A New Piano-Stool Ruthenium(II) P-Cymene-Based Complex: Crystallographic, Hirshfeld Surface, DFT, and Luminescent Studies
Abstract
:1. Introduction
2. Experimental
2.1. Starting Materials
2.2. Synthesis
3. Results and Discussion
3.1. Synthetic Methodology
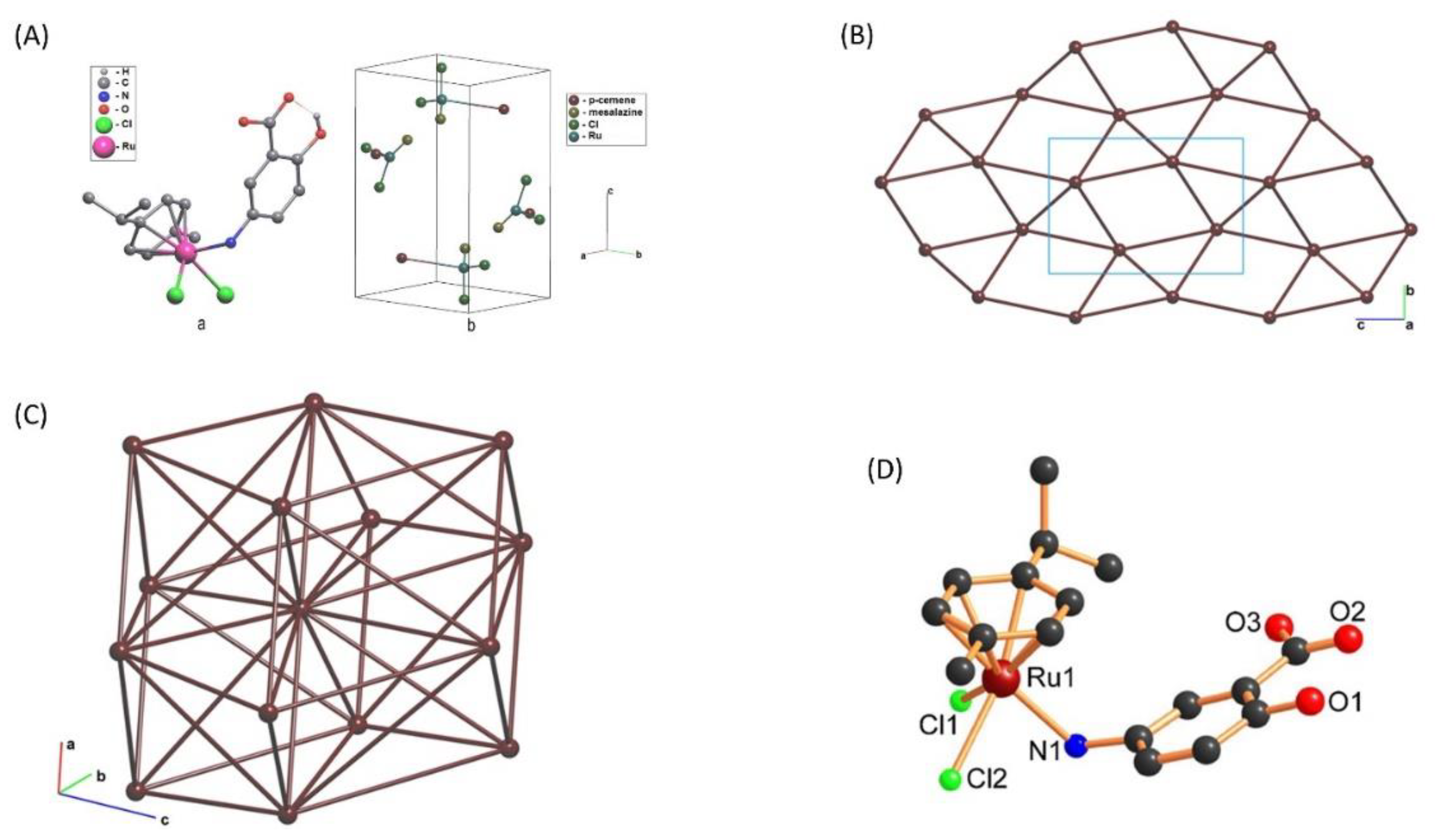
3.2. X-ray Structure Investigation
3.3. NMR Spectroscopy
3.4. IR Spectroscopy
3.5. Thermogravimetric Analysis
3.6. Absorption Study
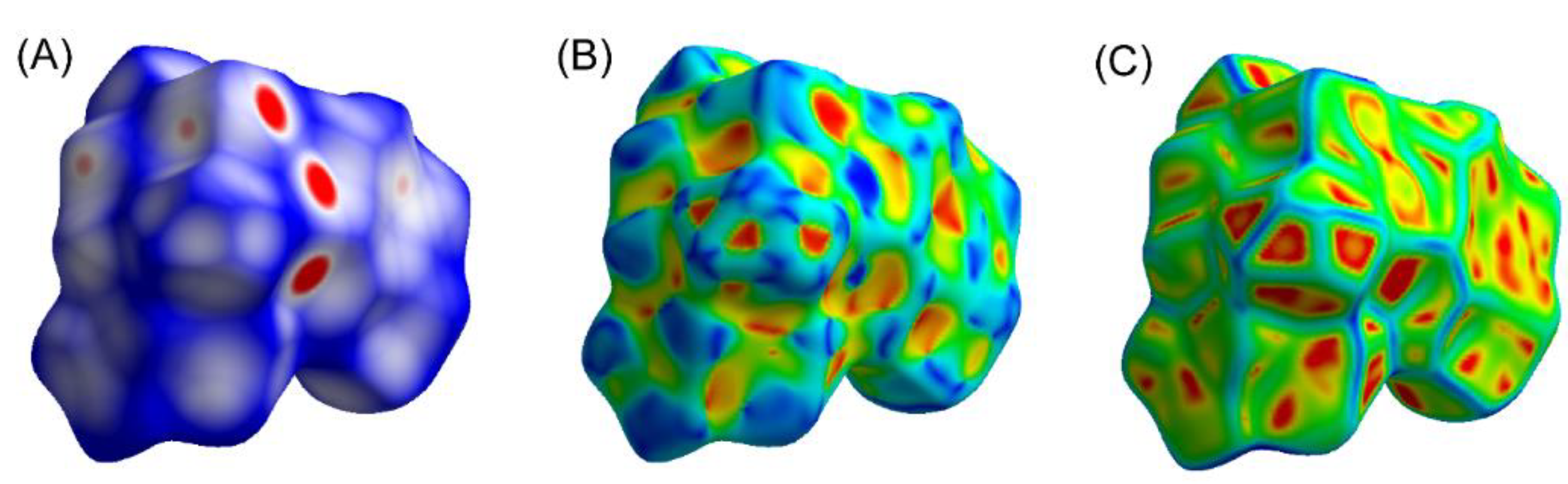
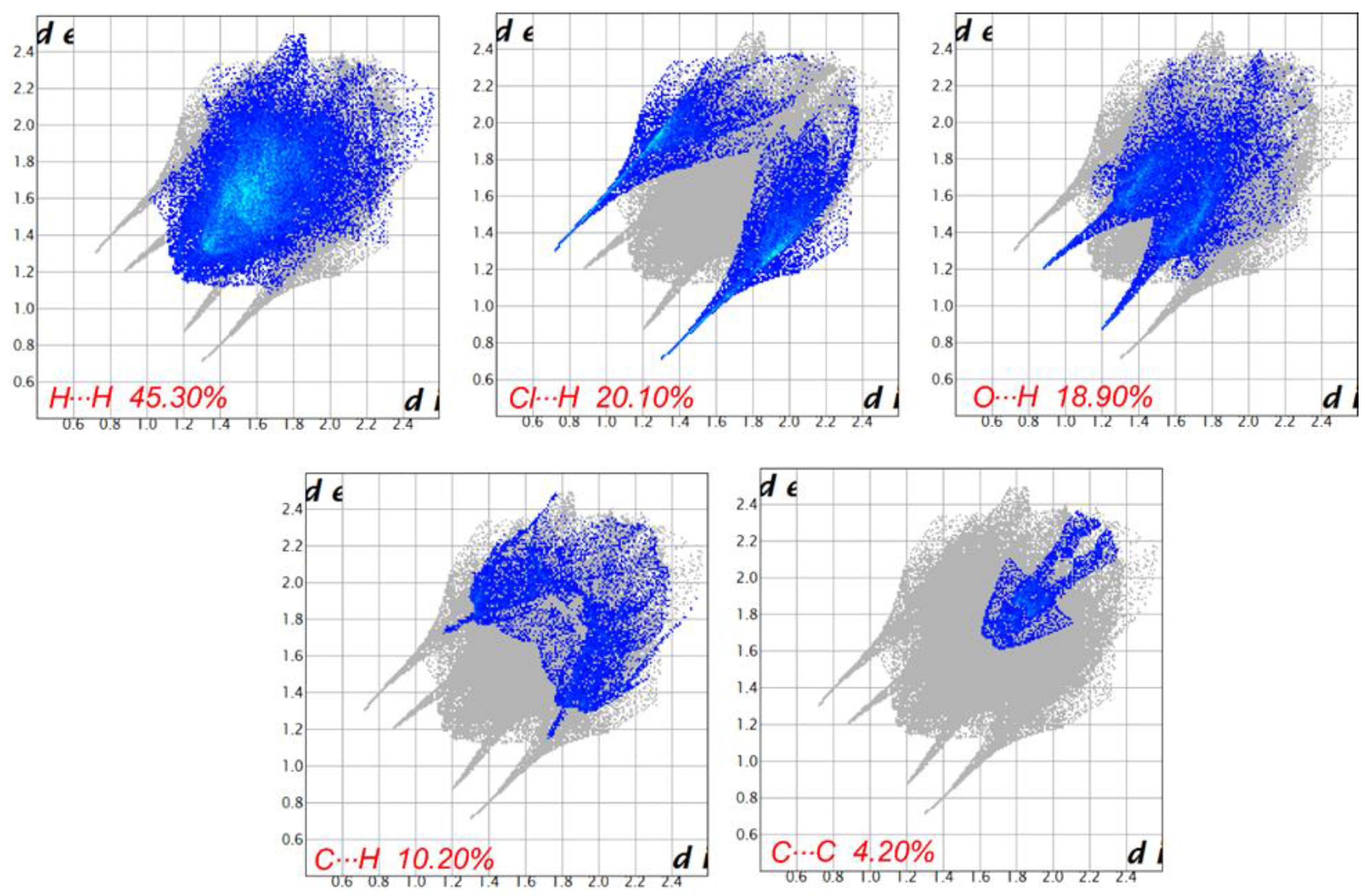
3.7. Hirshfeld Surface Analyses
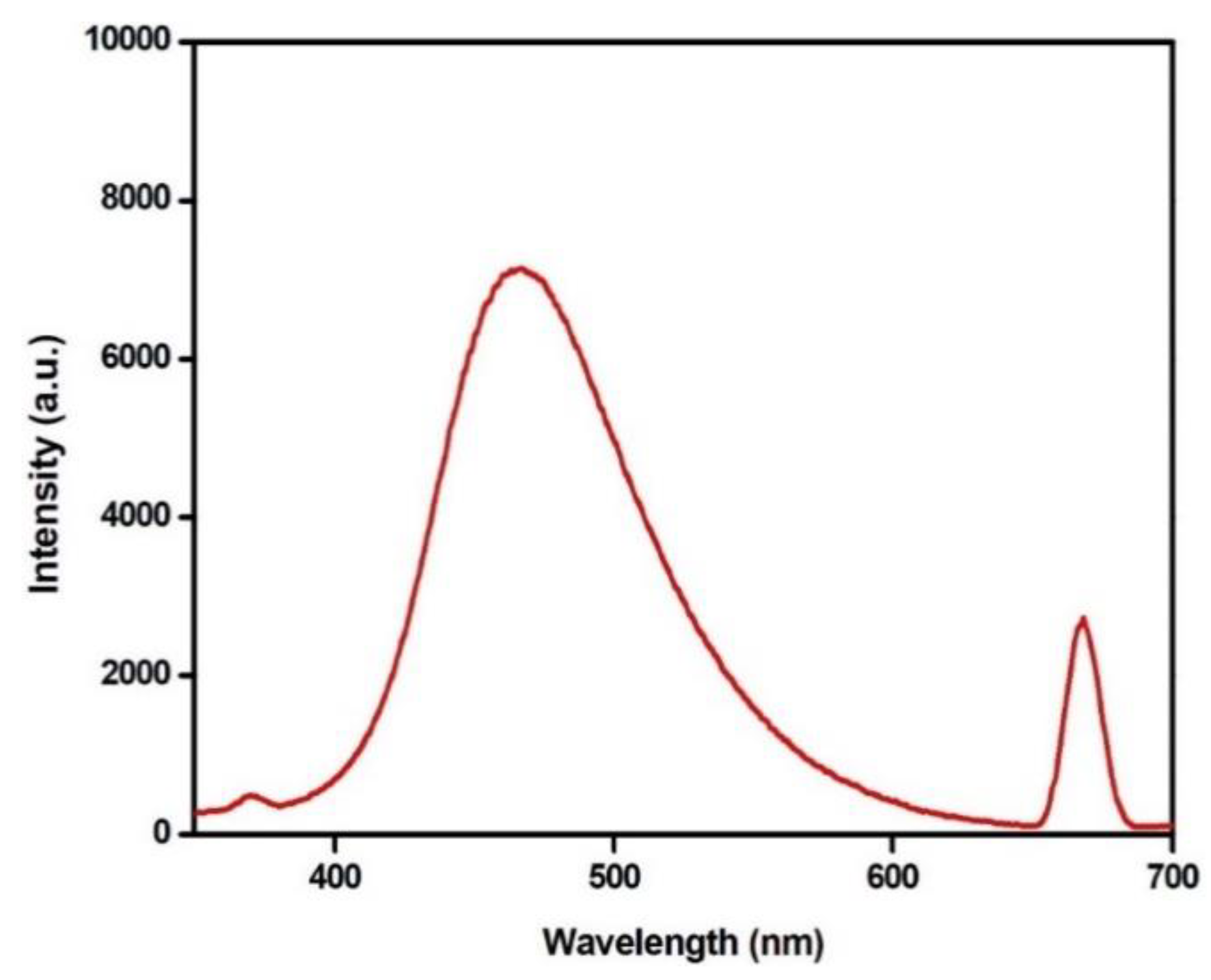
3.8. Photoluminescence Property
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sarı, Y.; Gürses, C.; Celepci, D.B.; Keleştemur, Ü.; Aktaş, A.; Yüksel, Ş.; Ateş, B.; Gök, Y. 4-Vinylbenzyl and 2-morpholinoethyl substituted ruthenium(II) complexes: Design, synthesis, and biological evaluation. J. Mol. Struct. 2020, 1202, 127355. [Google Scholar] [CrossRef]
- Reedijk, B.J. Metal-Ligand Exchange Kinetics in Platinum and Ruthenium Complexes. Platin. Met. Rev. 2008, 52, 2–11. [Google Scholar] [CrossRef]
- Notaro, A.; Gasser, G. Monomeric and dimeric coordinatively saturated and substitutionally inert Ru( II) polypyridyl complexes as anticancer drug candidates. Chem. Soc. Rev. 2017, 46, 7317–7337. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Vila, A.M.; Monsaert, S.; Bajek, A.; Verpoort, F. Ruthenium-Based Olefin Metathesis Catalysts Derived from Alkynes. Chem. Rev. 2010, 110, 4865–4909. [Google Scholar] [CrossRef]
- Liao, X.; Jiang, G.; Wang, J.; Duan, X.; Liao, Z.; Lin, X.; Shen, J.; Xiong, Y.; Jiang, G. Two ruthenium polypyridyl complexes functionalized with thiophen: Synthesis and antibacterial activity against Staphylococcus aureus. N. J. Chem. 2020, 44, 17215–17221. [Google Scholar] [CrossRef]
- Golbaghi, G.; Groleau, M.; López de los Santos, Y.; Doucet, N.; Déziel, E.; Castonguay, A. Cationic Ru II Cyclopentadienyl Complexes with Antifungal Activity against Several Candida Species. ChemBioChem 2020, 21, 3112–3119. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Deng, Y.; Li, B.; Li, C.; Lin, Y.; Yang, D.; Zhang, H.; Chen, L.; Wang, T. Novel cyclometalated Ru(II) complexes containing isoquinoline ligands: Synthesis, characterization, cellular uptake and in vitro cytotoxicity. Eur. J. Med. Chem. 2020, 203, 112562. [Google Scholar] [CrossRef]
- Murugan, K.; Vijayapritha, S.; Kavitha, V.; Viswanathamurthi, P. Versatile formation of Ru(II) hydrazone complexes: Structure, theoretical studies and catalytic activity in α-alkylation. Polyhedron 2020, 190, 114737. [Google Scholar] [CrossRef]
- Trnka, T.M.; Grubbs, R.H. The Development of L 2 X 2 RuCHR Olefin Metathesis Catalysts: An Organometallic Success Story. Acc. Chem. Res. 2001, 34, 18–29. [Google Scholar] [CrossRef]
- Van der Drift, R.C.; Bouwman, E.; Drent, E. Homogeneously catalysed isomerisation of allylic alcohols to carbonyl compounds. J. Organomet. Chem. 2002, 650, 1–24. [Google Scholar] [CrossRef]
- Wang, H.-X.; Wu, K.; Che, C.-M. Metal-Quinoid Carbene Chemistry: From Bonding to C–H Activation Catalysis. Synlett 2020. [Google Scholar] [CrossRef]
- Jabłońska-Wawrzycka, A.; Rogala, P.; Michałkiewicz, S.; Hodorowicz, M.; Barszcz, B. Ruthenium complexes in different oxidation states: Synthesis, crystal structure, spectra and redox properties. Dalt. Trans. 2013, 42, 6092–6101. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Moradi Fili, S.; Shahlaei, M. Synthesis, characterization and comparative DNA interaction studies of new copper(II) and nickel(II) complexes containing mesalamine drug using molecular modeling and multispectroscopic methods. J. Coord. Chem. 2015, 68, 3667–3684. [Google Scholar] [CrossRef]
- Soliman, M.H.; Mohamed, G.G. Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II) and Zn(II) new complexes of 5-aminosalicylic acid: Spectroscopic, thermal characterization and biological activity studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 107, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, X.; Dai, Z. Synthesis and Characterization of Light Lanthanide Complexes with 5-Aminosalicylic Acid. Synth. React. Inorg. Met. Chem. 2004, 34, 1123–1134. [Google Scholar] [CrossRef]
- Arjmand, F.; Muddassir, M. Design and synthesis of heterobimetallic topoisomerase I and II inhibitor complexes: In vitro DNA binding, interaction with 5′-GMP and 5′-TMP and cleavage studies. J. Photochem. Photobiol. B Biol. 2010, 101, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Nigović, B.; Šimunić, B. Determination of 5-aminosalicylic acid in pharmaceutical formulation by differential pulse voltammetry. J. Pharm. Biomed. Anal. 2003, 31, 169–174. [Google Scholar] [CrossRef]
- Purkait, K.; Mukherjee, A. Cytotoxicity and reactivity of a redox active 1,4-quinone-pyrazole compound and its Ru(II)-p-cymene complex. Inorganica Chim. Acta 2020, 502, 119361. [Google Scholar] [CrossRef]
- Štarha, P.; Trávníček, Z. Non-platinum complexes containing releasable biologically active ligands. Coord. Chem. Rev. 2019, 395, 130–145. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) Compounds: Next-Generation Anticancer Metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.; Sava, G. Linking the future of anticancer metal-complexes to the therapy of tumour metastases. Chem. Soc. Rev. 2015, 44, 8818–8835. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.J. Ruthenium metallopharmaceuticals. Coord. Chem. Rev. 2002, 232, 69–93. [Google Scholar] [CrossRef]
- Motswainyana, W.M.; Ajibade, P.A. Anticancer Activities of Mononuclear Ruthenium(II) Coordination Complexes. Adv. Chem. 2015, 2015, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Ogo, S.; Uehara, K.; Abura, T.; Watanabe, Y.; Fukuzumi, S. Aqueous Polymerization of Styrene Promoted by Water-Soluble Robust Ruthenium Hydride Complexes. Organometallics 2004, 23, 3047–3052. [Google Scholar] [CrossRef]
- Demonceau, A.; Dragutan, I.; Dragutan, V.; Le Gendre, P. Olefin Metathesis as Key Step in the Synthesis of Bioactive Compounds: Challenges in the Total Synthesis of Iriomoteolides. Curr. Org. Synth. 2012, 9, 779–790. [Google Scholar] [CrossRef]
- Stumpf, A.W.; Saive, E.; Demonceau, A.; Noels, A.F. Ruthenium-based catalysts for the ring opening metathesis polymerisation of low-strain cyclic olefins and of functionalised derivatives of norbornene and cyclooctene. J. Chem. Soc. Chem. Commun. 1995, 1127–1128. [Google Scholar] [CrossRef]
- Canivet, J.; Karmazin-Brelot, L.; Süss-Fink, G. Cationic arene ruthenium complexes containing chelating 1,10-phenanthroline ligands. J. Organomet. Chem. 2005, 690, 3202–3211. [Google Scholar] [CrossRef] [Green Version]
- Šterk, D.; Stephan, M.S.; Mohar, B. New chiral N-(N,N-dialkylamino)sulfamoyl-1,2-diamine ligands for highly enantioselective transfer hydrogenation of ketones. Tetrahedron Asymmetry 2002, 13, 2605–2608. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Friedrich, H.B.; Omondi, B. Synthesis and characterization of piano-stool ruthenium complexes with N,N′-pyridine imine bidentate ligands and their application in styrene oxidation. J. Organomet. Chem. 2016, 808, 87–96. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Pettinari, R.; Cerquetella, A.; Di Nicola, C.; Macchioni, A.; Zuccaccia, D.; Monari, M.; Piccinelli, F. Synthesis and Intramolecular and Interionic Structural Characterization of Half-Sandwich (Arene)Ruthenium(II) Derivatives of Bis(Pyrazolyl)Alkanes. Inorg. Chem. 2008, 47, 11593–11603. [Google Scholar] [CrossRef] [PubMed]
- Rüther, T.; Woodward, C.P.; Jones, T.W.; Coghlan, C.J.; Hebting, Y.; Cordiner, R.L.; Dawson, R.E.; Robinson, D.E.J.E.; Wilson, G.J. Synthesis, characterisation, and properties of p-cymene Ruthenium(II) tetracarboxylate bipyridine complexes [(η6-p-cymene)Ru(Rn,Rn′-tcbpy)Cl][Cl]. J. Organomet. Chem. 2016, 823, 136–146. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Zakeeruddin, S.M.; Lagref, J.-J.; Liska, P.; Comte, P.; Barolo, C.; Viscardi, G.; Schenk, K.; Graetzel, M. Stepwise assembly of amphiphilic ruthenium sensitizers and their applications in dye-sensitized solar cell. Coord. Chem. Rev. 2004, 248, 1317–1328. [Google Scholar] [CrossRef]
- Barolo, C.; Yum, J.-H.; Artuso, E.; Barbero, N.; Di Censo, D.; Lobello, M.G.; Fantacci, S.; De Angelis, F.; Grätzel, M.; Nazeeruddin, M.K.; et al. A Simple Synthetic Route to Obtain Pure Trans -Ruthenium(II) Complexes for Dye-Sensitized Solar Cell Applications. ChemSusChem 2013, 6, 2170–2180. [Google Scholar] [CrossRef]
- Martinho, M.; Xue, G.; Fiedler, A.T.; Que, L., Jr.; Bominaar, E.L.; Münck, E. Mössbauer and DFT Study of the Ferromagnetically Coupled Diiron(IV) Precursor to a Complex with an FeIV2O2 Diamond Core. J. Am. Chem. Soc. 2009, 131, 5823–5830. [Google Scholar] [CrossRef] [Green Version]
- Kal, S.; Filatov, A.S.; Dinolfo, P.H. Structural, Electrochemical, and Spectroscopic Investigation of Acetate Bridged Dinuclear Tetrakis-Schiff Base Macrocycles of Mn and Zn. Inorg. Chem. 2013, 52, 13963–13973. [Google Scholar] [CrossRef]
- Maxwell, C.I.; Mosey, N.J.; Stan Brown, R. DFT Computational Study of the Methanolytic Cleavage of DNA and RNA Phosphodiester Models Promoted by the Dinuclear Zn (II) Complex of 1,3-Bis(1,5,9-triazacyclododec-1-yl)propane. J. Am. Chem. Soc. 2013, 135, 17209–17222. [Google Scholar] [CrossRef]
- Malenov, D.P.; Zarić, S.D. Stacking interactions between ruthenium p -cymene complexes: Combined crystallographic and density functional study. CrystEngComm 2019, 21, 7204–7210. [Google Scholar] [CrossRef]
- Mishra, A.; Lee, S.; Kim, H.; Cook, T.R.; Stang, P.J.; Chi, K.-W. Selective Detection of Multicarboxylate Anions based on “Turn on” Electron Transfer by Self-Assembled Molecular Rectangles. Chem. An. Asian J. 2012, 7, 2592–2599. [Google Scholar] [CrossRef] [Green Version]
- Vinoth, G.; Indira, S.; Bharathi, M.; Durgadevi, A.; Abinaya, R.; Alves, L.G.; Martins, A.M.; Bharathi, K.S. Synthesis of Imines via Reactions of Benzyl Alcohol with Amines Using Half-Sandwich (η6–p-cymene) Ruthenium(II) Complexes Stabilised by 2-aminofluorene Derivatives. Appl. Organomet. Chem. 2019, 33, e5200. [Google Scholar] [CrossRef]
- Alexandrov, E.V.; Blatov, V.A.; Kochetkov, A.V.; Proserpio, D.M. Underlying nets in three-periodic coordination polymers: Topology, taxonomy and prediction from a computer-aided analysis of the Cambridge Structural Database. CrystEngComm 2011, 13, 3947–3958. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Y.; Liu, M.; Wang, S.; Li, Y.; Liu, X.; Tian, L. Synthesis, structural characterization and cytotoxic activity of triorganotin 5-(salicylideneamino)salicylates. Appl. Organomet. Chem. 2019, 33, e4715. [Google Scholar] [CrossRef]
- Orioli, M.; Marinello, C.; Cozzi, R.; Piodi, L.P.; Carini, M. LC-MS/MS and FT-IR analyses of stones from a patient with Crohn’s disease: A case report. J. Pharm. Biomed. Anal. 2004, 35, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, J.J.; Fabbiani, F.P.A.; Spackman, M.A. Comparison of Polymorphic Molecular Crystal Structures through Hirshfeld Surface Analysis. Cryst. Growth Des. 2007, 7, 755–769. [Google Scholar] [CrossRef]
- Pettinari, C.; Pettinari, R.; Xhaferai, N.; Giambastiani, G.; Rossin, A.; Bonfili, L.; Maria Eleuteri, A.; Cuccioloni, M. Binuclear 3,3′,5,5′-tetramethyl-1H,H-4,4′-bipyrazole Ruthenium(II) complexes: Synthesis, characterization and biological studies. Inorg. Chim. Acta 2020, 513, 119902. [Google Scholar] [CrossRef]


| CCDC No. | 2038452 |
|---|---|
| Formula | C17H21Cl2NO3 Ru |
| Formula weight | 459.32 |
| Temperature/K | 150 |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a/Å b/Å c/Å | 8.6496(15) 12.026(3) 17.285(3) |
| α/° β/° γ/° | 90.00 91.976(6) 90.00 |
| Volume/Å3 | 1796.9(6) |
| Z | 4 |
| ρcalc/mg mm−3 | 1.698 |
| μ/mm−1 | 9.930 |
| F(000) | 928.0 |
| Index ranges | −7 ≤ h ≤ 10 −14 ≤ k ≤ 15 −21 ≤ l ≤ 19 |
| Complex 1 | ||||
|---|---|---|---|---|
| X-ray | DFT | |||
| Ru(1)-Cl(1) | 2.4005(16) | 2.453 | ||
| Ru(1)-Cl(2) | 2.4304(14) | 2.458 | ||
| Ru(1)-N(17) | 2.159(4) | 2.174 | ||
| Ru(1)-C(10) | 2.195(5) | 2.193 | ||
| Ru(1)-C(9) | 2.208(5) | 2.204 | ||
| Ru(1)-C(11) | 2.171(5) | 2.183 | ||
| Ru(1)-C(12) | 2.185(5) | 2.212 | ||
| Ru(1)-C(13) | 2.160(5) | 2.228 | ||
| Ru(1)-C(14) | 2.193(5) | 2.204 | ||
| Cl(1) | Ru(1) | Cl(2) | 86.89(5) | 88.54 |
| N(1) | Ru(1) | Cl(2) | 81.78(13) | 80.96 |
| N(1) | Ru(1) | Cl(1) | 81.41(13) | 82.15 |
| Complex 1 | ||
|---|---|---|
| Experimental | DFT | Inference |
| 574 | 590 | Out-of-plane ring deformation |
| 701 | 740 | O-H wagging |
| 743 | 817 | In-plane ring deformation |
| 874 | 878–925 | Ar-CH wagging |
| 999 | 997 | Ar-CH twisting |
| 1026 | 1027 | -CH3 rocking |
| 1053 | 1066 | -CH3 rocking |
| 1184 | 1131 | Ar-CH rocking |
| 1267 | 1264 | CH twisting |
| 1421 | 1427 | CH scissoring |
| 1492 | 1497 | Ar-C-C asymmetric stretching |
| 1531 | 1602 | -NH2 scissoring |
| 1593 | 1641 | C=C stretch and C-H bending |
| 1623 | 1684 | C=C stretch and C-H bending |
| 1666 | 1741 | -C=O symmetric stretching |
| 2870 | 3020–3040 | CH3 symmetric stretching |
| 2961 | 3104–3160 | CH Asymmetric stretching |
| 3043 | 3186–3231 | Ar-CH symmetric stretching |
| 3219 | 3363 | Ar-O-H (phenolic) symmetric stretching |
| 3285 | 3265 | -NH2 symmetric stretching |
| 3435 | 3560 | O-H (COOH) symmetric stretching |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muddassir, M.; Alarifi, A.; Afzal, M. A New Piano-Stool Ruthenium(II) P-Cymene-Based Complex: Crystallographic, Hirshfeld Surface, DFT, and Luminescent Studies. Crystals 2021, 11, 13. https://doi.org/10.3390/cryst11010013
Muddassir M, Alarifi A, Afzal M. A New Piano-Stool Ruthenium(II) P-Cymene-Based Complex: Crystallographic, Hirshfeld Surface, DFT, and Luminescent Studies. Crystals. 2021; 11(1):13. https://doi.org/10.3390/cryst11010013
Chicago/Turabian StyleMuddassir, Mohd., Abdullah Alarifi, and Mohd. Afzal. 2021. "A New Piano-Stool Ruthenium(II) P-Cymene-Based Complex: Crystallographic, Hirshfeld Surface, DFT, and Luminescent Studies" Crystals 11, no. 1: 13. https://doi.org/10.3390/cryst11010013
APA StyleMuddassir, M., Alarifi, A., & Afzal, M. (2021). A New Piano-Stool Ruthenium(II) P-Cymene-Based Complex: Crystallographic, Hirshfeld Surface, DFT, and Luminescent Studies. Crystals, 11(1), 13. https://doi.org/10.3390/cryst11010013





