Growth and Characterisation of Layered (BA)2CsAgBiBr7 Double Perovskite Single Crystals for Application in Radiation Sensing
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Microstructure
3.2. Crystal Structure
3.3. Electrical Characterisation
3.3.1. IV Measurements and SCLC Method
3.3.2. van der Pauw Measurements
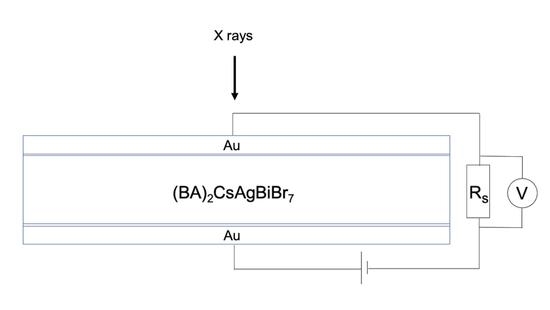
3.4. X-ray Photoresponse
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, H.; Fang, Y.; Mulligan, P.; Chuirazzi, W.; Fang, H.-H.; Wang, C.; Ecker, B.R.; Gao, Y.; Loi, M.A.; Cao, L.; et al. Sensitive X-ray Detectors Made of Methylammonium Lead Tribromide Perovskite Single Crystals. Nat. Photonics 2016, 10, 333–339. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Zhang, H.; Zhang, B.; Jie, W.; Sellin, P.J.; Hu, C.; Zeng, G.; Xu, Y. Enhanced X-ray Sensitivity of MAPbBr3 Detector by Tailoring the Interface-States Density. ACS Appl. Mater. Interfaces 2019, 11, 7522–7528. [Google Scholar] [CrossRef]
- Geng, X.; Feng, Q.; Zhao, R.; Hirtz, T.; Dun, G.; Yan, Z.; Ren, J.; Zhang, H.; Liang, R.; Tian, H.; et al. High-Quality Single Crystal Perovskite for Highly Sensitive X-Ray Detector. IEEE Electron. Device Lett. 2020, 41, 256–259. [Google Scholar] [CrossRef]
- Xu, Q.; Shao, W.; Li, Y.; Zhang, X.; Ouyang, X.; Liu, J.; Liu, B.; Wu, Z.; Ouyang, X.; Tang, X.; et al. High-Performance Surface Barrier X-Ray Detector Based on Methylammonium Lead Tribromide Single Crystals. ACS Appl. Mater. Interfaces 2019, 11, 9679–9684. [Google Scholar] [CrossRef]
- Ye, F.; Lin, H.; Wu, H.; Zhu, L.; Huang, Z.; Ouyang, D.; Niu, G.; Choy, W.C.H. High-Quality Cuboid CH3NH3PbI3 Single Crystals for High Performance X-Ray and Photon Detectors. Adv. Funct. Mater. 2019, 29, 1806984–1806991. [Google Scholar] [CrossRef]
- Murgulov, V.; Daub, M.; Hillebrecht, H.; Fiederle, M.; Franc, J.; Dědič, V. Growth and Characterization of Radiation Sensors Based on Single Crystals of Hybrid Metal–Organic Methylammonium Lead Bromide and Iodide Perovskite. Cryst. Res. Technol. 2020, 55, 2000112–2000124. [Google Scholar] [CrossRef]
- Pan, W.; Wu, H.; Luo, J.; Deng, Z.; Ge, C.; Chen, C.; Jiang, X.; Yin, W.-J.; Niu, G.; Zhu, L.; et al. Cs2AgBiBr6 Single-Crystal X-Ray Detectors with a Low Detection Limit. Nat. Photonics 2017, 11, 726–732. [Google Scholar] [CrossRef]
- Yin, L.; Wu, H.; Pan, W.; Yang, B.; Li, P.; Luo, J.; Niu, G.; Tang, J. Controlled Cooling for Synthesis of Cs2AgBiBr6 Single Crystals and Its Application for X-Ray Detection. Adv. Opt. Mater. 2019, 7, 1900491–1900499. [Google Scholar] [CrossRef]
- Yuan, W.; Niu, G.; Xian, Y.; Wu, H.; Wang, H.; Yin, H.; Liu, P.; Li, W.; Fan, J. In Situ Regulating the Order–Disorder Phase Transition in Cs2AgBiBr6 Single Crystal toward the Application in an X-Ray Detector. Adv. Funct. Mater. 2019, 29, 1900234. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Z.; Liang, R.; Zheng, X.; Geng, X.; Zhao, Y.; Xie, D.; Hong, J.; Tian, H.; Yang, Y.; et al. X-ray Detector Based on All-Inorganic Lead-Free Cs2AgBiBr6 Perovskite Single Crystal. IEEE Trans. Electron. Devices 2019, 66, 2224–2229. [Google Scholar] [CrossRef]
- Su, Y.; Ma, W.; Yang, Y. Perovskite Semiconductors for Direct X-Ray Detection and Imaging. J. Semicond. 2020, 41, 051204. [Google Scholar] [CrossRef]
- Wu, H.; Ge, Y.; Niu, G.; Tang, J. Metal Halide Perovskites for X-Ray Detection and Imaging. Matter 2021, 4, 144–163. [Google Scholar] [CrossRef]
- Connor, B.A.; Leppert, L.; Smith, M.D.; Neaton, J.B.; Karunadasa, H.I. Layered Halide Double Perovskites: Dimensional Reduction of Cs2AgBiBr6. J. Am. Chem. Soc. 2018, 140, 5235–5240. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, X.; Li, Y.; Liu, X.; Yang, T.; Ji, C.; Han, S.; Xu, Y.; Luo, J.; Sun, Z. Exploring Lead-Free Hybrid Double Perovskite Crystals of (BA)2CsAgBiBr7 with Large Mobility-Lifetime Product toward X-Ray Detection. Angew. Chem. Int. Ed. 2019, 58, 15757–15761. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, H.; Pan, S.; Li, H.; Zhang, J.; Gong, Z.; Zhang, Y.; Pan, J. Growth and Properties of Centimeter-Sized Lead Free All Inorganic Perovskite Cs2AgBiBr6 Crystal by Additive CH3COONa. J. Cryst. Growth 2020, 532, 125440–125447. [Google Scholar] [CrossRef]
- Duijnstee, E.A.; Ball, J.M.; Le Corre, V.M.; Koster, L.J.A.; Snaith, H.J.; Lim, J. Toward Understanding Space-Charge Limited Current Measurements on Metal Halide Perovskites. ACS Energy Lett. 2020, 5, 376–384. [Google Scholar] [CrossRef]
- Röhr, J.A.; Kirchartz, T.; Nelson, J. On the Correct Interpretation of the Low Voltage Regime in Intrinsic Single-Carrier Devices. J. Phys. Condens. Matter 2017, 29, 205901–205910. [Google Scholar] [CrossRef]
- Röhr, J.A.; Moia, D.; Haque, S.A.; Kirchartz, T.; Nelson, J. Exploring the Validity and Limitations of the Mott–Gurney Law for Charge-Carrier Mobility Determination of Semiconducting Thin-Films. J. Phys. Condens. Matter 2018, 30, 105901–105914. [Google Scholar] [CrossRef] [Green Version]
- Röhr, J.A. Direct Determination of Built-in Voltages in Asymmetric Single-Carrier Devices. Phys. Rev. Appl. 2019, 11, 054079–054084. [Google Scholar] [CrossRef]
- Lampert, M.A. Simplified Theory of Space-Charge-Limited Currents in an Insulator with Traps. Phys. Rev. 1956, 103, 1648–1656. [Google Scholar] [CrossRef]
- Lampert, M.A.; Mark, P. Current Injections in Solids; Electrical current series; Academic Press: Cambridge, MA, USA, 1970; p. 354. [Google Scholar]
- Mott, N.F.; Gurney, R.W. Electronic Processes in Ionic Crystals, 2nd ed.; International Series of Monographs on Physics; Oxford University Press: Oxford, UK, 1940; p. 275. [Google Scholar]
- Le Corre, V.M.; Duijnstee, E.A.; El Tambouli, O.; Ball, J.M.; Snaith, H.J.; Lim, J.; Koster, L.J.A. Revealing Charge Carrier Mobility and Defect Densities in Metal Halide Perovskites via Space-Charge-Limited Current Measurements. ACS Energy Lett. 2021, 6, 1087–1094. [Google Scholar] [CrossRef]





| Sample | Contacts | L (mm) | U (V) | ρ (Ωcm) | VTFL (V) | μ (cm2V−1s−1) | ntrap (cm−3) |
|---|---|---|---|---|---|---|---|
| Exp1 | T+ B− | 0.75 | −3 to 20 | 1.14 × 1012 | 37.4 | 3.20 × 10−5 | 1.32 × 1011 |
| 43.3 | 8.65 × 10−5 | 1.53 × 1011 | |||||
| B+ T− | 1.45 × 1012 | ||||||
| Exp2 | T+ B− | 0.75 | −30 to 8 | 4.19 × 1011 | |||
| B+ T− | 5.40 × 1011 | ||||||
| Exp3 | T+ B− | 0.75 | −100 to 100 | 2.67 × 1012 | |||
| B+ T− | 2.56 × 1012 | ||||||
| Exp4 | T+ B− | 0.75 | −36 to 32 | 7.09 × 1011 | 49.9 | 9.11 × 10−5 | 1.76 × 1011 |
| B+ T− | 6.94 × 1011 | 31.7 | 1.34 × 10−4 | 1.12 × 1011 | |||
| 33.1 | 1.44 × 10−4 | 1.17 × 1011 |
| Sample | Setup | ρ (Ωcm) |
|---|---|---|
| Exp1-Ag glue | 2 | 7.67 × 1010 |
| 1 | 6.99 × 1010 | |
| 3 | 3.03 × 1010 | |
| Exp2-Ag glue | 2 | 9.16 × 1010 |
| 1 | 6.99 × 1010 | |
| 3 | 2.06 × 1010 | |
| Exp3-Ag glue | 2 | 8.90 × 1010 |
| 3 | 3.41 × 1010 | |
| 1 | 5.80 × 109 | |
| Exp4-Ag glue | 2 | 3.01 × 1010 |
| 1 | 2.19 × 1010 | |
| 3 | 1.65 × 1010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgulov, V.; Schweinle, C.; Daub, M.; Hillebrecht, H.; Fiederle, M.; Dědič, V.; Franc, J. Growth and Characterisation of Layered (BA)2CsAgBiBr7 Double Perovskite Single Crystals for Application in Radiation Sensing. Crystals 2021, 11, 1208. https://doi.org/10.3390/cryst11101208
Murgulov V, Schweinle C, Daub M, Hillebrecht H, Fiederle M, Dědič V, Franc J. Growth and Characterisation of Layered (BA)2CsAgBiBr7 Double Perovskite Single Crystals for Application in Radiation Sensing. Crystals. 2021; 11(10):1208. https://doi.org/10.3390/cryst11101208
Chicago/Turabian StyleMurgulov, Valeria, Catherine Schweinle, Michael Daub, Harald Hillebrecht, Michael Fiederle, Václav Dědič, and Jan Franc. 2021. "Growth and Characterisation of Layered (BA)2CsAgBiBr7 Double Perovskite Single Crystals for Application in Radiation Sensing" Crystals 11, no. 10: 1208. https://doi.org/10.3390/cryst11101208
APA StyleMurgulov, V., Schweinle, C., Daub, M., Hillebrecht, H., Fiederle, M., Dědič, V., & Franc, J. (2021). Growth and Characterisation of Layered (BA)2CsAgBiBr7 Double Perovskite Single Crystals for Application in Radiation Sensing. Crystals, 11(10), 1208. https://doi.org/10.3390/cryst11101208