Synthesis, Crystal Structures and Characterization of Two Nonmetal Cation Tetrafluoroborates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of [H3tren](BF4)3 (I) and [H3tren](BF4)3•HF (II)
2.2. Single-Crystal Data Collection and Structure Determination
2.2.1. Structure Determination of [H3tren](BF4)3 (I)
2.2.2. Structure Determination of [H3tren](BF4)3 HF (II)
3. Results
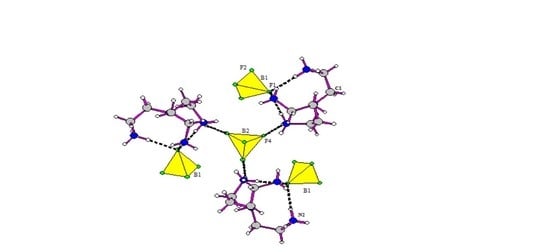
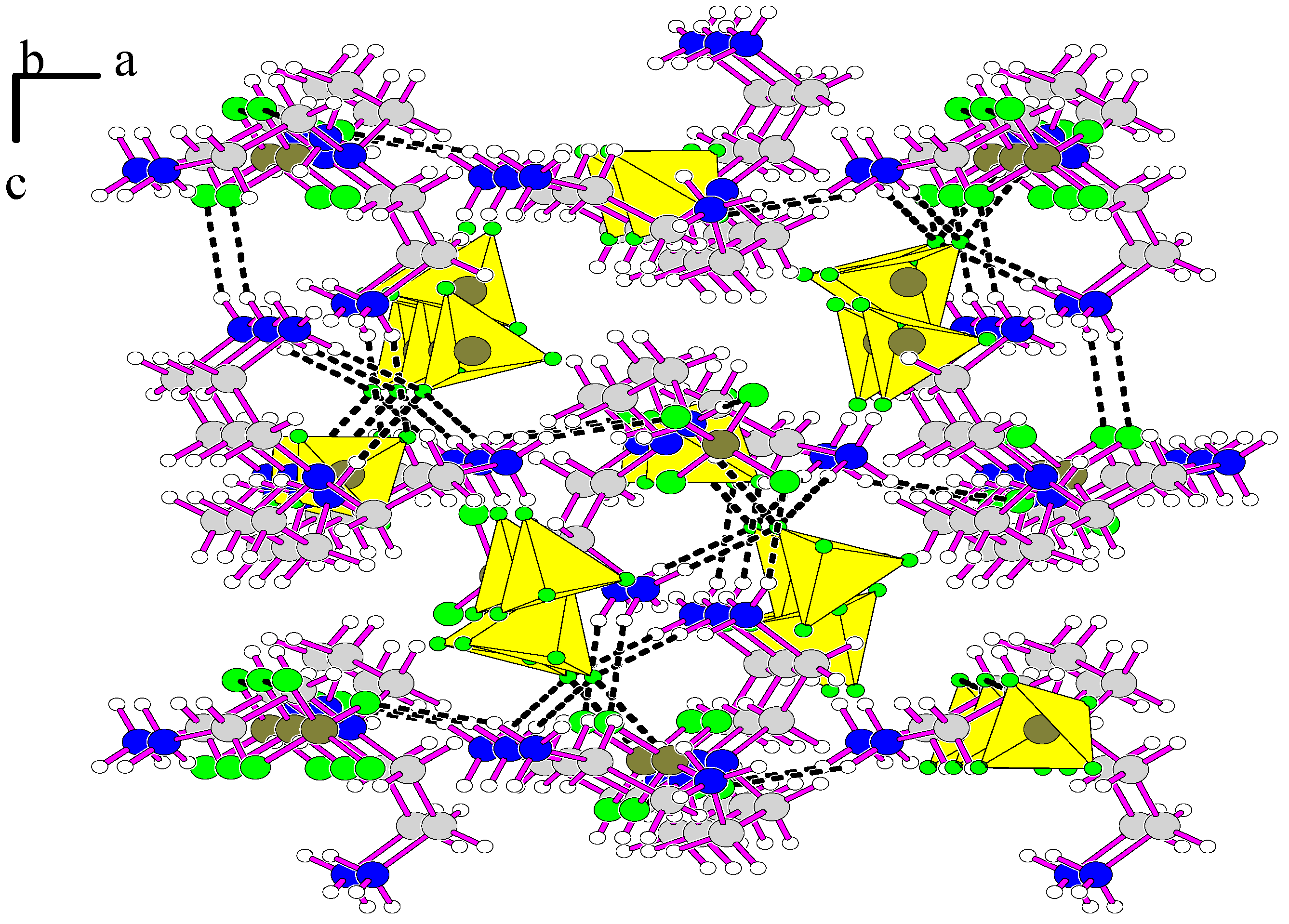
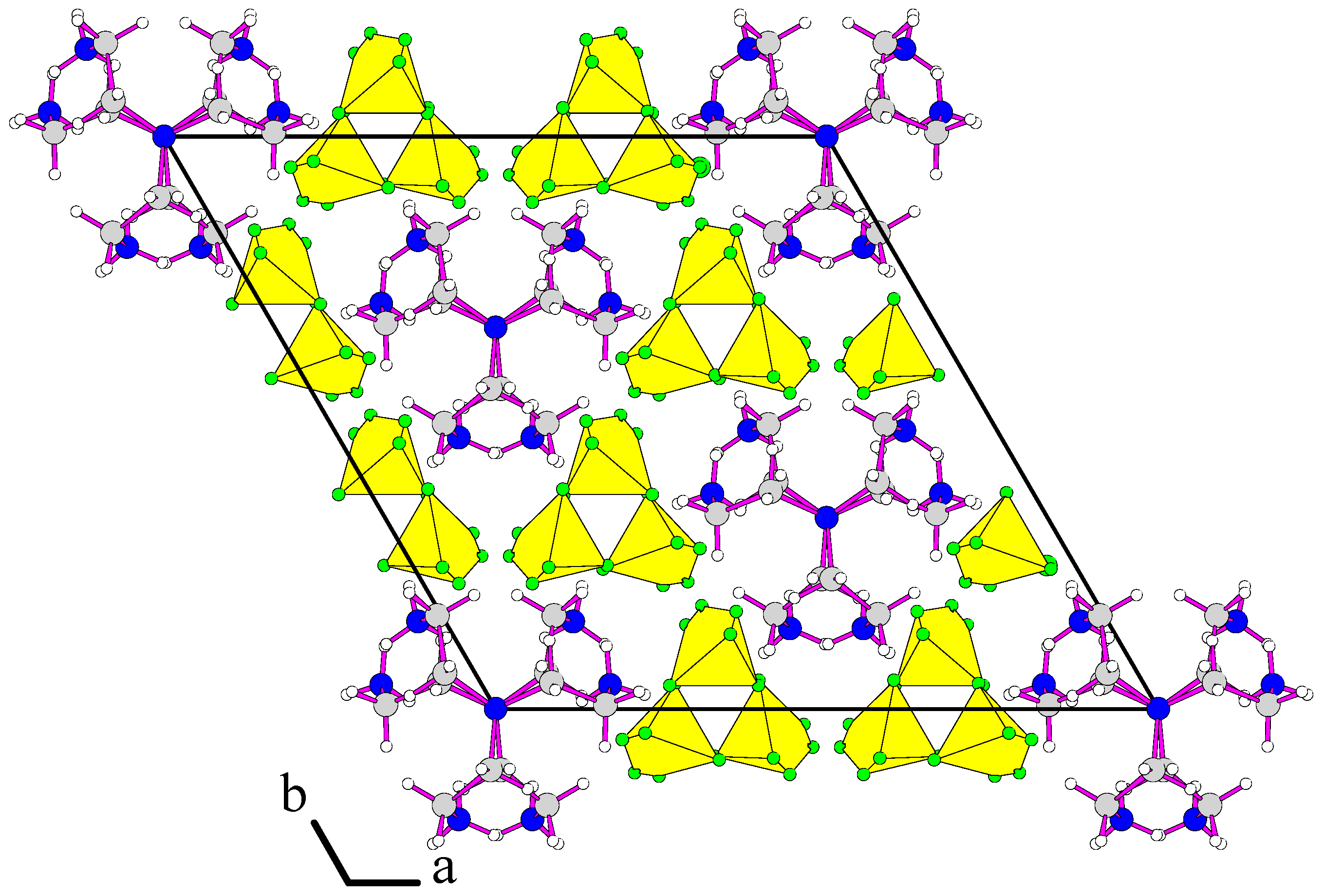
3.1. Structure Description of [H3tren](BF4)3 (I)
3.2. Structure Description of [H3tren](BF4)3 HF (II)
3.3. Thermal Analysis
3.4. IR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jayasundera, C.A.; Goff, R.J.; Li, J.; Lightfoot, P. Solvothermal indium fluoride chemistry: Syntheses and crystal structures of K5In3F14, β-(NH4)3InF6 and [NH4]3[C6H21N4]2[In4F21]. J. Solid State Chem. 2010, 183, 356. [Google Scholar] [CrossRef]
- Adil, A.; Leblanc, M.; Maisonneuve, V.; Lightfoot, P. Structural chemistry of organically-templated metal fluorides. Dalton Trans. 2010, 39, 5983. [Google Scholar] [CrossRef] [PubMed]
- Lhoste, J.; Rocquefelte, X.; Dessapt, R.; Jobic, S. A New Organic–Inorganic Hybrid Oxyfluorotitanate [Hgua]2·(Ti5O5F12) as a Transparent UV Filter. Inorg. Chem. 2011, 50, 5671. [Google Scholar] [CrossRef] [PubMed]
- Abdi, I.; Lhoste, J.; Leblanc, M.; Maisonneuve, V.; Grenèche, J.-M.; Viau, G.; Ben Ali, A. [H2amtaz]+ iron fluorides: Synthesis, crystal structures, magnetic and Mössbauer studies. J. Fluo. Chem. 2015, 173, 23. [Google Scholar] [CrossRef]
- Cadiau, A.; Martineau, C.; Taulelle, F.; Adil, K. Investigation of the composition space diagram of the ZnF2–3,5-diamino-1,2,4-triazole–HF–H2O chemical system and structural characterization of a new fluorinated guanazolate MOF [Zn3F2]·(Am2TAZ)4. J. Fluorine Chem. 2013, 150, 104. [Google Scholar] [CrossRef]
- Nijem, N.; Wu, H.H.; Canepa, P.; Marti, A. Tuning the Gate Opening Pressure of Metal–Organic Frameworks (MOFs) for the Selective Separation of Hydrocarbons. J. Am. Chem. Soc. 2012, 134, 15201. [Google Scholar] [CrossRef] [Green Version]
- Benaouadj, M.; Aboubou, A.; Ayad, M.Y.; Becherif, M. Nonlinear Flatness Control Applied to Supercapacitors Contribution in Hybrid Power Systems Using Photovoltaic Source and Batteries. Energy Procedia 2014, 50, 333. [Google Scholar] [CrossRef] [Green Version]
- Luz, I.; Corma, A.; Llabres, F.X. Cu-MOFs as active, selective and reusable catalysts for oxidative C–O bond coupling reactions by direct C–H activation of formamides, aldehydes and ethers. Catal. Sci. Technol. 2014, 4, 1829. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232. [Google Scholar] [CrossRef]
- Espa, D.; Pilia, L.; Makedonas, C.; Marchio, L.; Mercuri, M.L. Role of the Acceptor in Tuning the Properties of Metal [M(II) = Ni, Pd, Pt] Dithiolato/Dithione (Donor/Acceptor) Second-Order Nonlinear Chromophores: Combined Experimental and Theoretical Studies. Inorg. Chem. 2014, 53, 1170. [Google Scholar] [CrossRef]
- Zidan, D.; Al-Ktaifani, M.M.; L-Daher, M.S.E.; Allahham, A.; Ghanem, A. Diffraction ring patterns and nonlinear measurements of the Tris(2′,2-bipyridyl)iron(II) tetrafluoroborate. Optics Laser Tech. 2020, 131, 106449. [Google Scholar] [CrossRef]
- Cornu, L.; Gaudon, M.; Jubera, V. ZnAl2O4 as a potential sensor: Variation of luminescence with thermal history. J. Mater. Chem. C 2013, 34, 5419. [Google Scholar] [CrossRef] [Green Version]
- Pardo, E.; Train, C.; Liu, H.; Chamoreau, L.M. Multiferroics by Rational Design: Implementing Ferroelectricity in Molecule-Based Magnets. Angew. Chem. Int. Ed. Engl. 2012, 51, 8356. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Han, W.; Deng, J.; Zhu, W.; Zhang, Y.; Wang, H. Photocatalytic conversion of lignin into chemicals and fuels. ChemSusChem 2020, 13, 1. [Google Scholar]
- Iao, K.; Hagiwara, H.; Yokoyama, C. Acidic ionic liquid modified silica gel as novel solid catalysts for esterification and nitration reactions. J. Mol. Catal. A Chem. 2006, 246, 65. [Google Scholar]
- Wiscons, A.; Zeller, M.; Rowsell, J.L.C. Anion Exchange in Cationic Frameworks: Structures of Channel-Forming Triarylpyrylium Tetrafluoroborate Salts. Cryst. Growth Des. 2016, 16, 4. [Google Scholar] [CrossRef]
- Enayati, M.; Faghihian, H. N-butyl-pyridinium tetrafluoroborate as a highly efficient ionic liquid for removal of dibenzothiophene from organic solutions. J. Fuel Chem. Technol. 2015, 43, 195. [Google Scholar] [CrossRef]
- Fleck, M.; Petrosyan, A.M. Salts of Amino Acids: Crystallization, Structure and Properties; Springer: Dordrecht, The Netherlands, 2014; p. 574. [Google Scholar]
- Liu, H.-X.; Liang, Y.-X.; Jiang, X. Synthesis, crystal structure and NLO property of a nonmetal pentaborate [C6H13N2][B5O6(OH)4]. J. Solid State Chem. 2008, 181, 3243. [Google Scholar] [CrossRef]
- Guieu, V.; Payrastre, C.; Madaule, Y.; Garcia-Alonso, S.; Lacroix, P.G.; Nakatani, K. Large Quadratic Nonlinear Optical Efficiencies in Pseudosymmetric Streptocyanine Dyes. Chem. Mater. 2006, 18, 3674. [Google Scholar] [CrossRef]
- Ramajothi, J.; Dhanuskodi, S. Large Quadratic Nonlinear Optical Efficiencies in Pseudosymmetric Streptocyanine Dyes. Cryst. Res. Technol. 2003, 38, 986. [Google Scholar]
- Rogers, R.D.; Seddon, K.R.; Volkov, S. (Eds.) Green Industrial Applications of Ionic Liquids; NATO Science Series; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Wasserscheid, P.; Welton, T. (Eds.) Ionic Liquids in Synthesis; Wiley VCH: Weinheim, Germany, 2002. [Google Scholar]
- Umebayashi, Y.; Mitsugi, T.; Fukuda, S.; Fujimori, T.; Fujii, K.; Kanzaki, R.; Takeuchi, M.; Ishiguro, S. Lithium Ion Solvation in Room-Temperature Ionic Liquids Involving Bis(trifluoromethanesulfonyl) Imide Anion Studied by Raman Spectroscopy and DFT Calculations. J. Phys. Chem. B 2007, 111, 13028. [Google Scholar] [CrossRef]
- Sheldrick, M. “SHELXS-97”, a Program for Automatic Solution of Crystal Structures, Release 97-2; Göttingen University: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, M. “SHELXL-97”, Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997; Available online: https://www.scienceopen.com/document?vid=527f1309-ef7d-4d71-9985-641965ab49ab (accessed on 13 September 2020).
- Farrugia, J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837. [Google Scholar] [CrossRef]
- Shannon, D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751. [Google Scholar] [CrossRef]
- Johnson, C.K.; Burnett, M.N. ORTEP-III Version 1.0.2.: Oak Ridge, Thermal Ellipsoid Plot Program for Crystal Structure Illustrations, Oak Ridge National Laboratory Report ORNL-6895, USA 1996. Available online: https://digital.library.unt.edu/ark:/67531/metadc678816/ (accessed on 13 September 2020).
- Ali, A.B.; Grenèche, J.-M.; Leblanc, M.; Maisonneuve, V. [H3tren]3+ templated iron fluorides; synthesis, crystal structures and Mössbauer studies. Solid State Sci. 2009, 9, 1631. [Google Scholar] [CrossRef]
- Adil, K.; Saada, M.A.; Ali, A.B.; Body, M.; Dang, M.T.; Hémon-Ribaud, A.; Leblanc, M.; Maisonneuve, V. Hydrogen bonded H3O+, H2O, HF, F− in fluoride metalates (Al, Cr, Fe, Zr, Ta) templated with tren (tris-(2-aminoethyl)amine). J. Fluor. Chem. 2007, 128, 404. [Google Scholar] [CrossRef]
- Adil, K.; Ali, A.B.; Dujardin, G.; Dhal, R.; Leblanc, M.; Maisonneuve, V. Ternary and tetrahedral symmetry in hybrid fluorides, fluoride carbonates and carbonates. J. Fluor. Chem. 2004, 125, 1709. [Google Scholar] [CrossRef]
- Stuart, B. (Ed.) Infrared Spectroscopy: Fundamentals and Applications; Wiley and Sons: Hoboken, NJ, USA, 2004; p. 104. [Google Scholar]
- Abdi, I.; Alzahrany, F.; Lhoste, J.; Greneche, J.-M.; Ben Ali, A. Synthesis, crystal structure and Mössbauer study of new iron fluoride [C2N5H6] 2•(FeF5 (H2O) 2H2O. J. Adv. Chem. 2014, 10, 2617. [Google Scholar]
- Chang, C.; Jiang, J.-C.; Liou, Y.-C.; Hung, C.-H.; Lai, T.-Y.; Lin, S.H. Local Structures of Water in 1-Butyl-3-methylimidazolium Tetrafluoroborate Probed by High-Pressure Infrared Spectroscopy. Anal. Sci. 2008, 24, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamoto, K. (Ed.) Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley and Sons: Hoboken, NJ, USA, 2009; p. 196. [Google Scholar]
- Sukiasyan, P.; Suponitsky, K.Y.; Atanesyan, A.K.; Danghyan, A.A.; Hovhannisyan, A.A.; Petrosyan, A.M. Crystal structures and vibrational spectra of L-argininium(2+) bis(tetrafluoroborate) and L-argininium(2+) bis(perchlorate). Spectrochim. Acta A 2020, 228, 117782. [Google Scholar] [CrossRef] [PubMed]



| I | II | |
|---|---|---|
| Formula | B3F12N4C6H21 | B3F13N4C6H22 |
| Formula weight (g mol−1) | 409.7 | 429.7 |
| Crystal system | cubic | trigonal |
| Space group | P213 (n°198) | R3c (n°161) |
| a (Å) | 11.688(1) | 15.297(6) |
| b (Å) | 11.688(1) | 15.297(6) |
| c (Å) | 11.688(1) | 12.0070(2) |
| V (Å3), Z | 1596.7(4); 4 | 2433.2(4); 6 |
| Wavelength (MoKα) (Å) | 0.71073 | |
| Dimensions (mm3) | 0.095 × 0.209 × 0.190 | 0.08 × 0.13 × 0.61 |
| μ(MoKα) (mm−1) | 0.2 | 0.2 |
| ρcalc. (g cm−3) | 1.704 | 1.682 |
| Temperature (K) | 298 | |
| 2θ range (°) | 3–55 | 3–60 |
| (hkl) limits (one unique set) | −10 ≤ h ≤ 10; | −21 ≤ h ≤ 10; |
| −10 ≤ k ≤ 10; | 0 ≤ k ≤ 21; | |
| −15 ≤ l ≤ 15 | 0 ≤ l ≤ 16 | |
| Scan mode | ω-2θ | |
| Reflexions measured/unique/(I > 2σ(I)) | 1430/1241/893 | 1581/825/748 |
| R(int)/R(sigma) | 0.0175/0.003 | 0.0152/0.0127 |
| Number of refined parameters (on F2) | 79 | 99 |
| aR/Rw | 0.062/0.190 | 0.070/0.212 |
| Goodness of fit | 1.089 | 0.994 |
| Difference Fourier residues (e Å−3) | 0.56/−0.27 | 0.47/−0.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzamil, N.O.; Al-Enzi, G.M.; Alamri, A.H.; Abdi, I.; BenAli, A. Synthesis, Crystal Structures and Characterization of Two Nonmetal Cation Tetrafluoroborates. Crystals 2020, 10, 812. https://doi.org/10.3390/cryst10090812
Alzamil NO, Al-Enzi GM, Alamri AH, Abdi I, BenAli A. Synthesis, Crystal Structures and Characterization of Two Nonmetal Cation Tetrafluoroborates. Crystals. 2020; 10(9):812. https://doi.org/10.3390/cryst10090812
Chicago/Turabian StyleAlzamil, Noura Othman, Ghareeba Mussad Al-Enzi, Aishah Hassan Alamri, Insaf Abdi, and Amor BenAli. 2020. "Synthesis, Crystal Structures and Characterization of Two Nonmetal Cation Tetrafluoroborates" Crystals 10, no. 9: 812. https://doi.org/10.3390/cryst10090812
APA StyleAlzamil, N. O., Al-Enzi, G. M., Alamri, A. H., Abdi, I., & BenAli, A. (2020). Synthesis, Crystal Structures and Characterization of Two Nonmetal Cation Tetrafluoroborates. Crystals, 10(9), 812. https://doi.org/10.3390/cryst10090812