Proton, UV, and X-ray Induced Luminescence in Tb3+ Doped LuGd2Ga2Al3O12 Phosphors
Abstract
1. Introduction
2. Experimental
3. Characterizations
4. Results and Discussion
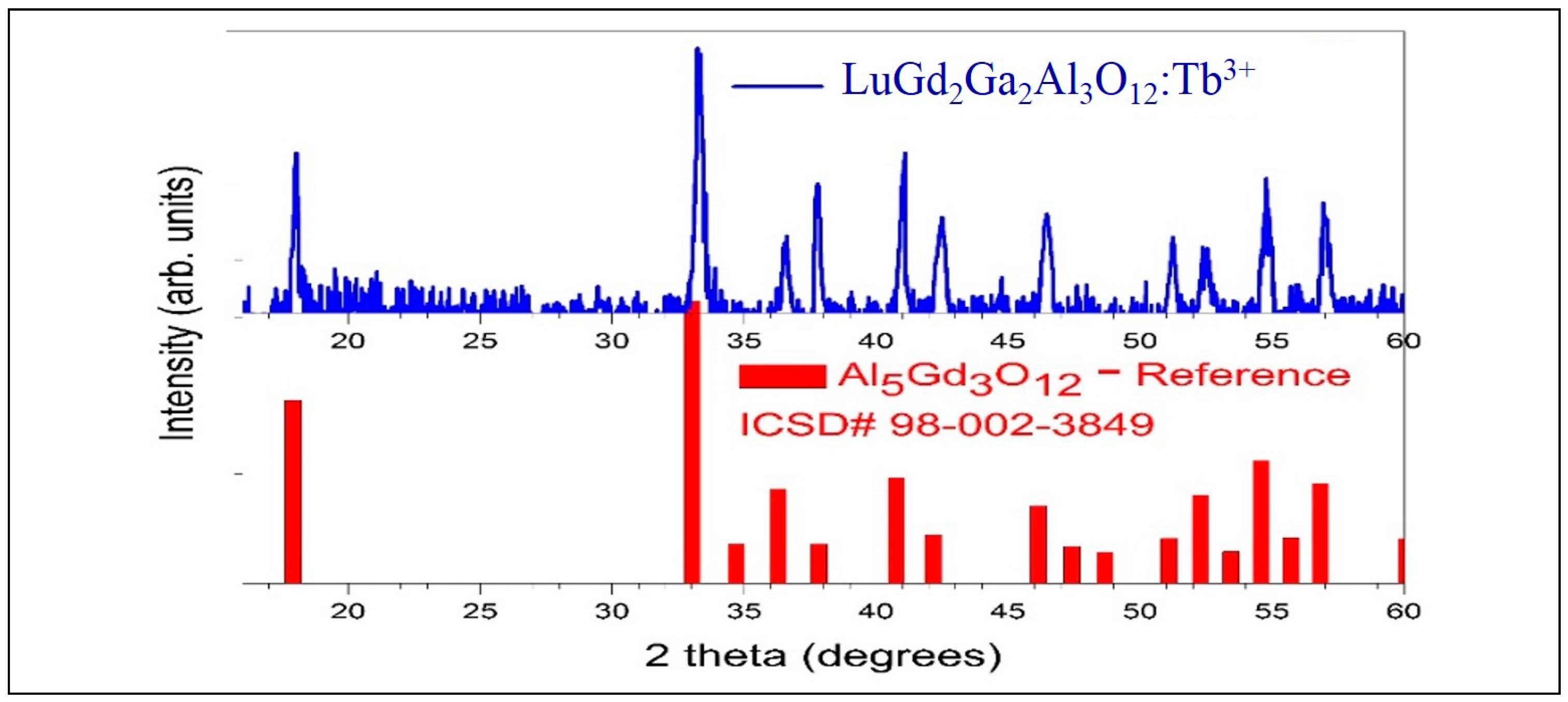
4.1. X-ray Diffraction Analysis
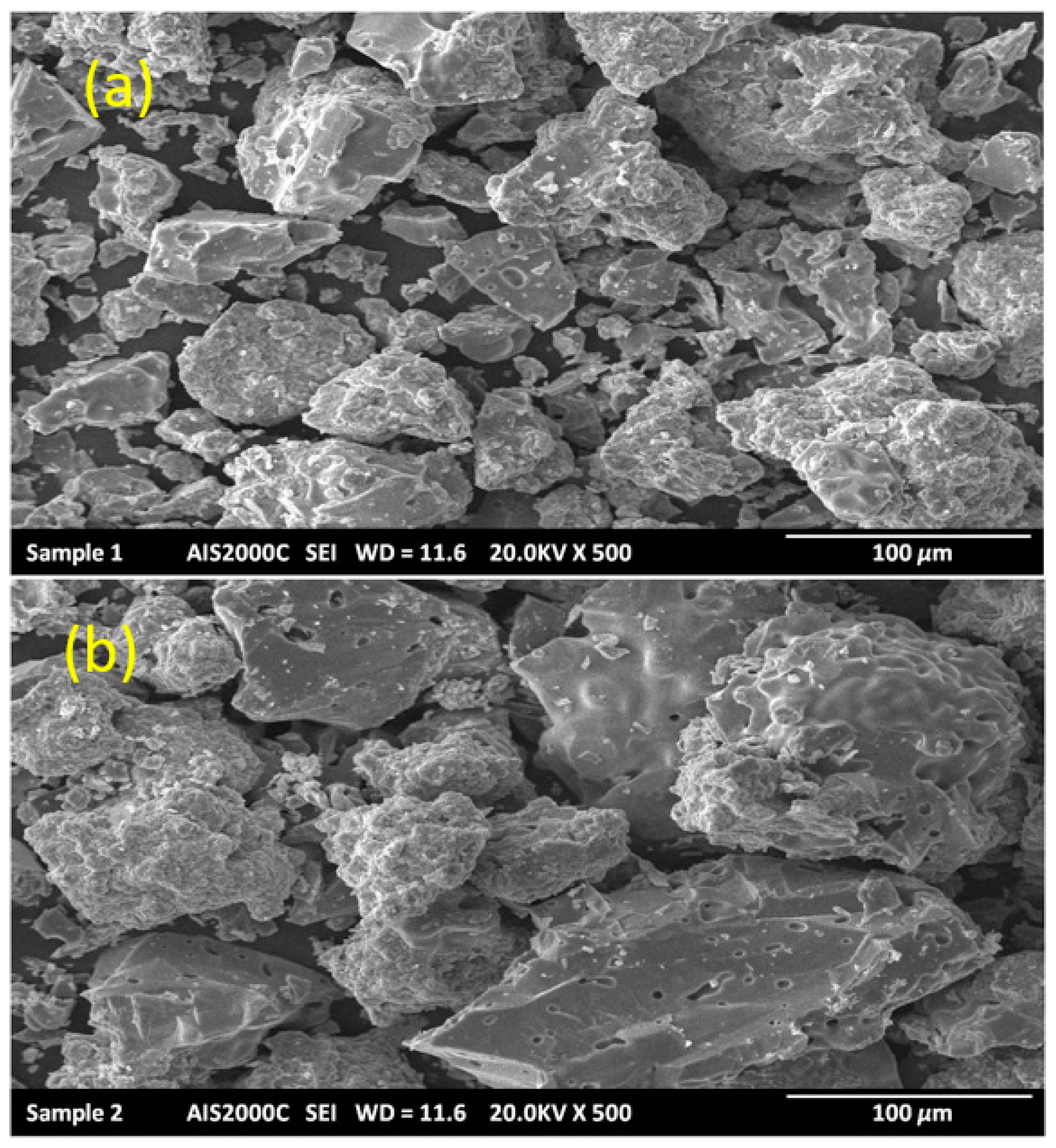
4.2. Scanning Electron Microscopy
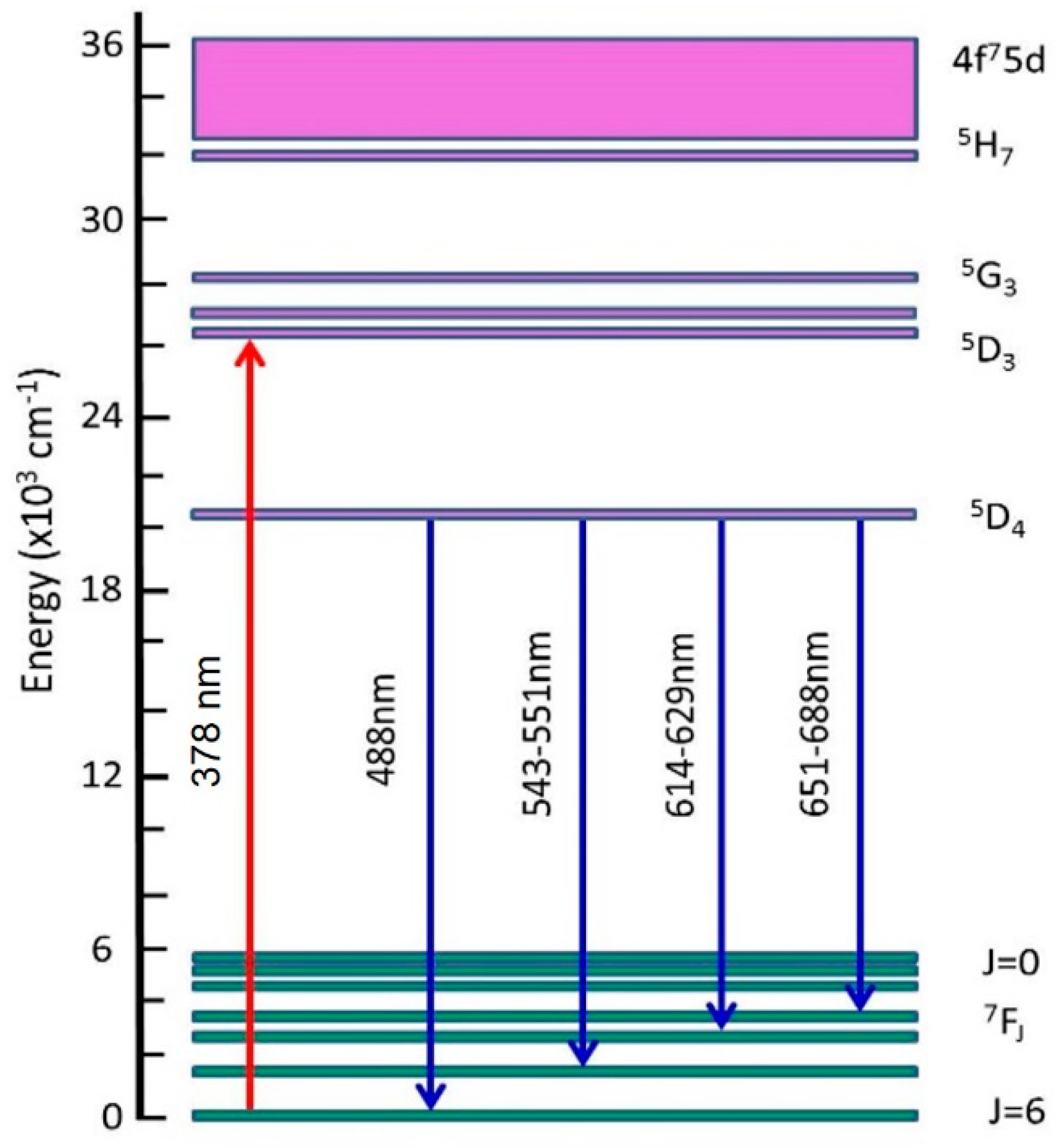
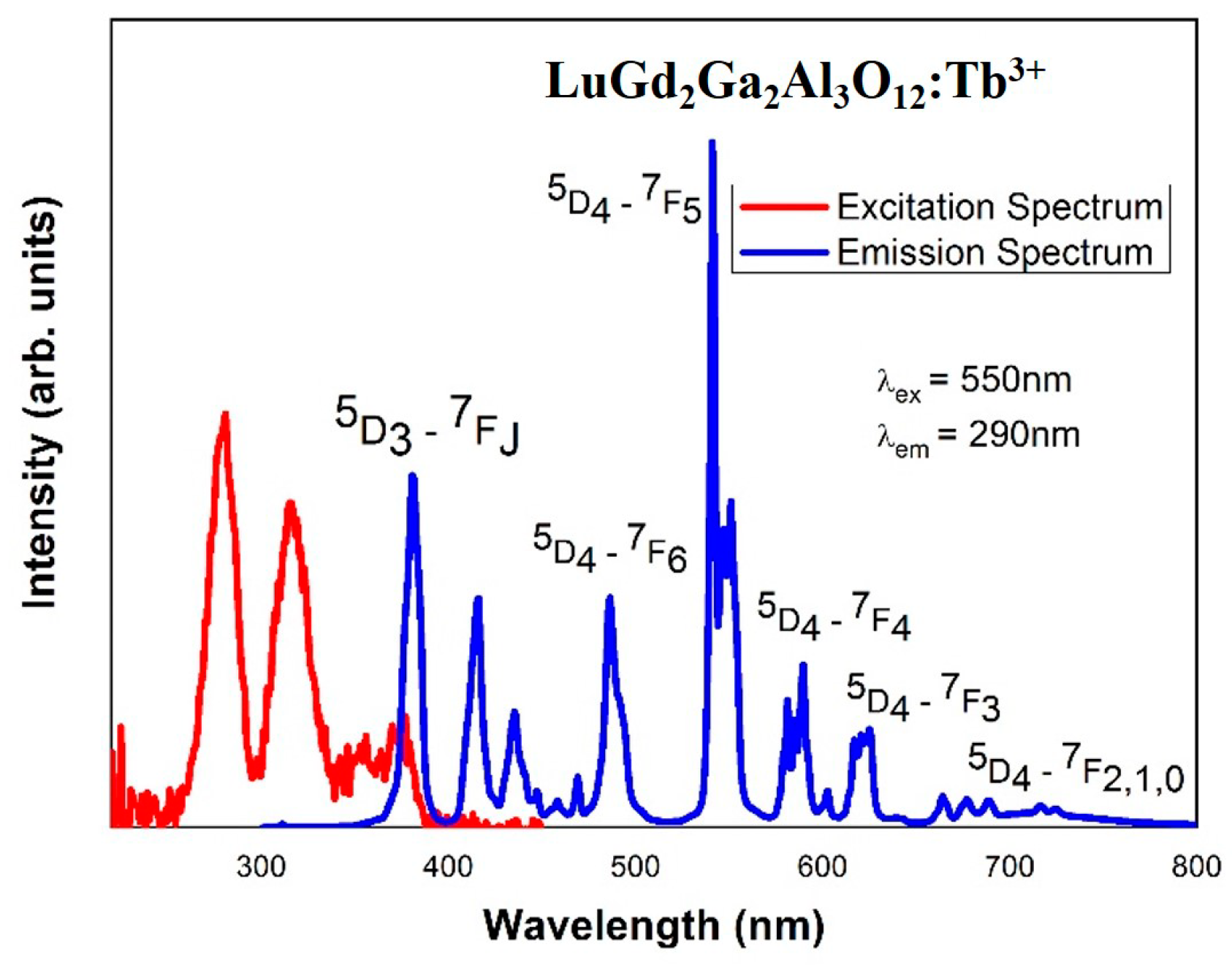
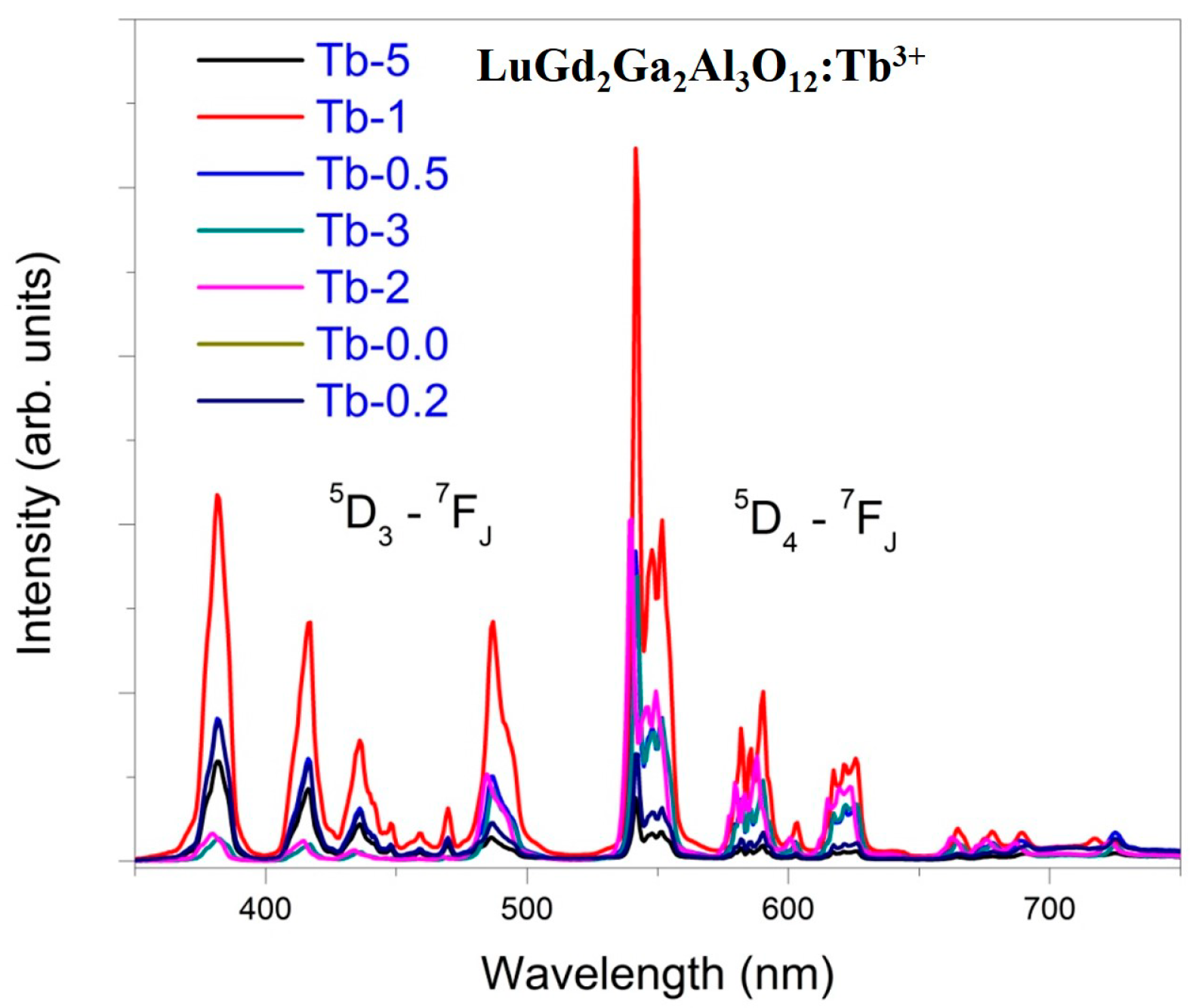
4.3. UV Induced Luminescence of LuGd2Ga2Al3O12:Tb3+
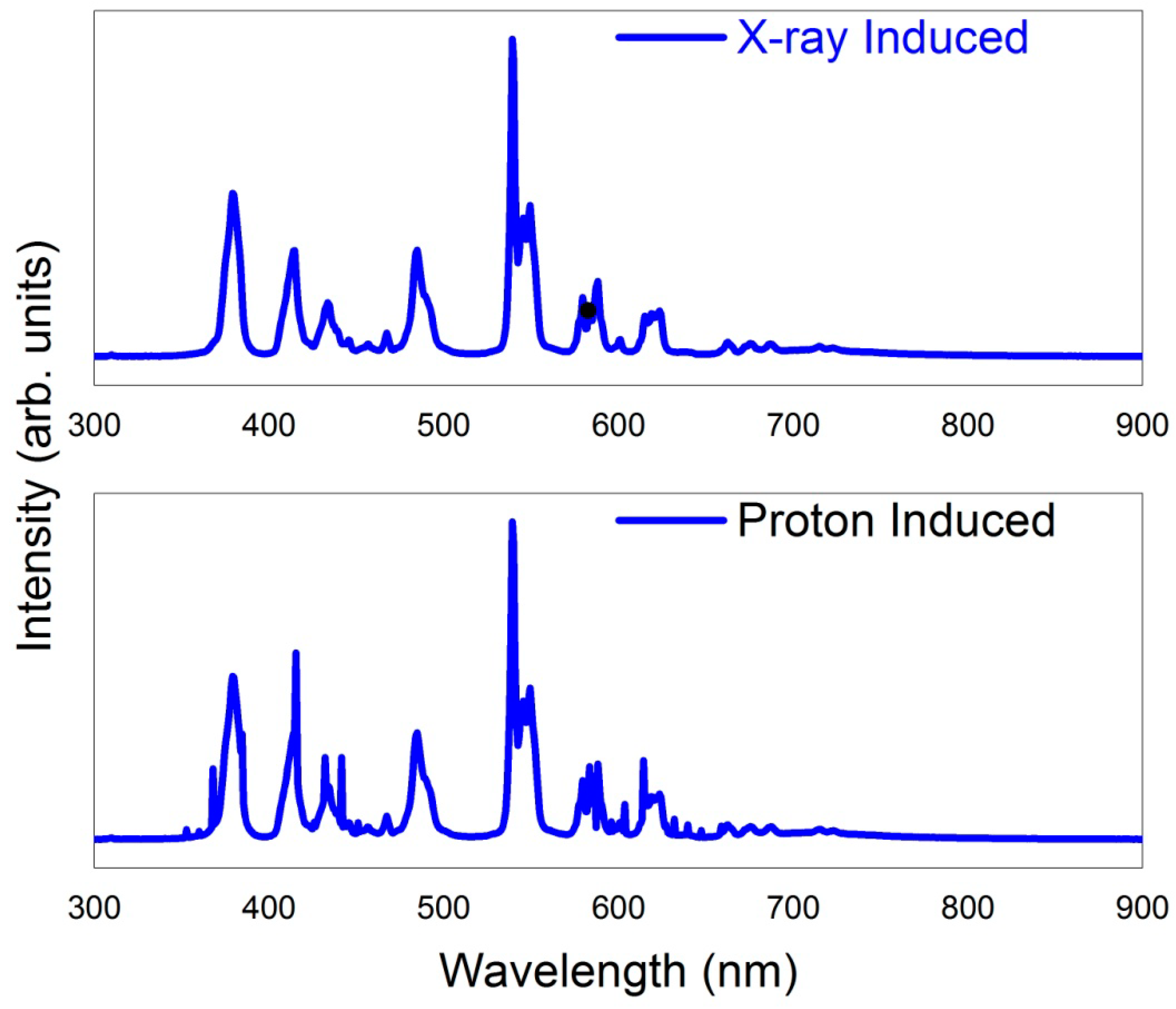
4.4. X-ray Induced Luminescence Spectroscopy
4.5. Optimization of Tb3+ Concentrations
4.6. Proton Induced Luminescence Spectroscopy
4.7. Luminescence Efficiency
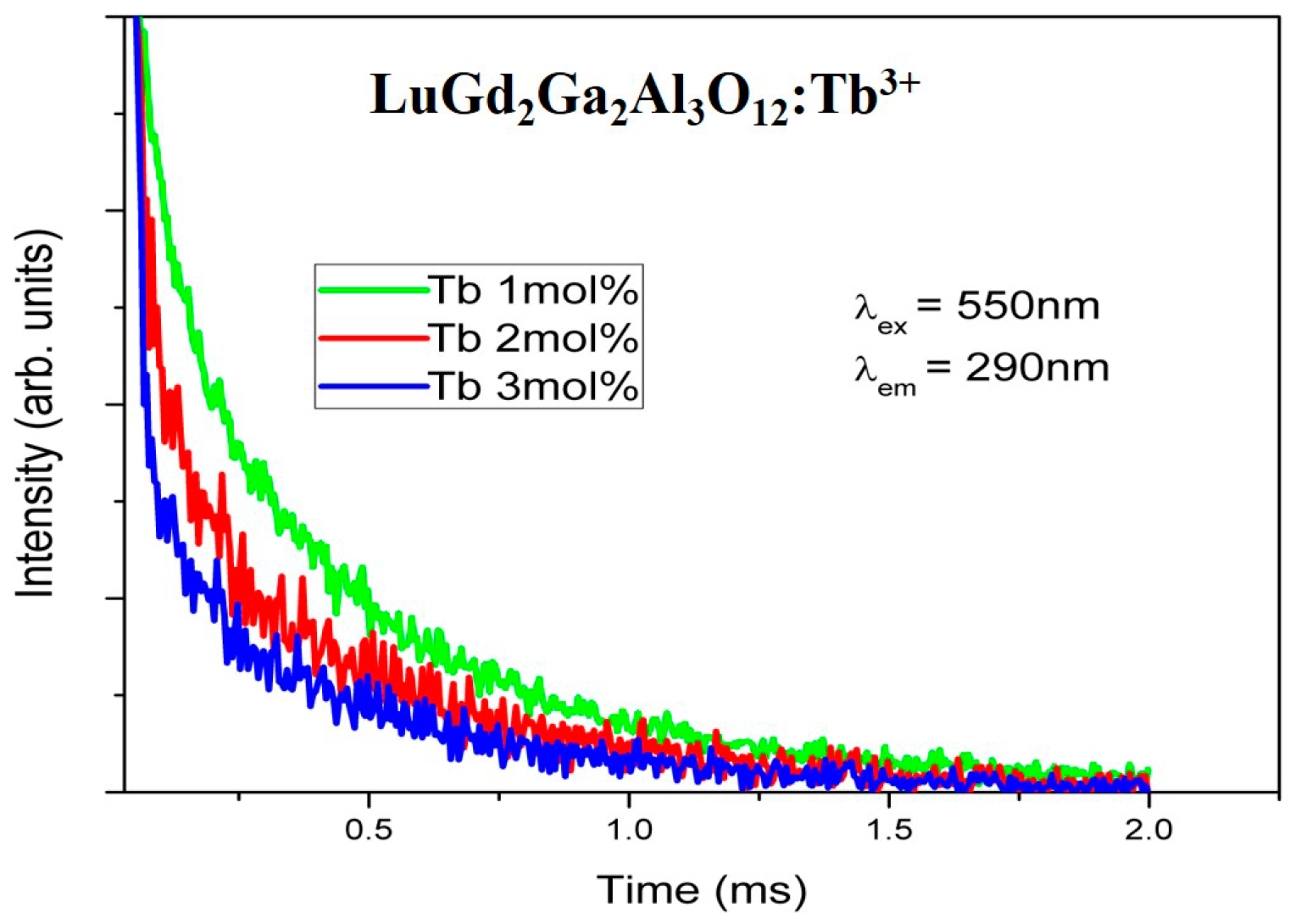
4.8. Decay Time Analysis
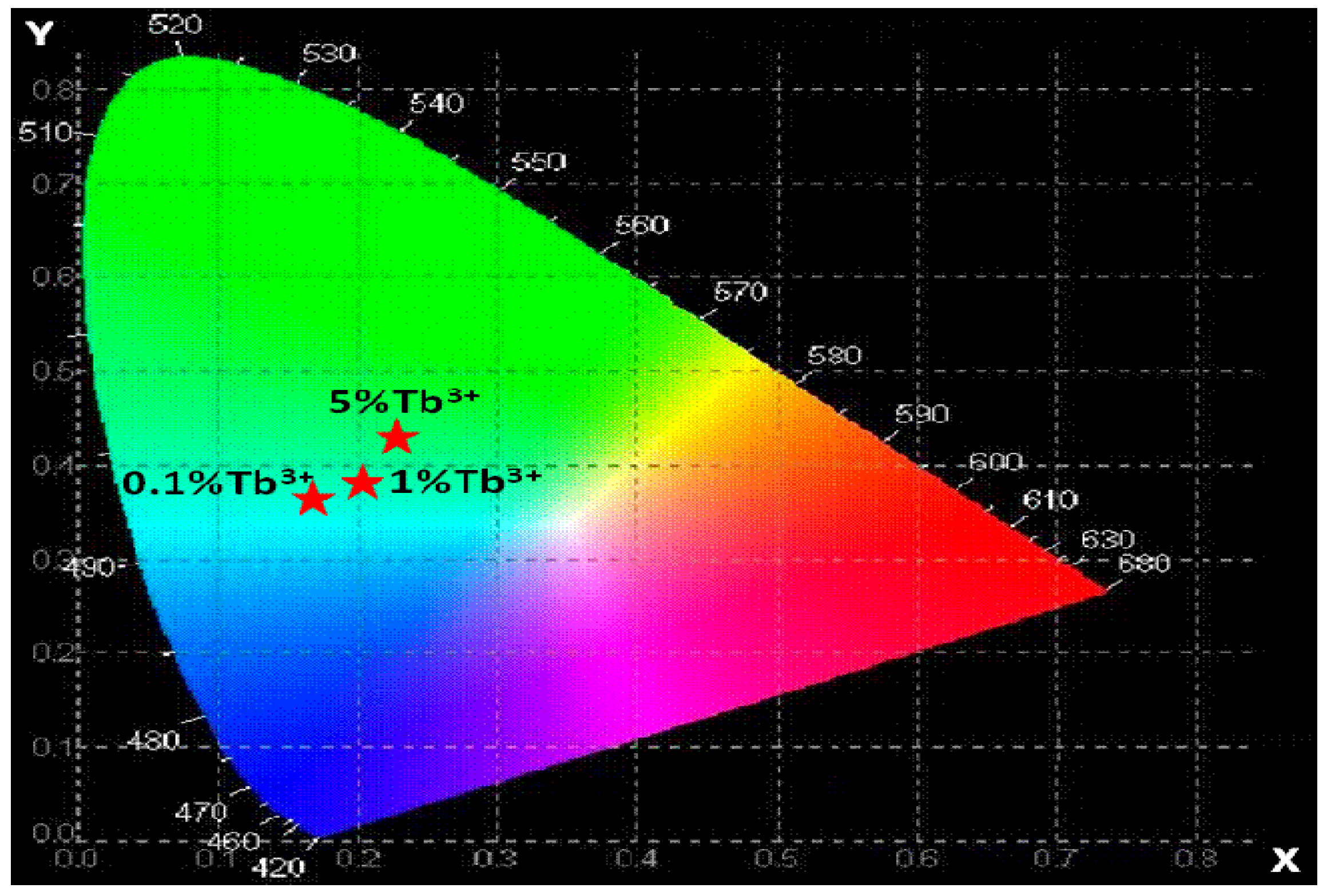
4.9. Chromaticity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tucureanu, V.; Matei, A.; Avram, A.M. Synthesis and characterization of YAG: Ce phosphors for white LEDs. Opto-Electron. Rev. 2015, 2323, 239–251. [Google Scholar]
- Dwivedi, J.; Kumar, P.; Kumar, A.; Singh, V.N.; Singh, B.P.; Dhawan, S.K.; Shanker, V.; Gupta, B.K. A commercial approach for the fabrication of bulk and nano phosphors converted into highly efficient white LEDs. RSC Adv. 2014, 4, 54936–54947. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, X.S.; Li, Y.F. RGO-supported β-SiC nanoparticles by a facile solvothermal route and their enhanced adsorption and photocatalytic activity. Mater. Lett. 2014, 132, 380–383. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Konstankevych, I. Single-crystalline films of Ce-doped YAG and LuAG phosphors: Advantages over bulk crystals analogues. J. Lumin. 2005, 114, 85–94. [Google Scholar] [CrossRef]
- Park, K.; Kim, T.; Yu, Y. Y/Gd-free yellow Lu3Al5O12: Ce3+ phosphor for white LEDs. J. Lumin. 2016, 173, 159–164. [Google Scholar] [CrossRef]
- Xia, Y.; Huang, X.; Wu, W. Multicolor persistent luminescence realized by persistent color conversion. J. Lumin. 2019, 207, 53–57. [Google Scholar] [CrossRef]
- Sankar, R. Efficient green luminescence in Tb3+-activated borates, A6MM′(BO3)6. Opt. Mater. 2008, 31, 268–275. [Google Scholar] [CrossRef]
- Lee, T.J.; Luo, L.Y.; Diau, E.W.G. Visible quantum cutting through downconversion in green-emitting K2GdF5: Tb3+ phosphors. Appl. Phys. Lett. 2006, 89, 131121. [Google Scholar] [CrossRef]
- Luchechko, A.; Kostyk, L.; Varvarenko, S. Green-Emitting Gd3Ga5O12: Tb3+ Nanoparticles Phosphor: Synthesis, Structure, and Luminescence. Nanoscale Res. Lett. 2017, 12, 263. [Google Scholar] [CrossRef]
- Li, G.; Cao, Q.; Li, Z. Photoluminescence properties of YAG: Tb nano-powders under vacuum ultraviolet excitation. J. Alloys Compd. 2009, 485, 561–564. [Google Scholar] [CrossRef]
- Jain, A.; González, C.A.E.; Tejeda, E.M. Covering the optical spectrum through different rare-earth ion-doping of YAG nanospheres produced by rapid microwave synthesis. Ceram. Int. 2018, 44, 1886–1893. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, J.; Zhang, C. Facile fabrication and luminescence characteristics of a mixture of phosphors (LuAG: Ce and CaAlSiN3: Eu) in glass for white LED. J. Non-Cryst. Solids 2018, 489, 57–63. [Google Scholar] [CrossRef]
- Van Krevel, J.W.H.; Hintzen, H.T.; Metselaar, R. Long wavelength Ce3+ emission in Y–Si–O–N materials. J. Alloys Compd. 1998, 268, 272–277. [Google Scholar] [CrossRef]
- Ozuna, O.; Hirata, G.A.; McKittrick, J. Luminescence enhancement in Eu3+-doped α-and γ-Al2O3 produced by pressure-assisted low-temperature combustion synthesis. Appl. Phys. Lett. 2004, 84, 1296–1298. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, H.J.; Hwang, Y.S. Two-dimensional beam profile monitoring by using a LYSO crystal. J. Korean Phys. Soc. 2010, 56, 2118–2121. [Google Scholar]
- Dorset, D.L. X-ray diffraction: A practical approach. Microsc. Microanal. 1998, 4, 513–515. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, C.; Zhan, Y. Luminescence and photocatalytic activity of ZnO nanocrystals: Correlation between structure and property. Inorg. Chem. 2007, 46, 6675–6682. [Google Scholar] [CrossRef]
- Bi, C.; Wang, Q.; Shao, Y. Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Rao, B.V.; Buddhudu, S. Emission analysis of RE3+ (Dy3+ or Tb3+): Ca3Ln (Y,Gd)(VO4)3 powder phosphors. Mater. Chem. Phys. 2008, 111, 65–68. [Google Scholar]
- Thakur, J.; Dutta, D.P.; Bagla, H. Effect of host structure and concentration on the luminescence of Eu3+ and Tb3+ in borate phosphors. J. Am. Ceram. Soc. 2012, 95, 696–704. [Google Scholar] [CrossRef]
- Fawad, U.; Oh, M.; Park, H. Luminescent investigations of Li6Lu (BO3)3: Tb3+, Dy3+ phosphors. J. Alloys Compd. 2014, 610, 281–287. [Google Scholar] [CrossRef]
- Manohara, B.M.; Nagabhushana, H.; Sunitha, D.V. Synthesis and luminescent properties of Tb3+ activated cadmium silicate nanophosphor. J. Alloys Compd. 2014, 592, 319–327. [Google Scholar] [CrossRef]
- Linganna, K.; Ju, S.; Basavapoornima, C. Luminescence and decay characteristics of Tb3+-doped fluorophosphate glasses. J. Asian Ceram. Soc. 2018, 6, 82–87. [Google Scholar] [CrossRef]
- Fawad, U.; Kim, H.J.; Khan, A. X-ray and Photoluminescence Study of Li6Gd (BO3)3: Tb3+, Dy3+ Phosphors. Sci. Adv. Mater. 2015, 7, 2536–2544. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Liu, Z. UV and X-ray excited luminescence of Tb3+-doped ZnGa2O4 phosphors. J. Alloys Compd. 2005, 391, 202–205. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, W.T.; Chu, C.I. UV/VUV switch-driven color-reversal effect for Tb-activated phosphors. Light Sci. Appl. 2016, 5, e16066. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Guo, Y. Synthesis and photoluminescence of double perovskite La2LiSbO6: Ln3+ (Ln = Eu, Tb, Tm, Sm, Ho) phosphors and enhanced luminescence of La2LiSbO6: Eu3+ red phosphor via Bi3+ doping for white light emitting diodes. J. Alloys Compd. 2019, 787, 1163–1172. [Google Scholar] [CrossRef]
- Cheng, S.D.; Kam, C.H.; Buddhudu, S. Enhancement of green emission from Tb3+: GdOBr phosphors with Ce3+ ion co-doping. Mater. Res. Bull. 2001, 36, 1131–1137. [Google Scholar] [CrossRef]
- Liu, X.; Yan, L.; Zou, J. Tunable cathodoluminescence properties of Tb3+-doped La2O3 nanocrystalline phosphors. J. Electrochem. Soc. 2009, 157, P1. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fawad, U.; Kim, H.J.; Gul, I.; Khan, M.; Tahir, S.; Jamal, T.; Muhammad, W. Proton, UV, and X-ray Induced Luminescence in Tb3+ Doped LuGd2Ga2Al3O12 Phosphors. Crystals 2020, 10, 844. https://doi.org/10.3390/cryst10090844
Fawad U, Kim HJ, Gul I, Khan M, Tahir S, Jamal T, Muhammad W. Proton, UV, and X-ray Induced Luminescence in Tb3+ Doped LuGd2Ga2Al3O12 Phosphors. Crystals. 2020; 10(9):844. https://doi.org/10.3390/cryst10090844
Chicago/Turabian StyleFawad, U., H. J. Kim, Ibrahim Gul, Matiullah Khan, Sajjad Tahir, Tauseef Jamal, and Wazir Muhammad. 2020. "Proton, UV, and X-ray Induced Luminescence in Tb3+ Doped LuGd2Ga2Al3O12 Phosphors" Crystals 10, no. 9: 844. https://doi.org/10.3390/cryst10090844
APA StyleFawad, U., Kim, H. J., Gul, I., Khan, M., Tahir, S., Jamal, T., & Muhammad, W. (2020). Proton, UV, and X-ray Induced Luminescence in Tb3+ Doped LuGd2Ga2Al3O12 Phosphors. Crystals, 10(9), 844. https://doi.org/10.3390/cryst10090844






