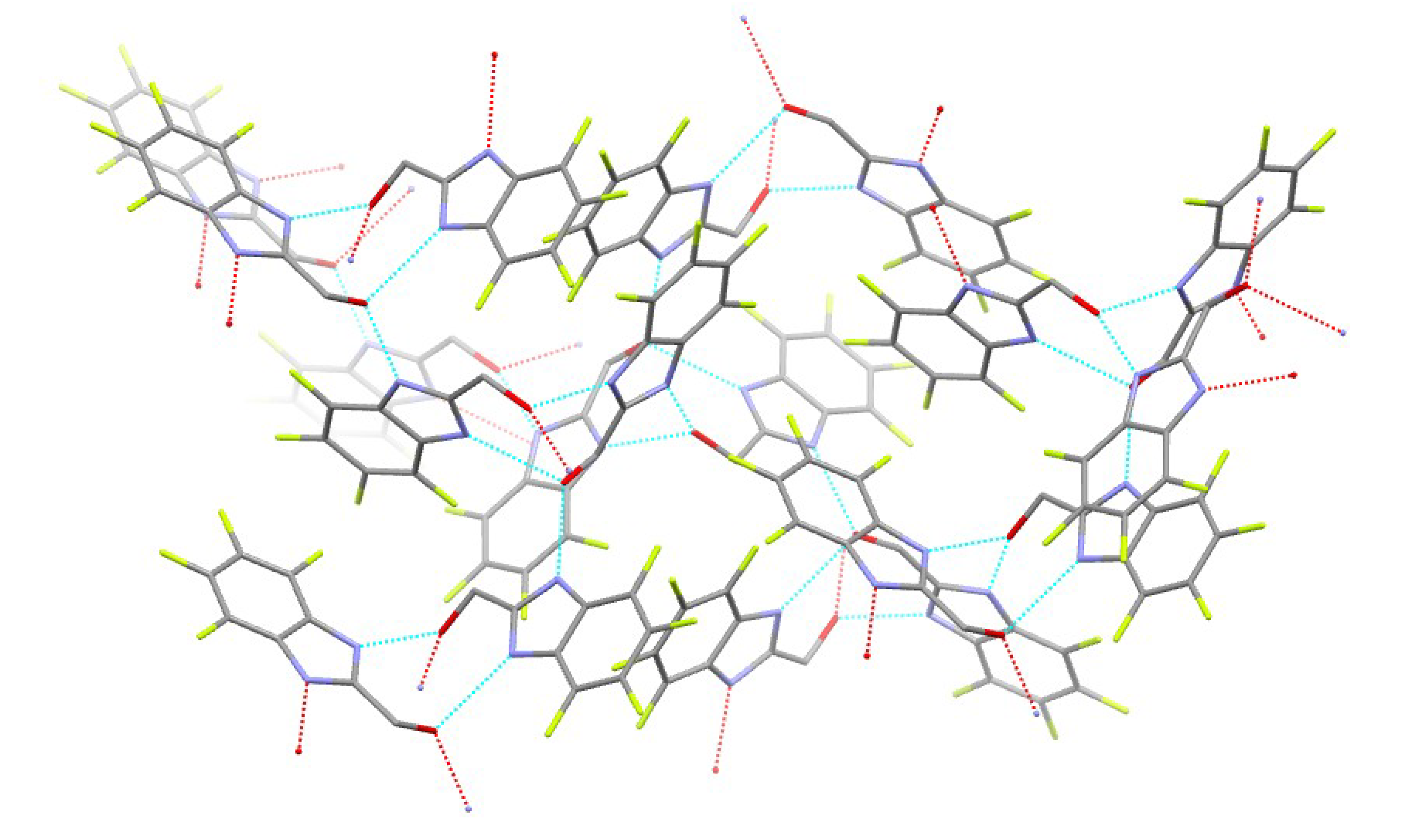
| Compound | H-Bond | D–H, Å | H…A, Å | D…A, Å | D–H…A, ° |
|---|---|---|---|---|---|
| 1b | N1–H…O9 | 0.87(3) | 1.91(3) | 2.766(3) | 170(2) |
| O9–H…N3 | 0.82(4) | 1.90(4) | 2.717(3) | 172(3) | |
| C8–H…F1′ | 0.97 | 2.43 | 3.238(6) | 141 | |
| 1c | N1–H…O9 | 0.88(2) | 2.03(2) | 2.849(2) | 155(2) |
| O9–H…N3 | 0.88(2) | 1.90(2) | 2.762(2) | 165(2) | |
| C7–H…F1 | 0.93 | 2.53 | 3.439(2) | 166 | |
| 1d | N1–H…O9 | 0.89(2) | 1.86(2) | 2.752(2) | 175(2) |
| O9–H…N3 | 0.92(4) | 1.80(2) | 2.705(2) | 172(2) | |
| 1e | N1–H…O9 | 0.91(3) | 1.91(3) | 2.801(3) | 166(2) |
| O9–H…N3 | 0.83(4) | 1.95(4) | 2.768(3) | 168(4) | |
| C7–H…F1 | 0.93 | 2.42 | 3.242(4) | 148 | |
| C8–H…F2 | 0.97 | 2.54 | 3.477(4) | 162 | |
| 1g | N1–H…O9 | 0.87(2) | 2.04(2) | 2.828(2) | 150(2) |
| O9–H…N3 | 0.81(2) | 1.99(2) | 2.781(2) | 165(2) | |
| C8–H…F3 | 0.97 | 2.44 | 3.241(2) | 139 | |
| 1i | N1–H…O9 | 0.85(3) | 1.92(3) | 2.753(3) | 165(2) |
| O9–H…N3 | 0.85(3) | 1.89(3) | 2.728(2) | 172(4) | |
| C8–H…F1 | 0.97 | 2.54 | 3.180(3) | 124 |
| Compound | 1b | 1c | 1d |
|---|---|---|---|
| Empirical formula | C8H7F1N2O | C8H6F2N2O | C8H6F2N2O |
| Formula weight | 166.16 | 184.15 | 184.15 |
| Temperature, K | 296(2) | 296(2) | 296(2) |
| Wavelength, Å | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| Space group | P-1 | C2/c | P21/c |
| Unit cell dimensions a, Å | 7.139(2)0 | 12.5909(4) | 9.7021(7) |
| b, Å | 7.321(2) | 8.4163(4) | 12.0410(8) |
| c, Å | 8.362(2) | 14.3912(5) | 7.3374(5) |
| α, o | 103.15(1) | 90 | 90 |
| β, o | 106.17(1) | 95.608(2) | 109.122(3) |
| γ, o | 102.63(1) | 90 | 90 |
| Volume, Å3 | 389.7(2) | 1517.7(1) | 809.9(1) |
| Z | 2 | 8 | 4 |
| Density (calcd.), Mg·m−3 | 1.416 | 1.612 | 1.510 |
| Abs. coefficient, mm−1 | 0.113 | 0.143 | 0.134 |
| F(000) | 172 | 752 | 376 |
| Crystal size, mm3 | 0.03 × 0.20 × 0.70 | 0.40 × 0.50 × 0.60 | 0.02 × 0.70 × 0.70 |
| Θ range for data collection, ° | 2.7–27.7 | 2.8–29.5 | 2.2–30.0 |
| Index ranges | −9 ≤ h ≤ 9, −9 ≤ k ≤ 9, −10 ≤ l ≤ 10 | −15 ≤ h ≤17, −10 ≤ k ≤ 11, −18 ≤ l ≤18 | −13≤ h ≤ 3, −16 ≤ k ≤ 16, −10 ≤ l ≤ 10 |
| Reflections collected | 6241 | 9855 | 9703 |
| Independent reflections | 1790 R(int) = 0.028 | 1831 R(int) = 0.057 | 2143 R(int) = 0.038 |
| Completeness to θ, % | 99.3 | 99.3 | 99.9 (θ ≤ 25°) |
| Data/restraints/parameters | 1790/0/125 | 1831/0/124 | 2143/0/124 |
| Goodness-of-fit on F2 | 1.02 | 1.02 | 1.02 |
| Final R indices I > 2σ(I) | R1 = 0.0519, wR2 = 0.1448 | R1 = 0.0442, wR2 = 0.1167 | R1 = 0.0423, wR2 = 0.1037 |
| Final R indices (all data) | R1 = 0.0672, wR2 = 0.1564 | R1 = 0.0514, wR2 = 0.1245 | R1 = 0.0583, wR2 = 0.1162 |
| Largest diff. peak/hole, e·Å−3 | 0.20/−0.15 | 0.22/−0.22 | 0.19/−0.21 |
| CCDC | 2016713 | 2016714 | 2016715 |
| Compound | 1e | 1g | 1i |
| Empirical formula | C8H6F2N2O | C8H5F3N2O | C8H4F4N2O |
| Formula weight | 184.15 | 202.14 | 220.13 |
| Temperature, K | 296(2) | 296(2) | 296(2) |
| Wavelength, Å | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Monoclinic | Tetragonal |
| Space group | P-1 | C2/c | P41212 |
| Unit cell dimensions a, Å | 7.2007(8) | 12.6623(8) | 10.8539(3) |
| b, Å | 7.2524(7) | 8.3996(8) | 10.8539(3) |
| c, Å | 8.7419(8) | 14.770(1) | 14.3674(4) |
| α, o | 107.112(3) | 90 | 90 |
| β, o | 108.156(3) | 92.769(4) | 90 |
| γ, o | 104.107(3) | 90 | 90 |
| Volume, Å3 | 385.27(7) | 1569.0(2) | 1692.6(1) |
| Z | 2 | 8 | 8 |
| Density (calcd.), Mg·m−3 | 1.587 | 1.711 | 1.728 |
| Abs. coefficient, mm−1 | 0.140 | 0.164 | 0.176 |
| F(000) | 188 | 816 | 880 |
| Crystal size, mm3 | 0.08 × 0.15 × 0.25 | 0.08 × 0.12 × 0.30 | 0.15 × 0.50 × 0.80 |
| Θ range for data collection, ° | 2.8–27.5 | 2.8–27.6 | 2.4–29.5 |
| Index ranges | −9 ≤ h ≤ 9, −9 ≤ k ≤ 9, −11 ≤ l ≤ 10 | −16 ≤ h ≤ 16, −10 ≤ k ≤ 10, −19 ≤ l ≤ 19 | −14 ≤ h ≤ 14, −14 ≤ k ≤ 15, −19 ≤ l ≤ 17 |
| Reflections collected | 6116 | 15,950 | 27,896 |
| Independent reflections | 1748 R(int) = 0.028 | 1804 R(int) = 0.045 | 2178 R(int) = 0.039 |
| Completeness to θ, % | 99.7 (θ ≤ 25°) | 99.8 | 100.0 (θ ≤ 25°) |
| Data/restraints/parameters | 1748/0/124 | 1804/0/133 | 2178/0/142 |
| Goodness-of-fit on F2 | 1.04 | 1.00 | 1.04 |
| Final R indices I > 2σ(I) | R1 = 0.0520, wR2 = 0.1406 | R1 = 0.0359, wR2 = 0.0872 | R1 = 0.0347, wR2 = 0.0835 |
| Final R indices (all data) | R1 = 0.0849, wR2 = 0.1656 | R1 = 0.0458, wR2 = 0.0948 | R1 = 0.0462, wR2 = 0.0932 |
| Largest diff. peak/hole, e·Å−3 | 0.47, −0.26 | 0.28, −0.15 | 0.14, −0.19 |
| CCDC | 2016716 | 2016717 | 2016718 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanov, V.; Tretyakov, E.; Selivanova, G.; Li, J.; Bagryanskaya, I.; Makarov, A.; Luneau, D. Synthesis and Structure of Fluorinated (Benzo[d]imidazol-2-yl)methanols: Bench Compounds for Diverse Applications. Crystals 2020, 10, 786. https://doi.org/10.3390/cryst10090786
Romanov V, Tretyakov E, Selivanova G, Li J, Bagryanskaya I, Makarov A, Luneau D. Synthesis and Structure of Fluorinated (Benzo[d]imidazol-2-yl)methanols: Bench Compounds for Diverse Applications. Crystals. 2020; 10(9):786. https://doi.org/10.3390/cryst10090786
Chicago/Turabian StyleRomanov, Vasily, Evgeny Tretyakov, Galina Selivanova, Jiayao Li, Irina Bagryanskaya, Alexander Makarov, and Dominique Luneau. 2020. "Synthesis and Structure of Fluorinated (Benzo[d]imidazol-2-yl)methanols: Bench Compounds for Diverse Applications" Crystals 10, no. 9: 786. https://doi.org/10.3390/cryst10090786
APA StyleRomanov, V., Tretyakov, E., Selivanova, G., Li, J., Bagryanskaya, I., Makarov, A., & Luneau, D. (2020). Synthesis and Structure of Fluorinated (Benzo[d]imidazol-2-yl)methanols: Bench Compounds for Diverse Applications. Crystals, 10(9), 786. https://doi.org/10.3390/cryst10090786








