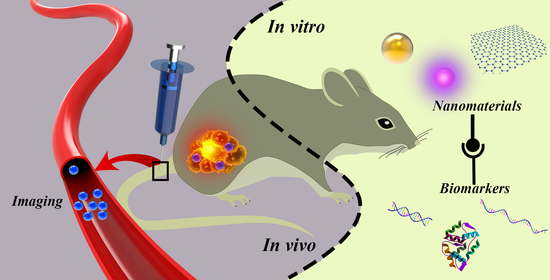
Recent Progress of Lung Cancer Diagnosis Using Nanomaterials
Abstract
:1. Introduction
2. Applications of Nanoparticles in Diagnosis of Cancer
2.1. In Vivo Examination
2.1.1. Single-Modal Imageological Examination-MRI
2.1.2. Single-Modal Imageological Examination-PET
2.1.3. Multi-Modal Imageological Examination
2.2. In Vitro Detection
2.2.1. Gold Nanoparticles
2.2.2. Quantum Dots (QDs)
2.2.3. Carbon Nanomaterials
2.2.4. Others
3. Cellular Uptake of Nanomaterials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.; Galeone, C.; Sverzellati, N.; Marchiano, A.; Calareso, G.; Sestini, S.; La Vecchia, C.; Sozzi, G.; Pelosi, G.; Pastorino, U. Screening with low-dose computed tomography does not improve survival of small cell lung cancer. J. Thorac. Oncol. 2016, 11, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doroudian, M.; MacLoughlin, R.; Poynton, F.; Prina-Mello, A.; Donnelly, S.C. Nanotechnology based therapeutics for lung disease. Thorax 2019, 74, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2013, 143, E93–E120. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Zhang, H.; Wei, Y.; Chen, H. Ambient mass spectrometry for the molecular diagnosis of lung cancer. Analyst 2020, 145, 313–320. [Google Scholar] [CrossRef]
- Khanmohammadi, A.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hajian, A.; Bagheri, H. Electrochemical biosensors for the detection of lung cancer biomarkers: A review. Talanta 2020, 206, 120251. [Google Scholar] [CrossRef]
- Salehi-Rad, R.; Li, R.; Paul, M.K.; Dubinett, S.M.; Liu, B. The biology of lung cancer: Development of more effective methods for prevention, diagnosis, and treatment. Clin. Chest Med. 2020, 41, 25–38. [Google Scholar] [CrossRef]
- Nardi-Agmon, I.; Peled, N. Exhaled breath analysis for the early detection of lung cancer: Recent developments and future prospects. Lung Cancer 2017, 8, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.D. How can nanotechnology be applied to sensors for breath analysis? Nanomedicine 2017, 12, 2695–2697. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Kohne, C.-H.; Lang, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor kras and braf mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef] [Green Version]
- Altelaar, A.F.M.; Munoz, J.; Heck, A.J.R. Next-Generation proteomics: Towards an integrative view of proteome dynamics. Nat. Rev. Genet. 2013, 14, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Capuano, R.; Catini, A.; Paolesse, R.; Di Natale, C. Sensors for lung cancer diagnosis. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Gharra, A.; Ivgi, H.; Khatib, S.; Badarneh, S.; Har-Shai, L.; et al. Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. ACS Nano 2017, 11, 112–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; Su, H.; Lin, G.; Li, S.; Yu, X.; Qin, A.; Zhao, Z.; Zhang, Z.; Tang, B.Z. Targeted imaging of EGFR overexpressed cancer cells by brightly fluorescent nanoparticles conjugated with cetuximab. Nanoscale 2016, 8, 15027–15032. [Google Scholar] [CrossRef]
- Lee, L.J.; Yang, Z.; Rahman, M.; Ma, J.; Kwak, K.J.; McElroy, J.; Shilo, K.; Goparaju, C.; Yu, L.; Rom, W.; et al. Extracellular mRNA detected by tethered lipoplex nanoparticle biochip for lung adenocarcinoma detection. Am. J. Respir. Crit. Care. Med. 2016, 193, 1431–1433. [Google Scholar] [CrossRef] [Green Version]
- Tartarone, A.; Lerose, R.; Aieta, M. Focus on lung cancer screening. J. Thorac. Dis. 2020, 12, 3815–3820. [Google Scholar] [CrossRef]
- Saadat, M.; Manshadi, M.K.D.; Mohammadi, M.; Zare, M.J.; Zarei, M.; Kamali, R.; Sanati-Nezhad, A. Magnetic particle targeting for diagnosis and therapy of lung cancers. J. Control. Release 2020, 328, 776–791. [Google Scholar] [CrossRef]
- Chen, K.N. The diagnosis and treatment of lung cancer presented as ground-glass nodule. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 697–702. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Farokhi, M.; Fatahi, Y.; Atyabi, F.; Dinarvand, R. New insights into designing hybrid nanoparticles for lung cancer: Diagnosis and treatment. J. Control. Release 2019, 295, 250–267. [Google Scholar] [CrossRef]
- Cryer, A.M.; Thorley, A.J. Nanotechnology in the diagnosis and treatment of lung cancer. Pharmacol. Ther. 2019, 198, 189–205. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chen, Y.D.; Guo, W.; Gao, Y.; Song, C.Q.; Zhang, Q.; Zheng, N.N.; Han, X.J.; Guo, C.S. Bismuth ferrite-based nanoplatform design: An ablation mechanism study of solid tumor and nir-triggered photothermal/photodynamic combination cancer therapy. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Cui, X.Y.; Cheng, W.L.; Xu, W.L.; Mu, W.; Han, X.J. Functional graphene derivatives for chemotherapy-based synergistic tumor therapy. NANO 2019, 14. [Google Scholar] [CrossRef]
- Cui, X.Y.; Cheng, W.L.; Han, X.J. Lipid bilayer modified gold nanorod@mesoporous silica nanoparticles for controlled drug delivery triggered by near-infrared light. J. Mater. Chem. 2018, 6, 8078–8084. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.Y.; Cheng, W.L.; Dong, M.D.; Han, X.J. A multifunctional biomimetic hybrid nanocarrier for the controlled delivery of chemotherapy drugs by near-infrared light. New J. Chem. 2019, 43, 2752–2757. [Google Scholar] [CrossRef]
- Yang, C.Y.; Guo, C.S.; Guo, W.; Zhao, X.L.; Liu, S.Q.; Han, X.J. Multifunctional bismuth nanoparticles as theranostic agent for PA/CT imaging and NIR laser-driven photothermal therapy. Acs. Appl. Nano Mater. 2018, 1, 820–830. [Google Scholar] [CrossRef]
- Yang, C.Y.; Huang, W.C.; Gao, Y.; Liu, Z.; An, N.; Mu, W.; Pan, Q.M.; Yang, B.; Guo, C.S.; Han, X.J. Phototherapy ablation of rabbit orthotopic tumors by non-stoichiometric BiPO4-x nanoparticles. Chem. Eng. J. 2020, 386. [Google Scholar] [CrossRef]
- Yang, C.Y.; Yu, H.H.; Gao, Y.; Guo, W.; Li, Z.Z.; Chen, Y.D.; Pan, Q.M.; Ren, M.; Han, X.; Guo, C.S. Surface-Engineered vanadium nitride nanosheets for an imaging-guided photothermal/photodynamic platform of cancer treatment. Nanoscale 2019, 11, 1968–1977. [Google Scholar] [CrossRef]
- Gu, L.; Deng, Z.J.; Roy, S.; Hammond, P.T. A combination RNAi-chemotherapy layer-by-layer nanoparticle for systemic targeting of KRAS/P53 with cisplatin to treat non-small cell lung cancer. Clin. Cancer Res. 2017, 23, 7312–7323. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Kuang, J.; Li, C.-X.; Zhang, M.; Zheng, D.; Zeng, X.; Liu, C.; Zhang, X.-Z. Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano 2018, 12, 1978–1989. [Google Scholar] [CrossRef]
- Van Rijt, S.H.; Boeluekbas, D.A.; Argyo, C.; Datz, S.; Lindner, M.; Eickelberg, O.; Koenigshoff, M.; Bein, T.; Meiners, S. Protease-Mediated release of chemotherapeutics from mesoporous silica nanoparticles to ex vivo human and mouse lung tumors. ACS Nano 2015, 9, 2377–2389. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, Y.; Ruan, W.; Liu, L.; Liu, M.; Chen, S.; Zhou, X. PH-Responsive theranostic nanocomposites as synergistically enhancing positive and negative magnetic resonance imaging contrast agents. J. Nanobiotechnol. 2018, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, L.; Guo, X.; Liu, T.; Xu, X.; Jiang, J.; Wang, F.; Cheng, Z.; Zhu, H.; Yang, Z. Multimodality imaging of naturally active melanin nanoparticles targeting somatostatin receptor subtype 2 in human small-cell lung cancer. Nanoscale 2019, 11, 14400–14409. [Google Scholar] [CrossRef] [PubMed]
- Mir, T.A.; Yoon, J.-H.; Gurudatt, N.G.; Won, M.-S.; Shim, Y.-B. Ultrasensitive cytosensing based on an aptamer modified nanobiosensor with a bioconjugate: Detection of human non-small-cell lung cancer cells. Biosens. Bioelectron. 2015, 74, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Cai, P.; Yang, W.; Xue, J.; Gao, L.; Liu, R.; Wang, Y.; Zhao, Y.; He, X.; Zhao, L.; et al. Ultrasmall Cu-64 Cu nanoclusters for targeting orthotopic lung tumors using accurate positron emission tomography imaging. ACS Nano 2015, 9, 4976–4986. [Google Scholar] [CrossRef]
- Di Domenico, M.; Pozzi, D.; Palchetti, S.; Digiacomo, L.; Iorio, R.; Astarita, C.; Fiorelli, A.; Pierdiluca, M.; Santini, M.; Barbarino, M.; et al. Nanoparticle-Biomolecular corona: A new approach for the early detection of non-small-cell lung cancer. J. Cell Physiol. 2019, 234, 9378–9386. [Google Scholar] [CrossRef]
- Card, J.W.; Zeldin, D.C.; Bonner, J.C.; Nestmann, E.R. Pulmonary applications and toxicity of engineered nanoparticles. Am. J. Physiol. Lung. 2008, 295, L400–L411. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of nanotechnology in cancer diagnosis and therapy—A mini-review. Int. J. Med Sci. 2020, 17, 2964–2973. [Google Scholar] [CrossRef]
- Barabadi, H.; Vahidi, H.; Kamali, K.D.; Hosseini, O.; Mahjoub, M.A.; Rashedi, M.; Shoushtari, F.J.; Saravanan, M. Emerging theranostic gold nanomaterials to combat lung cancer: A systematic review. J. Clust. Sci. 2019, 31, 323–330. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Hussain, S. Nanomedicine for Treatment of Lung Cancer. Adv. Exp. Med. Biol. 2016, 890, 137–147. [Google Scholar] [CrossRef]
- Madni, A.; Batool, A.; Noreen, S.; Maqbool, I.; Rehman, F.; Kashif, P.M.; Tahir, N.; Raza, A. Novel nanoparticulate systems for lung cancer therapy: An updated review. J. Drug Target 2017, 25, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Sim, A.J.; Kaza, E.; Singer, L.; Rosenberg, S.A. A review of the role of MRI in diagnosis and treatment of early stage lung cancer. Clin. Transl. Radiat. Oncol. 2020, 24, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wild, J.M.; Marshall, H.; Bock, M.; Schad, L.R.; Jakob, P.M.; Puderbach, M.; Molinari, F.; Van Beek, E.J.R.; Biederer, J. MRI of the lung (1/3): Methods. Insights Imaging 2012, 3, 345–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, A.; Dufort, S.; Lux, F.; Fortin, P.-Y.; Tassali, N.; Tillement, O.; Coll, J.-L.; Cremillieux, Y. Targeting and in vivo imaging of non-small-cell lung cancer using nebulized multimodal contrast agents. Proc. Natl. Acad. Sci. USA 2014, 111, 9247–9252. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lin, H.; Ma, L.; Jin, J.; Shen, T.; Wei, R.; Wang, X.; Ai, H.; Chen, Z.; Gao, J. Albumin-Based nanoparticles loaded with hydrophobic gadolinium chelates as T-1-T-2 dual-mode contrast agents for accurate liver tumor imaging. Nanoscale 2017, 9, 4516–4523. [Google Scholar] [CrossRef]
- Li, F.; Zhi, D.; Luo, Y.; Zhang, J.; Nan, X.; Zhang, Y.; Zhou, W.; Qiu, B.; Wen, L.; Liang, G. Core/Shell Fe3O4/Gd2O3 nanocubes as T-1-T-2 dual modal MRI contrast agents. Nanoscale 2016, 8, 12826–12833. [Google Scholar] [CrossRef]
- Guldris, N.; Argibay, B.; Kolen’ko, Y.V.; Carbo-Argibay, E.; Sobrino, T.; Campos, F.; Salonen, L.M.; Banobre-Lopez, M.; Castillo, J.; Rivas, J. Influence of of the separation procedure on the properties of magnetic nanoparticles: Gaining in vitro stability and T-1-T-2 magnetic resonance imaging performance. J. Colloid Interface Sci. 2016, 472, 229–236. [Google Scholar] [CrossRef]
- Hermann, P.; Kotek, J.; Kubicek, V.; Lukes, I. Gadolinium(III) complexes as MRI contrast agents: Ligand design and properties of the complexes. Dalton Trans. 2008, 23, 3027–3047. [Google Scholar] [CrossRef]
- Yoo, D.; Lee, J.-H.; Shin, T.-H.; Cheon, J. Theranostic magnetic nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef]
- Gao, W.; Wang, W.; Yao, S.; Wu, S.; Zhang, H.; Zhang, J.; Jing, F.; Mao, H.; Jin, Q.; Cong, H.; et al. Highly sensitive detection of multiple tumor markers for lung cancer using gold nanoparticle probes and microarrays. Anal. Chim. Acta 2017, 958, 77–84. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Zhang, J.; Liu, Y.; Wu, L.; Shen, J.; Zhang, Y.; Hu, Y.; Fan, Q.; Huang, W.; et al. Individual au-nanocube based plasmonic nanoprobe for cancer relevant MicroRNA biomarker detection. ACS Sensors 2017, 2, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zaki, A.A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teramoto, A.; Yamada, A.; Tsukamoto, T.; Imaizumi, K.; Toyama, H.; Saito, K.; Fujita, H. Decision support system for lung cancer using PET/CT and microscopic images. Adv. Exp. Med. Biol. 2020, 1213, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Yang, T.-H.O.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The immune landscape of cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [Green Version]
- Sanhai, W.R.; Sakamoto, J.H.; Canady, R.; Ferrari, M. Seven challenges for nanomedicine. Nat. Nanotechnol. 2008, 3, 242–244. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Li, R.; Ng, T.S.C.; Courties, G.; Rodell, C.B.; Prytyskach, M.; Kohler, R.H.; Pittet, M.J.; Nahrendorf, M.; Weissleder, R.; et al. Quantitative imaging of tumor-associated macrophages and their response to therapy using cu-64-labeled macrin. ACS Nano 2018, 12, 12015–12029. [Google Scholar] [CrossRef]
- Dufort, S.; Bianchi, A.; Henry, M.; Lux, F.; Duc, G.L.; Josserand, V.; Louis, C.; Perriat, P.; Cremillieux, Y.; Tillement, O.; et al. Nebulized gadolinium-based nanoparticles: A theranostic approach for lung tumor imaging and radiosensitization. Small 2015, 11, 215–221. [Google Scholar] [CrossRef]
- Li, Z.; Tan, S.; Li, S.; Shen, Q.; Wang, K. Cancer drug delivery in the nano era: An overview and perspectives (Review). Oncol. Rep. 2017, 38, 611–624. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Wang, R.; Yin, L.; Shang, X.; Zhang, R.; Zhang, P. CYFRA21-1 tests in the diagnosis of non-small cell lung cancer: A meta-analysis. Int. J. Biol. Markers 2019, 34, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Roointan, A.; Ahmad Mir, T.; Ibrahim Wani, S.; Mati Ur, R.; Hussain, K.K.; Ahmed, B.; Abrahim, S.; Savardashtaki, A.; Gandomani, G.; Gandomani, M.; et al. Early detection of lung cancer biomarkers through biosensor technology: A review. J. Pharm. Biomed. Anal. 2019, 164, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, S.; Wu, T.; Ni, D.; Fan, W.; Zhu, Y.; Qian, R.; Shi, J. Fe-Au nanoparticle-coupling for ultrasensitive detections of circulating tumor DNA. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Djureinovic, D.; Hallstrom, B.M.; Horie, M.; Mattsson, J.S.M.; Fleur, L.L.; Fagerberg, L.; Brunnstrom, H.; Lindskog, C.; Madjar, K.; Rahnenfuehrer, J.; et al. Profiling cancer testis antigens in non-small-cell lung cancer. JCI Insight. 2016, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.F.; Wang, M.; Xu, J.L. Thymidine kinase 1 combined with CEA, CYFRA21-1 and NSE improved its diagnostic value for lung cancer. Life Sci. 2018, 194, 1–6. [Google Scholar] [CrossRef]
- Millares, L.; Barreiro, E.; Cortese, R.; Martinez-Romero, A.; Balcells, C.; Cascante, M.; Enguita, A.B.; Alvarez, C.; Rami-Porta, R.; Sanchez de Cos, J.; et al. Tumor-Associated metabolic and inflammatory responses in early stage non-small cell lung cancer: Local patterns and prognostic significance. Lung Cancer 2018, 122, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Maharjan, N.; Thapa, N.; Tu, J. Blood-Based biomarkers for early diagnosis of lung cancer: A review article. JNMA J. Nepal. Med. Assoc. 2020, 58, 519–524. [Google Scholar] [CrossRef]
- Kalinke, L.; Thakrar, R.; Janes, S.M. The promises and challenges of early non-small cell lung cancer detection: Patient perceptions, low-dose CT screening, bronchoscopy and biomarkers. Mol. Oncol. 2020. [Google Scholar] [CrossRef]
- Saranya, G.; Joseph, M.M.; Karunakaran, V.; Nair, J.B.; Saritha, V.N.; Veena, V.S.; Sujathan, K.; Ajayaghosh, A.; Maiti, K.K. Enzyme-Driven switchable fluorescence-SERS diagnostic nanococktail for the multiplex detection of lung cancer biomarkers. ACS Appl. Mater. Interfaces 2018, 10, 38807–38818. [Google Scholar] [CrossRef]
- Qiao, X.; Su, B.; Liu, C.; Song, Q.; Luo, D.; Mo, G.; Wang, T. Selective surface enhanced raman scattering for quantitative detection of lung cancer biomarkers in superparticle@MOF structure. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Liu, L.; Wu, S.; Jing, F.; Zhou, H.; Jia, C.; Li, G.; Cong, H.; Jin, Q.; Zhao, J. Bead-Based microarray immunoassay for lung cancer biomarkers using quantum dots as labels. Biosens. Bioelectron. 2016, 80, 300–306. [Google Scholar] [CrossRef]
- Meng, X.; Chen, X.; Wu, W.; Zheng, W.; Deng, H.; Xu, L.; Chen, W.; Li, Z.; Peng, H. Electrochemiluminescent immunoassay for the lung cancer biomarker CYFRA21-1 using MoOx quantum dots. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef] [PubMed]
- Rakovich, T.Y.; Mahfoud, O.K.; Mohamed, B.M.; Prina-Mello, A.; Crosbie-Staunton, K.; Van den Broeck, T.; De Kimpe, L.; Sukhanova, A.; Baty, D.; Rakovich, A.; et al. Highly sensitive single domain antibody-quantum dot conjugates for detection of HER2 biomarker in lung and breast cancer cells. ACS Nano 2014, 8, 5682–5695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ke, H.; Wang, Y.; Li, P.; Huang, C.; Jia, N. 3D carbon nanosphereand gold nanoparticle-based voltammetric cytosensor for cell line A549 and forearly diagnosis of non-small cell lung cancer cells. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef]
- Yang, T.; Guo, X.; Wu, Y.; Wang, H.; Fu, S.; Wen, Y.; Yang, H. Facile and label-free detection of lung cancer biomarker in urine by magnetically assisted surface-enhanced raman scattering. ACS Appl. Mater. Interfaces 2014, 6, 20985–20993. [Google Scholar] [CrossRef] [PubMed]
- Bouillez, A.; Rajabi, H.; Jin, C.; Samur, M.; Tagde, A.; Alam, M.; Hiraki, M.; Maeda, T.; Hu, X.; Adeegbe, D.; et al. MUC1-C integrates PD-L1 induction with repression of immune effectors in non-small-cell lung cancer. Oncogene 2017, 36, 4037–4046. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Lai, Y.; Li, J.; Qin, L.; Xu, Y.; Zhao, R.; Li, B.; Lin, S.; Wang, S.; Wu, Q.; et al. PSCA and MUC1 in non-small-cell lung cancer as targets of chimeric antigen receptor T cells. Oncoimmunology 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Qiao, L.-L.; Yao, W.-J.; Zhang, Z.-Q.; Yang, X.; Zhao, M.-X. The biological activity research of the nano-drugs based on 5-fluorouracil-modified quantum dots. Int. J. Nanomed. 2020, 15, 2765–2776. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.-X.; Zeng, E.-Z.; Zhu, B.-J. The biological applications of inorganic nanoparticle drug carriers. Chemnanomat 2015, 1, 82–91. [Google Scholar] [CrossRef]
- Ren, D.; Wang, B.; Hu, C.; You, Z. Quantum dot probes for cellular analysis. Anal. Methods 2017, 9, 2621–2632. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Jian, M.; Deng, H.; Wang, W.; Huang, Z.; Huang, K.; Liu, A.; Chen, W. Valence states effect on electrogenerated chemiluminescence of gold nanocluster. ACS Appl. Mater. Interfaces 2017, 9, 14929–14934. [Google Scholar] [CrossRef]
- Even-Desrumeaux, K.; Nevoltris, D.; Lavaut, M.N.; Alim, K.; Borg, J.-P.; Audebert, S.; Kerfelec, B.; Baty, D.; Chames, P. Masked selection: A straightforward and flexible approach for the selection of binders against specific epitopes and differentially expressed proteins by phage display. Mol. Cell. Proteomics 2014, 13, 653–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Tang, Z.; Hu, Z.; Wang, Y.; Yang, X.; Mo, F.; Lu, X. Natural single-domain antibody-nanobody: A novel concept in the antibody field. J. Biomed. Nanotechnol. 2018, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.D.; Shandilya, R.; Bhargava, A.; Kumar, R.; Tiwari, R.; Chaudhury, K.; Srivastava, R.K.; Goryacheva, I.Y.; Mishra, P.K. Quantum dot based nano-biosensors for detection of circulating cell free miRNAs in lung carcinogenesis: From biology to clinical translation. Front Genet. 2018, 9, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Torre, P.; Perez-Lorenzo, M.J.; Alcazar-Garrido, A.; Flores, A.I. Cell-Based nanoparticles delivery systems for targeted cancer therapy: Lessons from anti-angiogenesis treatments. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Li, Z.; Guo, K.; Shao, T. Hierarchically ordered mesoporous carbon/graphene composites as supercapacitor electrode materials. Nanoscale 2016, 8, 15671–15680. [Google Scholar] [CrossRef] [PubMed]
- Shoja, Y.; Kermanpur, A.; Karimzadeh, F. Diagnosis of EGFR exon21 L858R point mutation as lung cancer biomarker by electrochemical DNA biosensor based on reduced graphene oxide/functionalized ordered mesoporous carbon/Ni-oxytetracycline metallopolymer nanoparticles modified pencil graphite electrode. Biosens. Bioelectron. 2018, 113, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Aasi, A.; Aghaei, S.M.; Panchapakesan, B. A density functional theory study on the interaction of toluene with transition metal decorated carbon nanotubes: A promising platform for early detection of lung cancer from human breath. Nanotechnology 2020, 31, 415707. [Google Scholar] [CrossRef]
- Wang, X.J.; Tian, L.F.; Du, H.; Li, M.; Mu, W.; Drinkwater, B.W.; Han, X.J.; Mann, S. Chemical communication in spatially organized protocell colonies and protocell/living cell micro-arrays. Chem. Sci. 2019, 10, 9446–9453. [Google Scholar] [CrossRef]
- Wang, X.J.; Tian, L.F.; Ren, Y.S.; Zhao, Z.Y.; Du, H.; Zhang, Z.Z.; Drinkwater, B.W.; Mann, S.; Han, X.J. Chemical information exchange in organized protocells and natural cell assemblies with controllable spatial positions. Small 2020, 16. [Google Scholar] [CrossRef]
- Zong, W.; Ma, S.H.; Zhang, X.N.; Wang, X.J.; Li, Q.C.; Han, X.J. A fissionable artificial eukaryote-like cell model. J. Am. Chem. Soc. 2017, 139, 9955–9960. [Google Scholar] [CrossRef]
- Li, Q.C.; Li, S.B.; Zhang, X.X.; Xu, W.L.; Han, X.J. Programmed magnetic manipulation of vesicles into spatially coded prototissue architectures arrays. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.C.; Han, X.J. Self-Assembled "breathing" grana-like cisternae stacks. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.C.; Han, X.J. Self-Assembled rough endoplasmic reticulum-like proto-organelles. Iscience 2018, 8, 138–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.J.; Du, H.; Wang, Z.; Mu, W.; Han, X.J. Versatile phospholipid assemblies for functional synthetic cells and artificial tissues. Adv. Mater. 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, M.; Liu, J.; Yang, Y.; Yu, Y.; Li, J.; Pan, W.; Fan, L.; Li, G.; Li, X.; et al. Inhibition of tumor metastasis by targeted daunorubicin and dioscin codelivery liposomes modified with PFV for the treatment of non-small- cell lung cancer. Int. J. Nanomed. 2019, 14, 4071–4090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adjei, I.M.; Sharma, B.; Labhasetwar, V. Nanoparticles: Cellular uptake and cytotoxicity. Adv. Exp. Med. Biol. 2014, 811, 73–91. [Google Scholar] [CrossRef]
- Matczuk, M.; Ruzik, L.; Aleksenko, S.S.; Keppler, B.K.; Jarosz, M.; Timerbaev, A.R. Analytical methodology for studying cellular uptake, processing and localization of gold nanoparticles. Anal. Chim. Acta 2019, 1052, 1–9. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Derk, R.; Davidson, D.C.; Manke, A.; Stueckle, T.A.; Rojanasakul, Y.; Wang, L. Potential in vitro model for testing the effect of exposure to nanoparticles on the lung alveolar epithelial barrier. Sens. Biosensing. Res. 2015, 3, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-Dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [Green Version]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Cui, H.; Ying, L.; Yu, X.F. Enhanced cytosolic delivery and release of CRISPR/Cas9 by black phosphorus nanosheets for genome editing. Angew. Chem. Int. Ed. Engl. 2018, 57, 10268–10272. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.; Lagzi, I.; Teraji, S.; Nakanishi, H.; Cervenak, L.; Zambo, D.; Deak, A.; Molnar, K.; Truszka, M.; Szekacs, I.; et al. Interaction of positively charged gold nanoparticles with cancer cells monitored by an in situ label-free optical biosensor and transmission electron microscopy. ACS Appl. Mater. Interfaces 2018, 10, 26841–26850. [Google Scholar] [CrossRef] [PubMed]
- Saadat, M.; Zahednezhad, F.; Zakeri-Milani, P.; Heidari, H.R.; Shahbazi-Mojarrad, J.; Valizadeh, H. Drug targeting strategies based on charge dependent uptake of nanoparticles into cancer cells. J. Pharm. Pharm. Sci. 2019, 22, 191–220. [Google Scholar] [CrossRef] [Green Version]

| Nanoparticles | Cancer Biomarkers | Limit of Detection (LOD) | Methods | Ref. |
|---|---|---|---|---|
| Gold nanoparticles | EGFR, CK, Nap | --- | Fluorescence and surface enhanced Raman scattering | [68] |
| CEA, CYFRA21-1, NSE, Dkk1 | <1000 pg/mL | Co-detection method based on NPs and microarrays | [50] | |
| MiR-205 | ---- | Localized surface plasmon resonance | [51] | |
| MUC1 | 8 cells/mL | Chronoamperometry | [33] | |
| Aldehydes | 10 ppb | Surface enhanced Raman scattering | [69] | |
| Quantum dots | CEA, CYFRA21-1, NSE | CEA: 190 pg/mL; CYFRA21-1: 970 pg/mL; NSE: 370 pg/mL | Microarray immunoassay (bead bases sandwich assay) | [70] |
| CYFRA21-1 | 0.3 pg/mL | Electrochemiluminescent immunoassay | [71] | |
| HER2 | --- | Western Blot, ELISA, confocal microscopy, flow cytometry | [72] | |
| Carbon nanomaterials | CEA/EGFR | 14 cells/mL | Differential pulse voltammetry | [73] |
| Liposome | TKTL1, TTF1 | --- | TIRF and TLN biochip | [15] |
| Fe0 nanomaterials | ctDNA | 0.1 pg/mL | Inductively coupled plasma mass spectrometry | [62] |
| Fluorescent nanoparticles | EGFR | ---- | Western Blot, confocal microscopy, flow cytometry | [14] |
| Fe3O4/Au/Ag nanocomposites | Adenosine | ---- | Surface enhanced Raman scattering | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Wang, Z.; Wei, F.; Mu, W.; Han, X. Recent Progress of Lung Cancer Diagnosis Using Nanomaterials. Crystals 2021, 11, 24. https://doi.org/10.3390/cryst11010024
Tang X, Wang Z, Wei F, Mu W, Han X. Recent Progress of Lung Cancer Diagnosis Using Nanomaterials. Crystals. 2021; 11(1):24. https://doi.org/10.3390/cryst11010024
Chicago/Turabian StyleTang, Xuefeng, Zhao Wang, Feng Wei, Wei Mu, and Xiaojun Han. 2021. "Recent Progress of Lung Cancer Diagnosis Using Nanomaterials" Crystals 11, no. 1: 24. https://doi.org/10.3390/cryst11010024
APA StyleTang, X., Wang, Z., Wei, F., Mu, W., & Han, X. (2021). Recent Progress of Lung Cancer Diagnosis Using Nanomaterials. Crystals, 11(1), 24. https://doi.org/10.3390/cryst11010024