Spectroscopic Insight into Tetrahedrally Distorted Square Planar Copper(II) Complex: XRD/HSA, Physicochemical, DFT, and Thermal Investigations
Abstract
:1. Introduction
2. Experimental
2.1. Computational
2.2. Materials and Synthesis
2.3. Synthesis of Copper(II) Complex
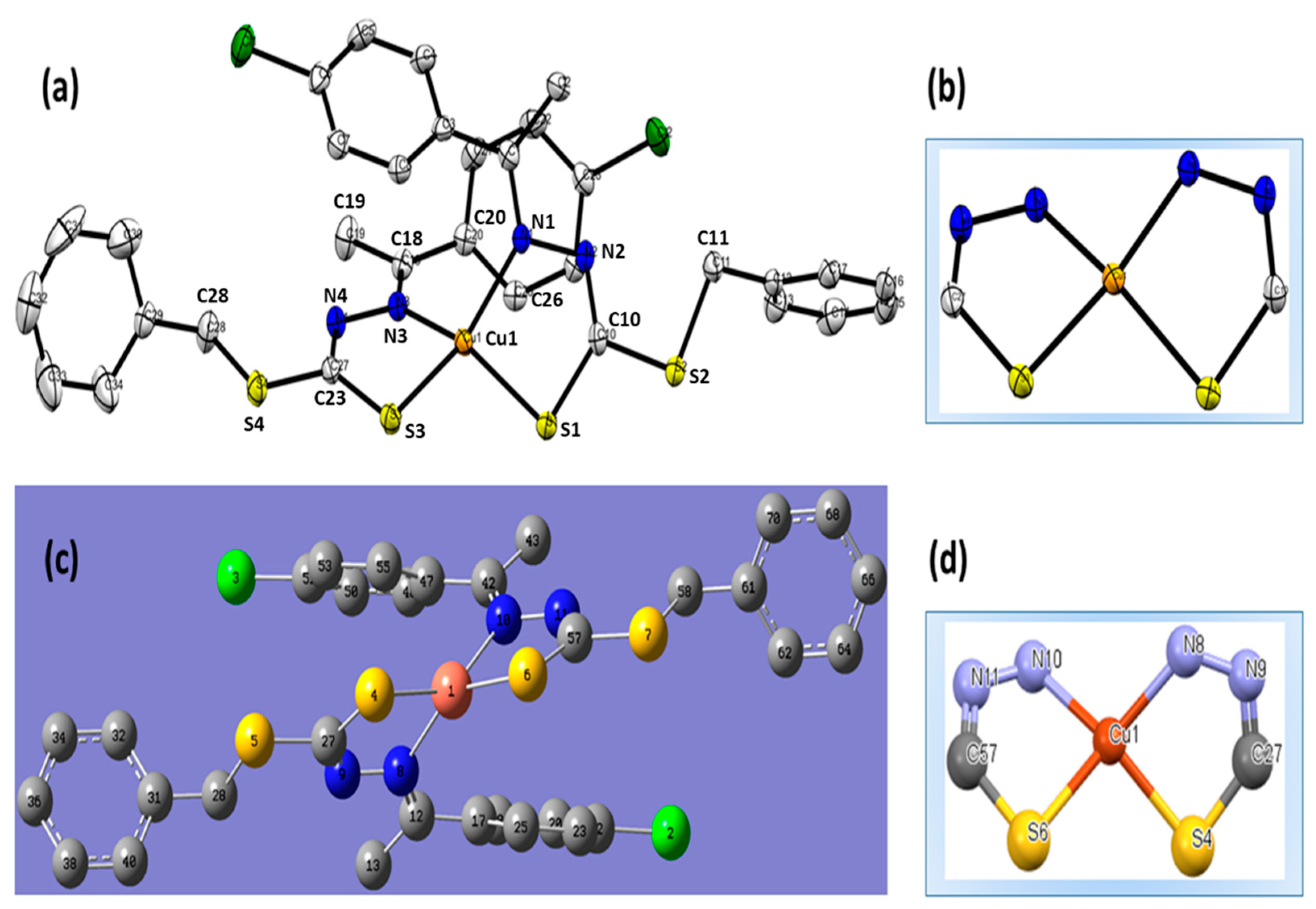
2.4. XRD-Structure
3. Results and Discussion
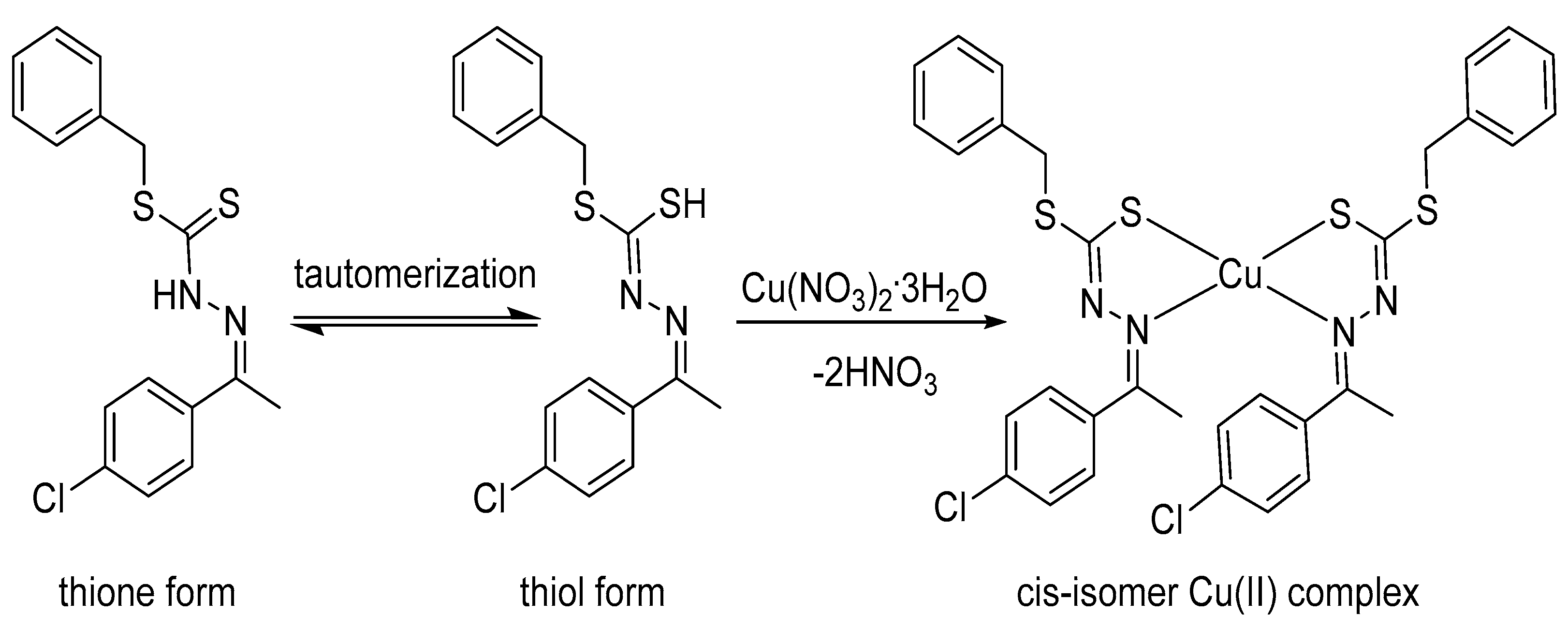
3.1. Synthesis of Copper(II) Complex
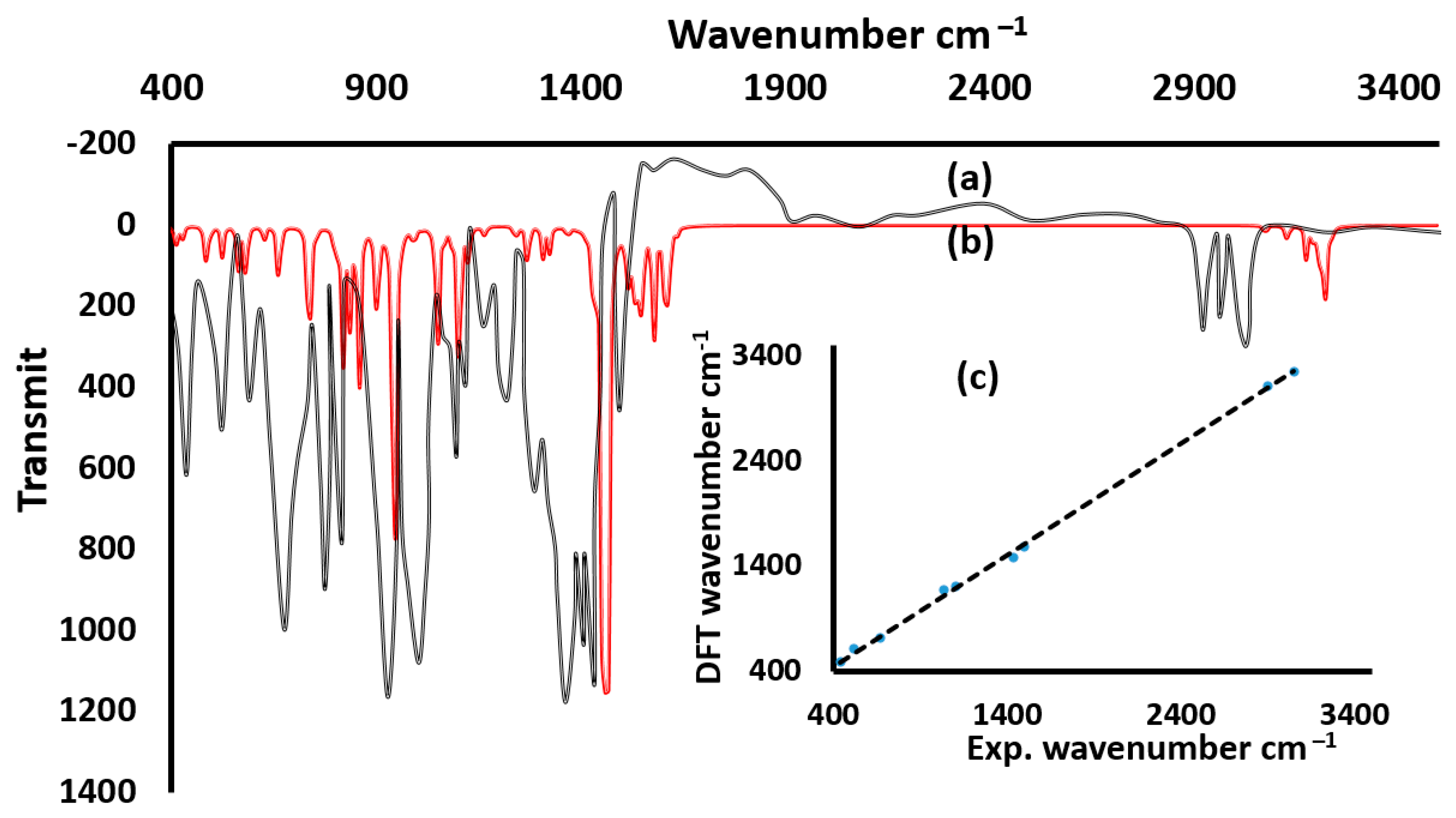
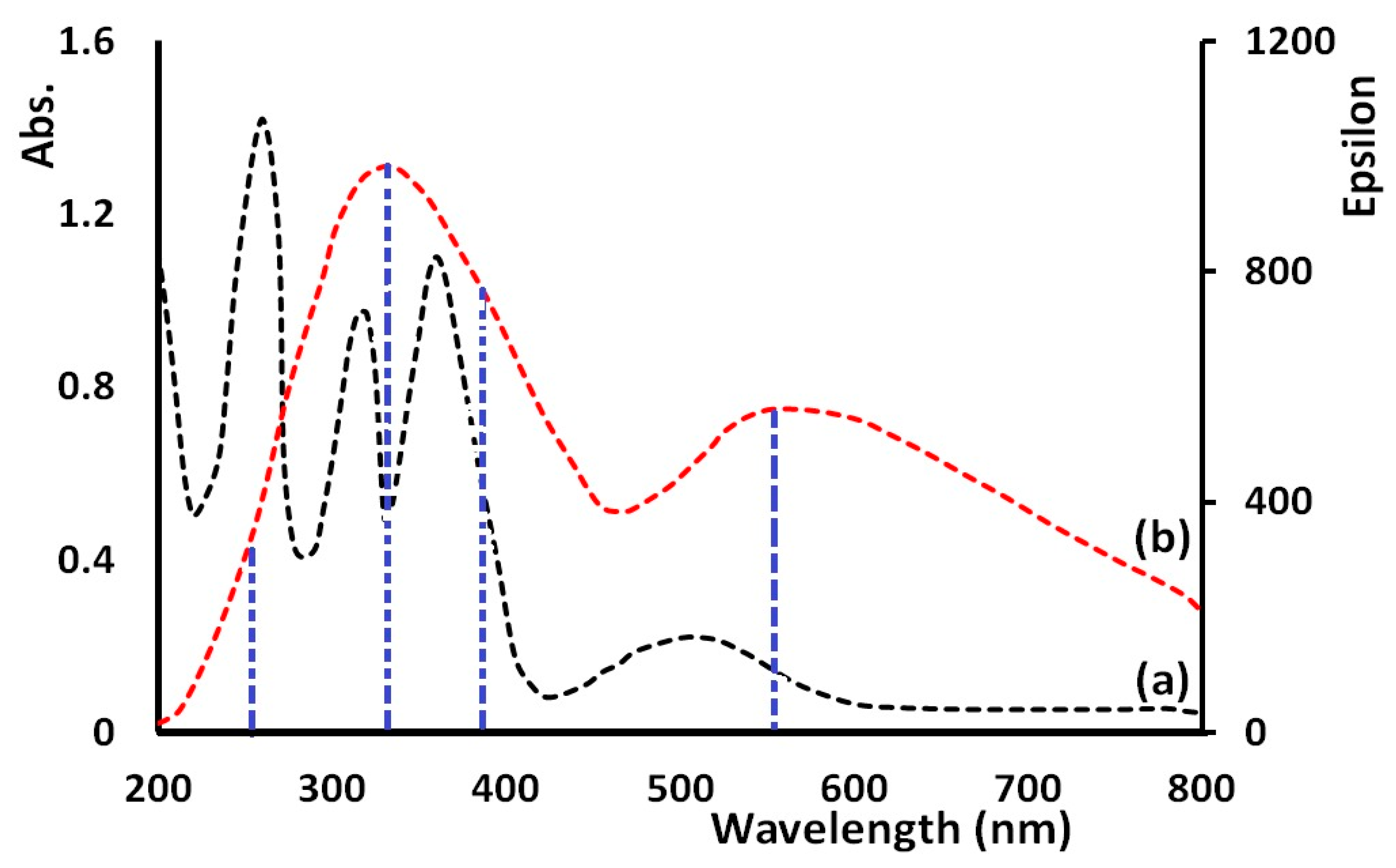
3.2. Infrared and Electronic Absorption Spectra
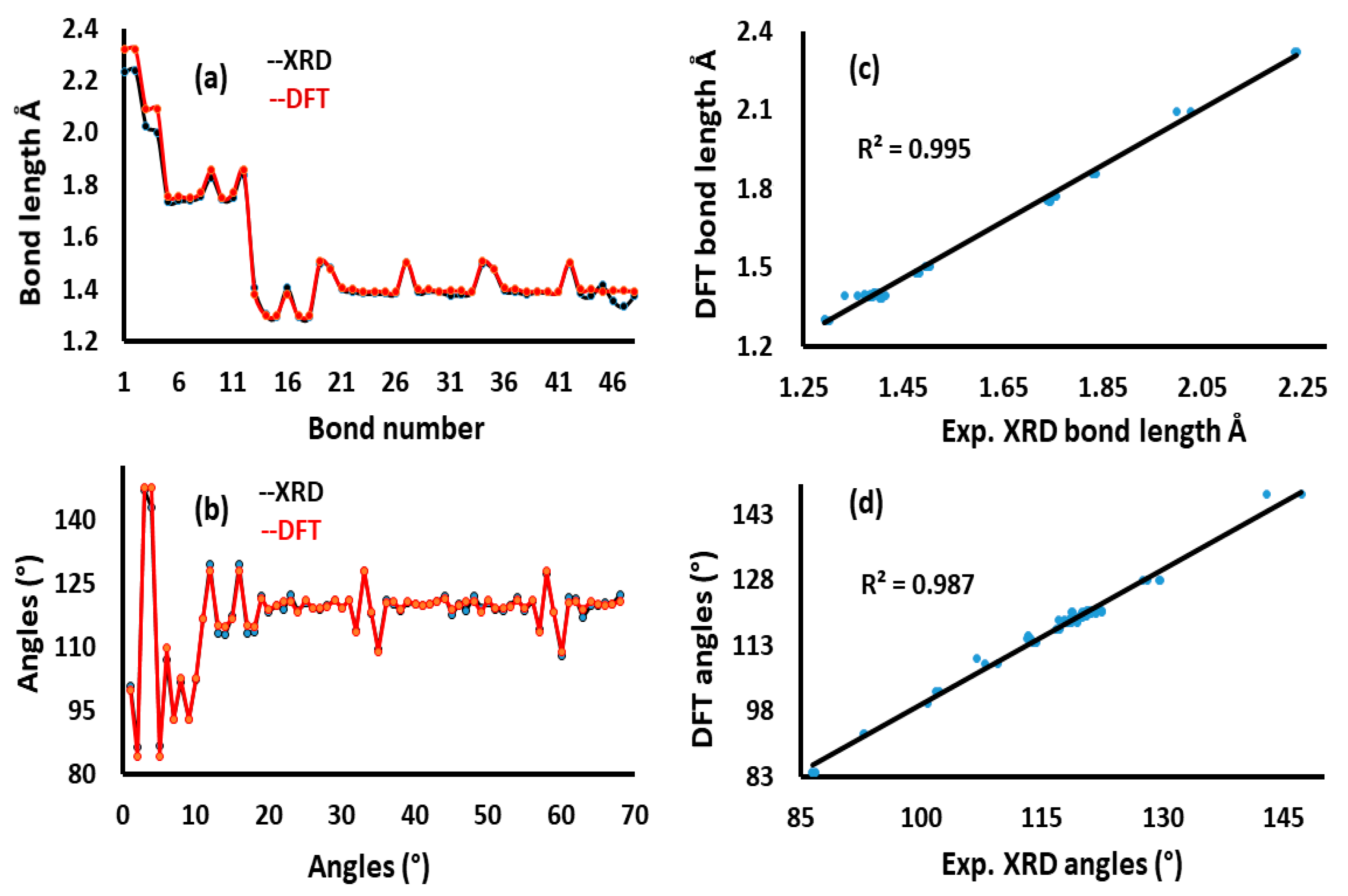
3.3. XRD and DFT
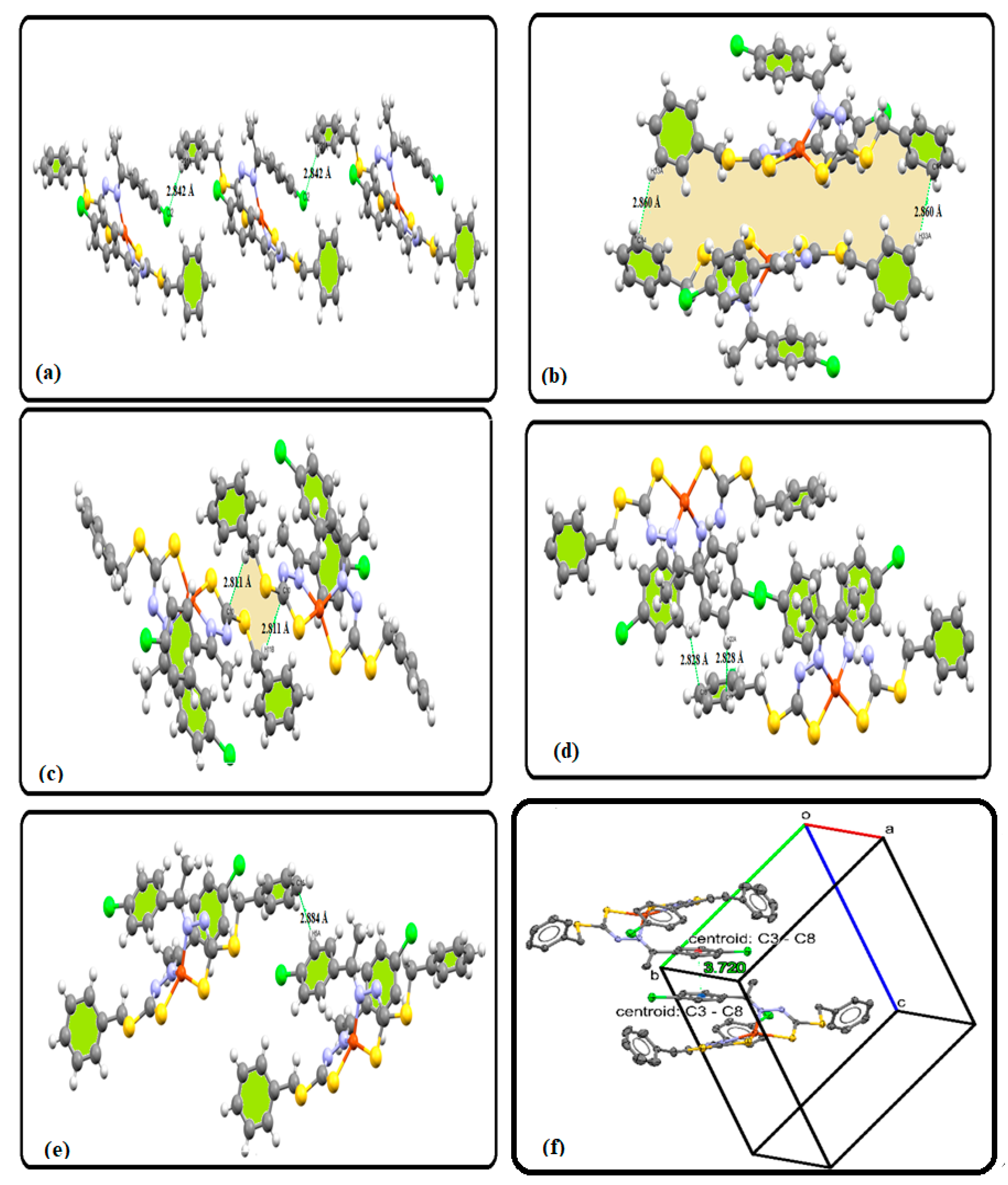
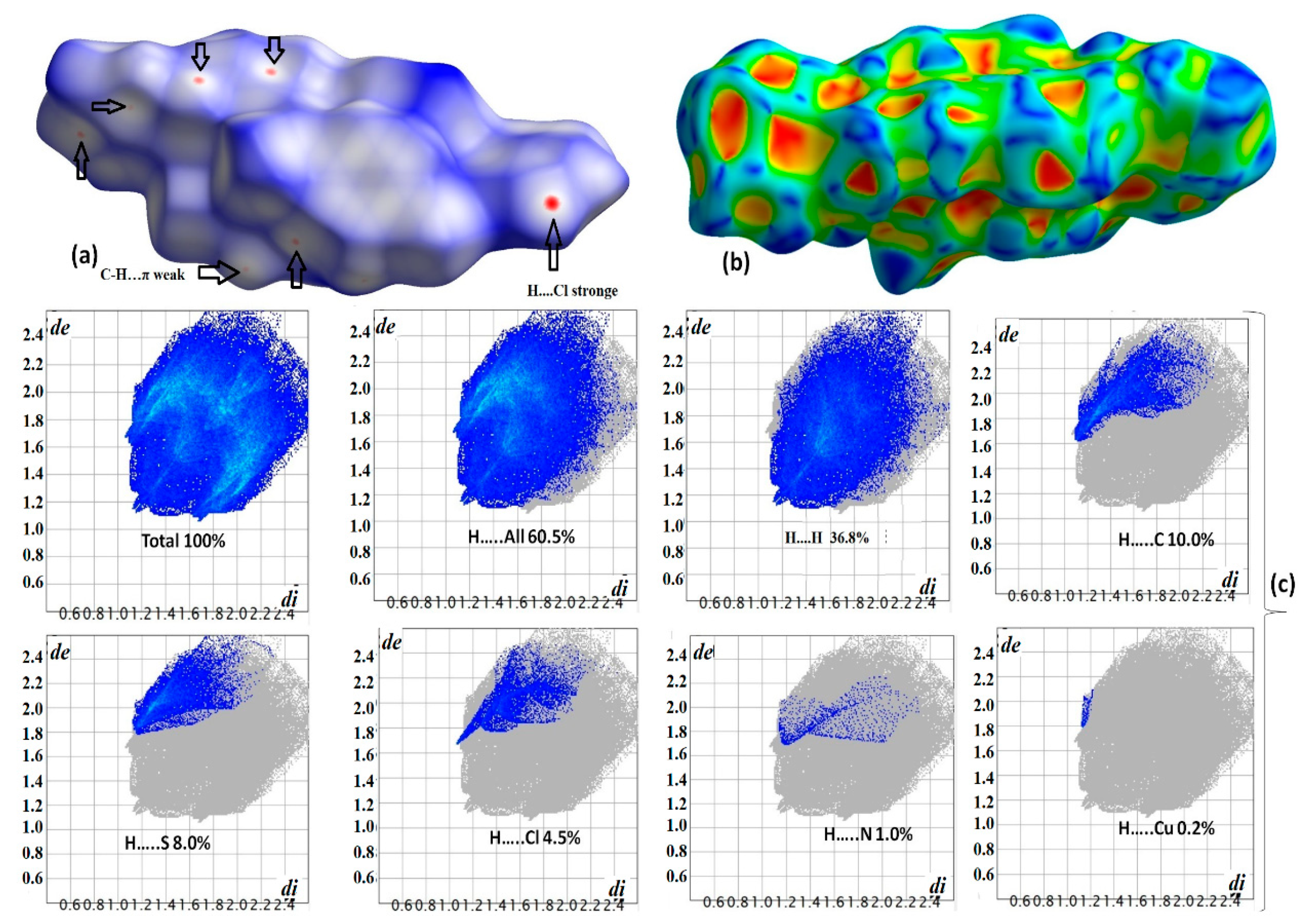
3.4. XRD Packing, HSA, and Molecular Electrostatic Potential (MEP) Investigation
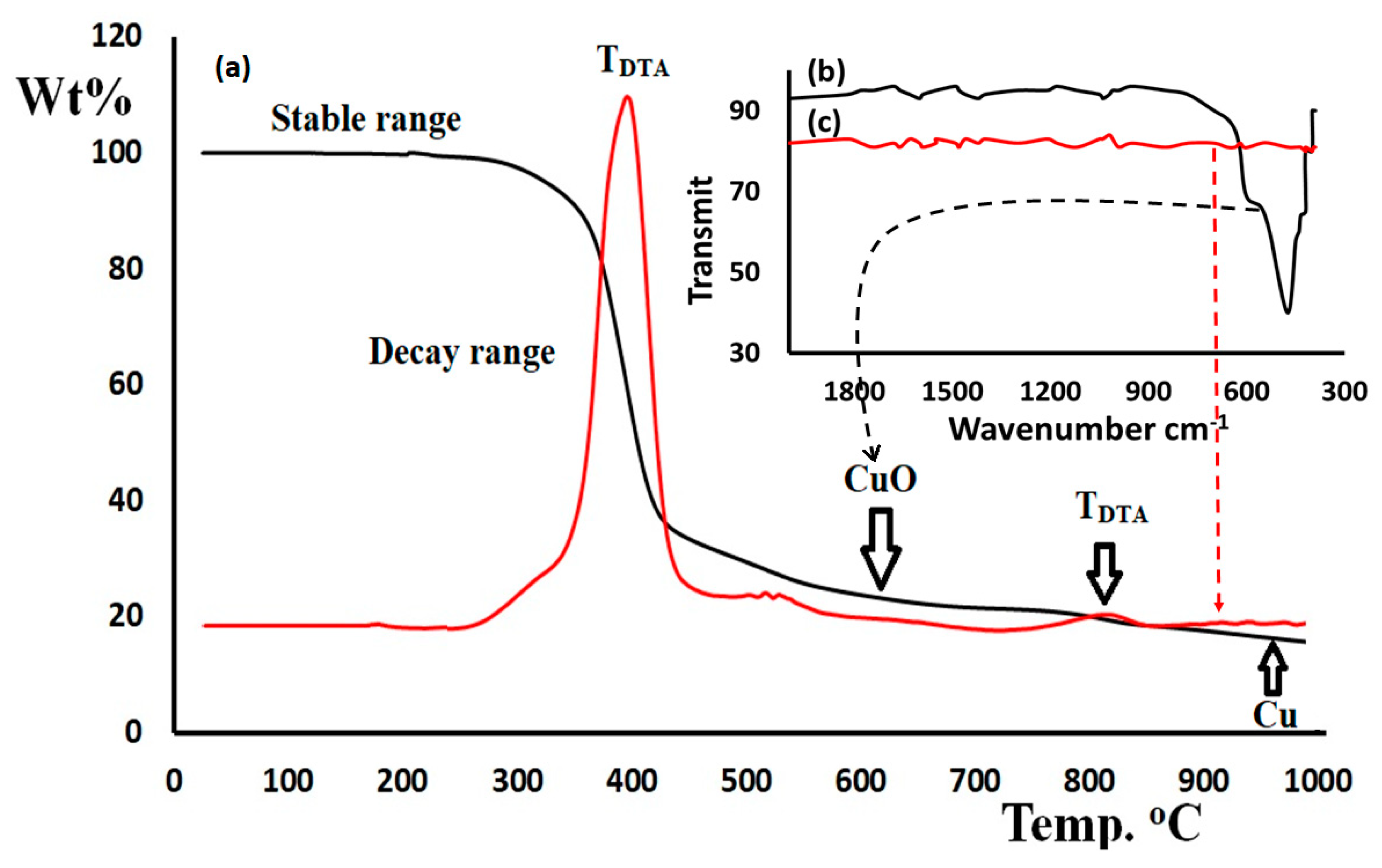
3.5. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Otero, L.; Vieites, M.; Boiani, L.; Denicola, A.; Rigol, C.; Opazo, L.; Olea-Azar, C.; Maya, J.D.; Morello, A.; Krauth-Siegel, R.I.; et al. Novel antitrypanosomal agents based on palladium nitrofurylthiosemicarbazone complexes: DNA and redox metabolism as potential therapeutic targets. J. Med. Chem. 2006, 49, 3322–3331. [Google Scholar] [CrossRef]
- Rosu, T.; Gulea, A.; Nicolae, A.; Georgescu, R. Complexes of 3d(n) metal ions with thiosemicarbazones: Synthesis and antimicrobial activity. Molecules 2007, 12, 782–796. [Google Scholar] [CrossRef]
- Pelosi, G.; Bisceglie, F.; Bignami, F.; Ronzi, P.; Schiavone, P.; Re, M.C.; Casoli, C.; Pilotti, E. Antiretroviral activity of thiosemicarbazone metal complexes. J. Med. Chem. 2010, 53, 8765–8769. [Google Scholar] [CrossRef]
- Li, M.X.; Chen, C.L.; Zhang, D.; Niu, J.Y.; Ji, B.S. Mn(II), Co(II) and Zn(II) complexes with heterocyclic substituted thiosemicarbazones: Synthesis, characterization, X-ray crystal structures and antitumor comparison. Eur. J. Med. Chem. 2010, 45, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, E.; Kalaivani, P.; Prabhakaran, R.; Rath, N.P.; Brinda, S.; Poornima, P.; Padma, V.V.; Natarajan, K. Synthesis, X-ray crystal structure, DNA binding, antioxidant and cytotoxicity studies of Ni(ii) and Pd(ii) thiose micarbazone complexes. Metallomics 2012, 4, 218–227. [Google Scholar]
- Lewis, N.A.; Liu, F.; Seymour, L.; Magnusen, A.; Erves, T.R.; Arca, J.F.; Beckford, F.A.; Venkatraman, R.; Gonzalez-Sarrias, A.; Fronczek, F.R.; et al. Synthesis, characterization, and preliminary in vitro studies of vanadium(IV) complexes with a Schiff base and thiosemicarbazones as mixed-ligands. Eur. J. Inorg. Chem. 2012, 2012, 664–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.S.; Peng, B.; Ma, L.; Cao, S.L.; Bai, L.L.; Yang, C.R.; Wan, C.Q.; Yan, H.J.; Ding, P.P.; Li, Z.F.; et al. Synthesis, crystal structures and antitumor activity of two platinum(II) complexes with methyl hydrazinecarbodithioate derivatives of indolin-2-one. Eur. J. Med. Chem. 2017, 127, 137–146. [Google Scholar] [CrossRef]
- Li, M.X.; Zhang, L.Z.; Chen, C.L.; Niu, J.Y.; Ji, B.S. Synthesis, crystal structures, and biological evaluation of Cu(II) and Zn(II) complexes of 2-benzoylpyridine Schiff bases derived from S-methyl- and S-phenyldithiocarbazates. J. Inorg. Biochem. 2012, 106, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Sasmal, P.K.; Patra, A.K.; Chakravarty, A.R. Synthesis, structure, DNA binding and DNA cleavage activity of oxovanadium(IV) N-salicylidene-S-methyldithiocarbazate complexes of phenanthroline bases. J. Inorg. Biochem. 2008, 102, 1463–1472. [Google Scholar] [CrossRef]
- Maia, P.I.; Fernandes, A.G.; Silva, J.J.; Andricopulo, A.D.; Lemos, S.S.; Lang, E.S.; Abram, U.; Deflon, V.M. Dithiocarbazate complexes with the [M(PPh3)]2+ (M = Pd or Pt) moiety: Synthesis, characterization and anti-Trypanosoma cruzi activity. J. Inorg. Biochem. 2010, 104, 1276–1282. [Google Scholar] [CrossRef]
- Islam, M.A.A.A.; Tarafder, M.T.H.; Chanmiya Sheikh, M.; Ashraful Alam, M.; Zangrando, E. Coordination chemistry of [methyl-3-(4-benzyloxyphenyl)methylene]dithiocarbazate with divalent metal ions: Crystal structures of the N,S Schiff base and of its bis-chelated nickel(II) complex. Transit. Met. Chem. 2011, 36, 531–537. [Google Scholar] [CrossRef]
- Break, M.K.; Tahir, M.I.; Crouse, T.J.; Khoo, T.J. Synthesis, Characterization, and Bioactivity of Schiff Bases and Their Cd(2+), Zn(2+), Cu(2+), and Ni(2+) Complexes Derived from Chloroacetophenone Isomers with S-Benzyldithiocarbazate and the X-Ray Crystal Structure of S-Benzyl- beta -N-(4-chlorophenyl)methylenedithiocarbazate. Bioinorg. Chem. Appl. 2013, 2013, 362513. [Google Scholar] [PubMed] [Green Version]
- Hernandez, W.; Paz, J.; Carrasco, F.; Vaisberg, J.A.; Spodine, E.; Manzur, J.; Hennig, L.; Sieler, J.; Blaurock, S.; Beyer, L. Synthesis and Characterization of New Palladium(II) Thiosemicarbazone Complexes and Their Cytotoxic Activity against Various Human Tumor Cell Lines. Bioinorg. Chem. Appl. 2013, 2013, 524701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejidike, I.P.; Ajibade, P.A. Transition metal complexes of symmetrical and asymmetrical Schiff bases as antibacterial, antifungal, antioxidant, and anticancer agents: Progress and prospects. Rev. Inorg. Chem. 2015, 35, 191–224. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.; Takjoo, R.; Frank, W.; Aghaei Kaju, A. The preparation, spectroscopic characterization and X-ray crystal structures of the pyrrole-2-carboxaldehyde Schiff base of S-allyldithiocarbazate (HL) and its nickel(II) complex ([Ni(L)2]). J. Coord. Chem. 2009, 62, 3651–3660. [Google Scholar] [CrossRef]
- Bakherad, M.; Keivanloo, A.; Samangooei, S. Solvent-free Heck and copper-free Sonogashira cross-coupling reactions catalyzed by a polystyrene-anchored Pd(II) phenyldithiocarbazate complex. Tetrahedron Lett. 2012, 53, 5773–5776. [Google Scholar] [CrossRef]
- Basha, M.T.; Chartres, J.D.; Pantarat, N.; Ali, M.A.; Mirza, A.H.; Kalinowski, D.S.; Richardson, D.R.; Bernhardt, P.V. Heterocyclic dithiocarbazate iron chelators: Fe coordination chemistry and biological activity. Dalton Trans. 2012, 41, 6536–6548. [Google Scholar] [CrossRef] [Green Version]
- Takjoo, R.; Centore, R.; Hayatolgheibi, S.S. Mixed ligand complexes of cadmium(II) and copper(II) dithiocarbazate: Synthesis, spectral characterization, X-ray crystal structure. Inorg. Chim. Acta 471 2018, 471, 587–594. [Google Scholar] [CrossRef]
- Mumit, M.A.; Pal, T.K.; Alam, M.A.; Islam, M.A.A.A.; Paul, S.; Sheikh, M.C. DFT studies on vibrational and electronic spectra, HOMO–LUMO, MEP, HOMA, NBO and molecular docking analysis of benzyl-3-N-(2,4,5-trimethoxyphenylmethylene)hydrazinecarbodithioate. J. Mol. Struct. 2020, 1220, 128715. [Google Scholar] [CrossRef]
- Santra, A.; Brandao, P.; Mondal, G.; Bera, P.; Jana, A.; Bhattacharyya, I.; Pramanik, C.; Bera, P. Monomeric and dimeric cadmium(II) complexes of S-alkyl/aryl dithiocarbazate as single-source precursors for cadmium sulfide nanoparticles: An experimental, theoretical interpretation in the stability of precursor and visible light dye degradation study. Inorg. Chim. Acta. 2020, 501, 119315. [Google Scholar] [CrossRef]
- Cox, D.N.; Mingos, D.M.P.; Hoffmann, R. Extended Hückel molecular-orbital calculations on dodecahedral metalloboranes which do not conform to the polyhedral skeletal electron-pair theory. J. Chem. Soc. Dalton Trans. 1981, 1788–1797. [Google Scholar] [CrossRef]
- Abe, Y.; Nakazima, N.; Tanase, T.; Katano, S.; Mukai, H.; Ohta, K. Syntheses, Structures, and Mesomorphism of a Series of Cu(II) Salen Complexes with 4-Substituted Long Alkoxy Chains. Mol. Cryst. Liq. Cryst. 2007, 466, 129–147. [Google Scholar] [CrossRef]
- Morris, R.J.; Girolami, G.S. A square-planar oxo-alkyl of manganese(III). Synthesis and X-ray crystal structure of {Li2[MnOMe3]·2Li2(Meacac)·tmed}2. Polyhedron 1988, 7, 2001–2008. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer 17; University of Western: Perth, Australia, 2017. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Gonzalez, J.A.; Pople, J.A. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Caro, L.D.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELX-97, Release 97-2; University of Goettingen: Goettingen, Germany, 1998. [Google Scholar]
- Akbar Ali, M.; Tarafdar, M.H.H. Metal complexes of sulphur and nitrogen-containing ligands: Complexes of s-benzyldithiocarbazate and a schiff base formed by its condensation with pyridine-2-carboxaldehyde. J. Inorg. Nucl. Chem. 1977, 39, 1785–1791. [Google Scholar] [CrossRef]
- Guerraoui, A.; Djedouani, A.; Jeanneau, E.; Boumaza, A.; Alsalme, A.; Zarrouk, A.; Salih, K.S.M.; Warad, I. Crystal structure and spectral of new hydrazine-pyran-dione derivative: DFT enolhydrazone tautomerization via zwitterionic intermediate, hirshfeld analysis and optical activity studies. J. Mol. Struct. 2020, 1220, 128728–128738. [Google Scholar] [CrossRef]
- Tabti, S.; Djedouani, A.; Aggoun, D.; Warad, I.; Rahmouni, S.; Romdhane, S.; Fouzi, H. New Cu (II), Co(II) and Ni(II) complexes of chalcone derivatives: Synthesis, X-ray crystal structure, electrochemical properties and DFT computational studies. J. Mol. Struct. 2018, 1155, 11–20. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flörke, U.; Boshaala, A. Experimental Crystal Structure Determination. CSDCommun. CCDC 2016, 1503558. Available online: https://www.ccdc.cam.ac.uk/structures/search?id=doi:10.5517/ccdc.csd.cc1mgkwj&sid=DataCite (accessed on 15 September 2021). [CrossRef]
- Hema, M.K.; Karthik, C.S.; Warad, I.; Lokanath, N.K.; Zarrouk, A.; Kumara, K.; Pampa, K.J.; Mallu, P. Regular square planer bis-(4,4,4-trifluoro-1-(thiophen-2-yl)butane-1,3-dione)/copper(II) complex: Trans/cis-DFT isomerization, crystal structure, thermal, solvatochromism, hirshfeld surface and DNA-binding analysis. J. Mol. Struct. 2018, 1157, 69–77. [Google Scholar] [CrossRef]
- Warad, I.; Musameh, S.; Badran, I.; Nassar, N.N.; Brandao, P.; Tavares, C.J.; Barakat, A. Synthesis, solvatochromism and crystal structure of trans-[Cu(Et2 NCH2 CH2 NH2)2·H2O](NO3)2 complex: Experimental with DFT combination. J. Mol. Struct. 2017, 11148, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Aouad, M.R.; Messali, M.; Rezki, N.; Al-Zaqri, N.; Warad, I. Single proton intramigration in novel 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione: XRD-crystal interactions, physicochemical, thermal, Hirshfeld surface, DFT realization of thiol/thione tautomerism. J. Mol. Liq. 2018, 264, 621–630. [Google Scholar] [CrossRef]
- Badran, I.; Abdallah, L.; Mubarakeh, R.; Warad, I. Effect of alkyl derivation on the chemical and antibacterial properties of newly synthesized Cu(II)-diamine complexes. Mor. J. Chem. 2019, 7, 161–170. [Google Scholar]
- Hema, M.K.; Karthik, C.S.; Lokanath, N.K.; Mallu, P.; Zarrouk, A.; Salih, K.S.M.; Warad, I. Synthesis of novel Cubane [Ni4(O∩O)4(OCH3)4(OOH)4] cluster: XRD/HSA-interactions, spectral, DNA-binding, docking and subsequent thermolysis to NiO nanocrystals. J. Mol. Liq. 2020, 315, 113756–113766. [Google Scholar] [CrossRef]
- Al-Zaqri, N.; Salih, K.S.M.; Awwadi, F.F.; Alsalme, A.; Alharthi, F.A.; Alsyahi, A.; Ali, A.A.; Zarrouk, A.; Aljohani, M.; Chetouni, A.; et al. Synthesis, physicochemical, thermal, and XRD/HSA interactions of mixed [Cu(Bipy)(Dipn)](X)2 complexes: DNA binding and molecular docking evaluation. J. Coord. Chem. 2020, 73, 3236–3248. [Google Scholar] [CrossRef]
- Badran, I.; Tighadouini, S.; Radi, S.; Zarrouk, A.; Warad, I. Experimental and first-principles study of a new hydrazine derivative for DSSC applications. J. Mol. Struct. 2021, 1229, 129799. [Google Scholar] [CrossRef]


| Chemical Formula | C32H28Cl2CuN4S4 |
|---|---|
| CCDC No. | 1,503,558 |
| Mr | 731.26 |
| Crystal system, space group | Triclinic, P-1 |
| Temperature K | 130 |
| a, b, c (Å) | 9.5051 (11), 13.7130 (16), 14.3488 (16) |
| α, β, γ (°) | 63.012 (2), 75.674 (2), 89.656 (2) |
| V (Å3) | 1602.5 (3) |
| Z | 2 |
| µ (mm−1) | 1.14 |
| Radiation type | Mo-Kα |
| Crystal size (mm) | 0.41 × 0.20 × 0.18 |
| No. of measured, independent and observed [I > 2.0 σ(I)] reflections | 15,300, 7589, 6391 |
| Rint | 0.023 |
| Tmin, Tmax | 0.652, 0.821 |
| (sin θ/λ)max (Å−1) | 0.658 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.034, 0.087, 1.04 |
| No. of reflections, No. of parameters | 7589, 390 |
| Δρmax, Δρmin (e Å−3) | 0.62, −0.34 |
| No. | Bond Type | Bond Length [Å] | Angle Type | Angle Value (°) | |||||
|---|---|---|---|---|---|---|---|---|---|
| XRD | DFT | XRD | DFT | ||||||
| 1 | Cu1 | S1 | 2.235 | 2.320 | S1 | Cu1 | S3 | 100.7 | 99.7 |
| 2 | Cu1 | S3 | 2.239 | 2.320 | S1 | Cu1 | N1 | 86.4 | 84.1 |
| 3 | Cu1 | N1 | 2.025 | 2.093 | S1 | Cu1 | N3 | 147.2 | 147.6 |
| 4 | Cu1 | N3 | 1.999 | 2.093 | S3 | Cu1 | N1 | 142.9 | 147.6 |
| 5 | Cl1 | C6 | 1.738 | 1.757 | S3 | Cu1 | N3 | 86.8 | 84.1 |
| 6 | Cl2 | C23 | 1.739 | 1.757 | N1 | Cu1 | N3 | 106.9 | 109.9 |
| 7 | S1 | C10 | 1.742 | 1.750 | Cu1 | S1 | C10 | 93.0 | 92.8 |
| 8 | S2 | C10 | 1.756 | 1.769 | C10 | S2 | C11 | 101.8 | 102.5 |
| 9 | S2 | C11 | 1.829 | 1.855 | Cu1 | S3 | C27 | 92.8 | 92.8 |
| 10 | S3 | C27 | 1.744 | 1.750 | C27 | S4 | C28 | 102.2 | 102.5 |
| 11 | S4 | C27 | 1.752 | 1.769 | Cu1 | N1 | N2 | 116.7 | 116.7 |
| 12 | S4 | C28 | 1.836 | 1.855 | Cu1 | N1 | C1 | 129.6 | 127.9 |
| 13 | N1 | N2 | 1.407 | 1.381 | N2 | N1 | C1 | 113.3 | 115.3 |
| 14 | N1 | C1 | 1.302 | 1.299 | N1 | N2 | C10 | 113.1 | 114.7 |
| 15 | N2 | C10 | 1.293 | 1.300 | Cu1 | N3 | N4 | 117.2 | 116.7 |
| 16 | N3 | N4 | 1.403 | 1.381 | Cu1 | N3 | C18 | 129.5 | 127.9 |
| 17 | N3 | C18 | 1.295 | 1.299 | N4 | N3 | C18 | 113.2 | 115.2 |
| 18 | N4 | C27 | 1.292 | 1.300 | N3 | N4 | C27 | 113.6 | 114.7 |
| 19 | C1 | C2 | 1.498 | 1.505 | N1 | C1 | C2 | 122.0 | 121.5 |
| 20 | C1 | C3 | 1.481 | 1.478 | N1 | C1 | C3 | 118.3 | 118.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boshaala, A.; Abrahem, A.F.; Almughery, A.A.; Al-Zaqri, N.; Zarrouk, A.; Lgaz, H.; Warad, I. Spectroscopic Insight into Tetrahedrally Distorted Square Planar Copper(II) Complex: XRD/HSA, Physicochemical, DFT, and Thermal Investigations. Crystals 2021, 11, 1179. https://doi.org/10.3390/cryst11101179
Boshaala A, Abrahem AF, Almughery AA, Al-Zaqri N, Zarrouk A, Lgaz H, Warad I. Spectroscopic Insight into Tetrahedrally Distorted Square Planar Copper(II) Complex: XRD/HSA, Physicochemical, DFT, and Thermal Investigations. Crystals. 2021; 11(10):1179. https://doi.org/10.3390/cryst11101179
Chicago/Turabian StyleBoshaala, Ahmed, Abrahem F. Abrahem, Abdulla Ali Almughery, Nabil Al-Zaqri, Abdelkader Zarrouk, Hassane Lgaz, and Ismail Warad. 2021. "Spectroscopic Insight into Tetrahedrally Distorted Square Planar Copper(II) Complex: XRD/HSA, Physicochemical, DFT, and Thermal Investigations" Crystals 11, no. 10: 1179. https://doi.org/10.3390/cryst11101179








