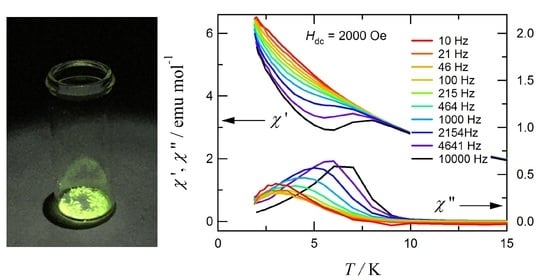
Polymeric Terbium(III) Squarate Hydrate as a Luminescent Magnet
Abstract
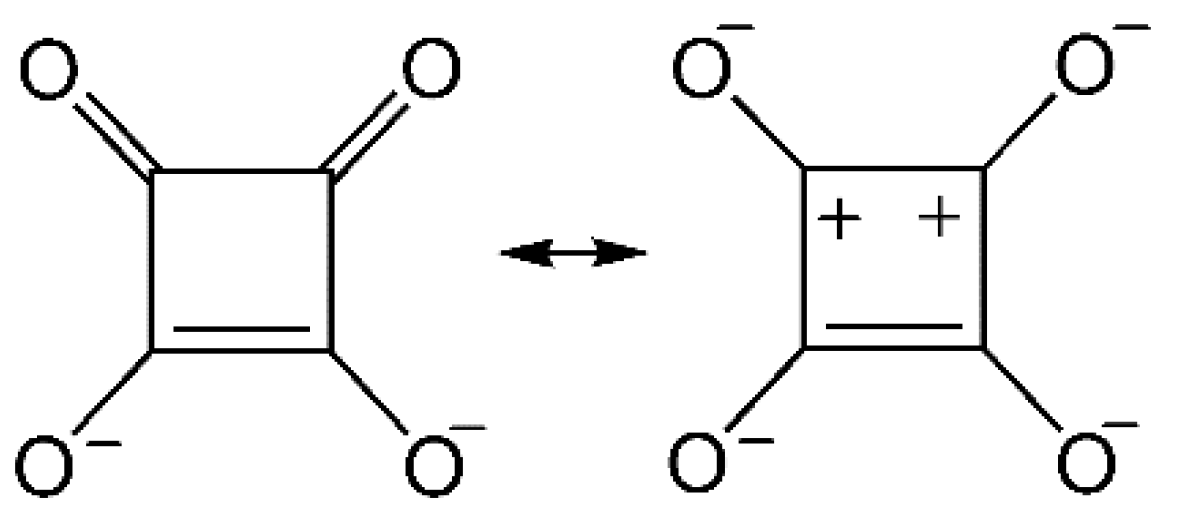
:1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Structural Analysis
2.3. Photoluminescent Measurements
2.4. Magnetic Measurements
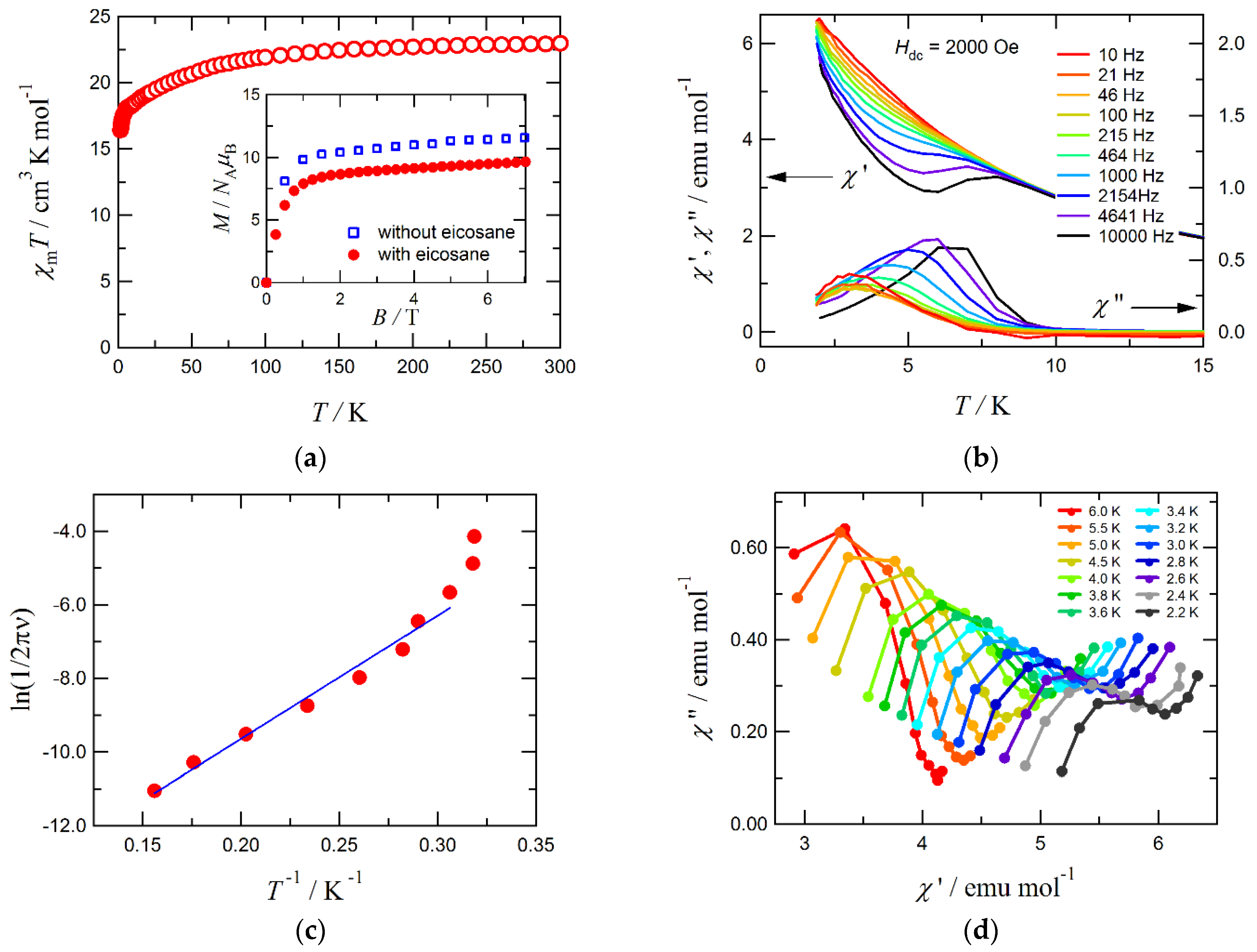
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- West, R. Oxocarbons; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Brenner, N.; Sperling, J.M.; Poe, T.N.; Celis-Barros, C.; Brittain, K.; Villa, E.M.; Albrecht-Schmitt, T.E.; Polinski, M.J. Trivalent f-element squarates, squarate-oxalates, and cationic materials, and the determination of the nine-coordinate ionic radius of Cf(III). Inorg. Chem. 2020, 59, 9384–9395. [Google Scholar] [CrossRef]
- Lin, X.Y.; Zhao, L.M.; Wang, D.H.; Wang, Y.K.; Li, M.; Li, H.H.; Chen, Z.R. Structural diversities of squarate-based complexes: Photocurrent responses and thermochromic behaviours enchanced by viologens. Inorg. Chem. Front. 2018, 5, 189–199. [Google Scholar] [CrossRef]
- Goswami, S.; Saha, R.; Steele, I.M.; Dasgupta, P.; Poddar, A.; Kumar, S. An unusual μ-1,2,3-squarato- bridged two dimensional coordination polymer: Crystal structure, thermal, photoluminescence and magnetic studies. Inorg. Chim. Acta 2014, 410, 111–117. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Tu, R.; Zhang, W.; Zhang, J.; Wang, M.; Zhang, F.; Yang, K.; Li, J.; Pan, H.; Bernards, M.T.; Xie, P.; et al. Squarate-calcium metal–organic framework for molecular sieving of CO2 from flue gas with high water vapor resistance. Energy Fuel. 2021, 35, 13900–13907. [Google Scholar] [CrossRef]
- Babaryk, A.A.; Contreras Almengor, O.R.; Cabrero-Antonino, M.; Navalón, S.; García, H.; Horcajada, P. A semiconducting Bi2O2(C4O4) coordination polymer showing a photoelectric response. Inorg. Chem. 2020, 59, 3406–3416. [Google Scholar] [CrossRef]
- Goswami, S.; Jena, H.S.; Konar, S. Study of Heterogeneous Catalysis by iron-squarate based 3D metal organic framework for the transformation of tetrazines to oxadiazole derivatives. Inorg. Chem. 2014, 53, 7071–7073. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, L.; Zhang, Z.; Yang, Q.; Yang, Y.; Bao, Z.; Ren, Q.; Li, J. A robust squarate-based metal–organic framework demonstrates record-high affinity and selectivity for xenon over krypton. J. Am. Chem. Soc. 2019, 141, 9358–9364. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Mautner, F.A. Polynuclear and polymeric squarato-bridged coordination compounds. CrystEngComm 2015, 17, 7604–7617. [Google Scholar] [CrossRef]
- Petit, J.F.; Gleizes, A.; Trombe, J.C. Lanthanide(III) squarates 1. Five families of compounds obtained from aqueous solutions in an open system. Crystal structure and thermal behaviour. Inorg. Chim. Acta 1990, 167, 51–68. [Google Scholar] [CrossRef]
- Huskowska, E.; Glowiak, T.; Legendziewicz, J.; Oremek, G. Luminescence and crystal structure of neodymium and europium squarate hydrates. J. Alloys Compd. 1992, 179, 13–25. [Google Scholar] [CrossRef]
- Kanetomo, T.; Ishida, T. Luminescent single-ion magnets from lanthanoid(III) complexes with monodentate ketone ligands. AIP Conf. Proc. 2016, 1709, 020015. [Google Scholar]
- Biswas, S.; Adhikary, A.; Goswami, S.; Konar, S. Observation of a large magnetocaloric effect in a 2D Gd(III)-based coordination polymer. Dalton Trans. 2013, 42, 13331–13334. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.Y.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- Bernot, K.; Bogani, L.; Caneschi, A.; Gatteschi, D.; Sessoli, R. A family of rare-earth-based single chain magnets: Playing with anisotropy. J. Am. Chem. Soc. 2006, 128, 7947–7956. [Google Scholar] [CrossRef] [PubMed]
- Armelao, L.; Quici, S.; Barigelletti, F.; Accorsi, G.; Bottaro, G.; Cavazzini, M.; Tondello, E. Design of luminescent lanthanide complexes: From molecules to highly efficient photo-emitting materials. Coord. Chem. Rev. 2010, 254, 487–505. [Google Scholar] [CrossRef]
- Yan, B. Lanthanide-functionalized metal–organic framework hybrid systems to create multiple luminescent centers for chemical sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef]
- Kido, J.; Okamoto, Y. Organo lanthanide metal complexes for electroluminescent materials. Chem. Rev. 2002, 102, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Xu, J.; Gulzar, A.; Yang, P.; Bi, H.; Yang, D.; Gai, S.; He, F.; Lin, J.; Xing, B.; Jin, D. Recent advances in near-infrared emitting lanthanide-doped nanoconstructs: Mechanism, design and application for bioimaging. Coord. Chem. Rev. 2019, 381, 104–134. [Google Scholar] [CrossRef]
- Quici, S.; Cavazzini, M.; Marzanni, G.; Accorsi, G.; Armaroli, N.; Ventura, B.; Barigelletti, F. Visible and near-infrared intense luminescence from water-soluble lanthanide [Tb(III), Eu(III), Sm(III), Dy(III), Pr(III), Ho(III), Yb(III), Nd(III), Er(III)] complexes. Inorg. Chem. 2005, 44, 529–537. [Google Scholar] [CrossRef]
- Sahadevan, S.A.; Monni, N.; Oggianu, M.; Abhervé, A.; Marongiu, D.; Saba, M.; Mura, A.; Bongiovanni, G.; Mameli, V.; Cannas, C.; et al. Heteroleptic NIR-Emitting YbIII/Anilate-based neutral coordination polymer nanosheets for solvent sensing. ACS Appl. Nano. Mater. 2020, 3, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Trombe, J.C.; Petit, J.F.; Gleizes, A. Lanthanide(III) squarates 2. High diversity of rare coordination modes of the squarate anion in a series of weakly hydrated cerium(III) squarates prepared by pseudo-hydrothermal methods. Inorg. Chim. Acta 1990, 167, 69–81. [Google Scholar] [CrossRef]
- Wang, L.; Gu, W.; Deng, X.J.; Zeng, L.F.; Liao, S.Y.; Zhang, M.; Yang, L.-Y.; Liu, X. A Series of 2D and 3D Novel Lanthanide Complexes Constructed from Squarate C4O42–: Syntheses, Structures, Magnetic Properties, and Near-infrared Emission Properties. Aust. J. Chem. 2011, 64, 1373–1382. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, Q.G.; Li, S.N.; Jiang, Y.C.; Hu, M.C. Synthesis, crystal structures, and solid-state luminescent properties of diverse Ln–Pyridine-3,5-Dicarboxylate coordination polymers modulated by the ancillary ligand. Cryst. Growth Des. 2014, 14, 177–188. [Google Scholar] [CrossRef]
- Lluncll, M.; Casanova, D.; Circra, J.; Bofill, J.M.; Alcmany, P.; Alvarez, S.; Pinsky, M.; Avnir, D. SHAPE, version 2.1; University of Barcelona: Barcelona, Spain; Hebrew University of Jerusalem: Jerusalem, Israel, 2005. [Google Scholar]
- Leonard, J.P.; Gunnlaugsson, T. Luminescent Eu(III) and Tb(III) complexes: Developing lanthanide luminescent-based devices. J. Fluoresc. 2005, 15, 585–595. [Google Scholar] [CrossRef]
- Ribeiro, S.J.; Gonçalves, R.R.; de Oliveira, L.F.; Santos, P.S. Spectroscopic study of lanthanide squarate hydrates. J. Alloys Compd. 1994, 216, 61–66. [Google Scholar] [CrossRef]
- Moore, E.G.; Samuel, A.P.; Raymond, K.N. From antenna to assay: Lessons learned in lanthanide luminescence. Acc. Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Marin, R.; Brunet, G.; Murugesu, M. Shining New Light on Multifunctional Lanthanide Single-Molecule Magnets. Angew. Chem. Int. Ed. 2021, 60, 1728–1746. [Google Scholar] [CrossRef]
- Karbowiak, M.; Rudowicz, C.; Ishida, T. Determination of crystal-field energy levels and temperature dependence of magnetic susceptibility for Dy3+ in [Dy2Pd] heterometallic complex. Inorg. Chem. 2013, 52, 13199–13206. [Google Scholar] [CrossRef]
- Ehama, K.; Ohmichi, Y.; Sakamoto, S.; Fujinami, T.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Tsuchimoto, M.; Re, N. Synthesis, structure, luminescent, and magnetic properties of carbonato-bridged ZnII2LnIII2 complexes [(μ4-CO3)2{ZnIILnLnIII(NO3)}2] (LnIII = GdIII, TbIII, DyIII.; L1 = N,N′-Bis(3-methoxy-2-oxybenzylidene)-1,3-propanediaminato, L2 = N,N′-Bis(3-ethoxy-2-oxybenzylidene)-1,3-propanediaminato). Inorg. Chem. 2013, 52, 12828–12841. [Google Scholar]
- Yamashita, K.; Miyazaki, R.; Kataoka, Y.; Nakanishi, T.; Hasegawa, Y.; Nakano, M.; Yamamura, T.; Kajiwara, T. A luminescent single-molecule magnet: Observation of magnetic anisotropy using emission as a probe. Dalton Trans. 2013, 42, 1987–1990. [Google Scholar] [CrossRef]
- Karbowiak, M.; Rudowicz, C.; Nakamura, T.; Murakami, R.; Ishida, T. Spectroscopic and magnetic studies of erbium(III)-TEMPO complex as a potential single-molecule magnet: Interplay of the crystal-field and exchange coupling effects. Chem. Phys. Lett. 2016, 662, 163–168. [Google Scholar] [CrossRef]
- Castro, S.L.; Sun, Z.; Grant, C.M.; Bollinger, J.C.; Hendrickson, D.N.; Christou, G. Single-molecule magnets: Tetranuclear vanadium(III) complexes with a butterfly structure and an S = 3 ground state. J. Am. Chem. Soc. 1998, 120, 2365–2375. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Giansiracusa, M.; Gransbury, G.; Chilton, N.; Mills, D. Single Molecule Magnets. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons: New York, NY, USA, 2021. [Google Scholar]
- Sessoli, R.; Powell, A.K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Yamauchi, S.; Fujinami, T.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Watanabe, M.; Tsuchimoto, M.; Coletti, C.; Re, N. Synthesis, Structure, Luminescence, and Magnetic Properties of a Single-Ion Magnet “mer”-[Tris(N-[(imidazol-4-yl)- methylidene]-dl-phenylalaninato)terbium(III) and Related “fac”-dl-Alaninato Derivative. Inorg. Chem. 2014, 53, 5961–5971. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Long, J.; Guari, Y.; Ferreira, R.A.; Carlos, L.D.; Larionova, J. Recent advances in luminescent lanthanide based Single-Molecule Magnets. Coord. Chem. Rev. 2018, 363, 57–70. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takano, R.; Ishida, T. Polymeric Terbium(III) Squarate Hydrate as a Luminescent Magnet. Crystals 2021, 11, 1221. https://doi.org/10.3390/cryst11101221
Takano R, Ishida T. Polymeric Terbium(III) Squarate Hydrate as a Luminescent Magnet. Crystals. 2021; 11(10):1221. https://doi.org/10.3390/cryst11101221
Chicago/Turabian StyleTakano, Rina, and Takayuki Ishida. 2021. "Polymeric Terbium(III) Squarate Hydrate as a Luminescent Magnet" Crystals 11, no. 10: 1221. https://doi.org/10.3390/cryst11101221