Influence of Milling Route on the Corrosion Passivation of Al-2%SiC Nanocomposites in Chloride Solutions
Abstract
:1. Introduction
2. Experimental Part
2.1. Chemicals, Materials, and Fabrication of Al-SiC Composites
2.2. Electrochemical Experiments
2.3. Surface Analyses Techniques
3. Results and Discussion
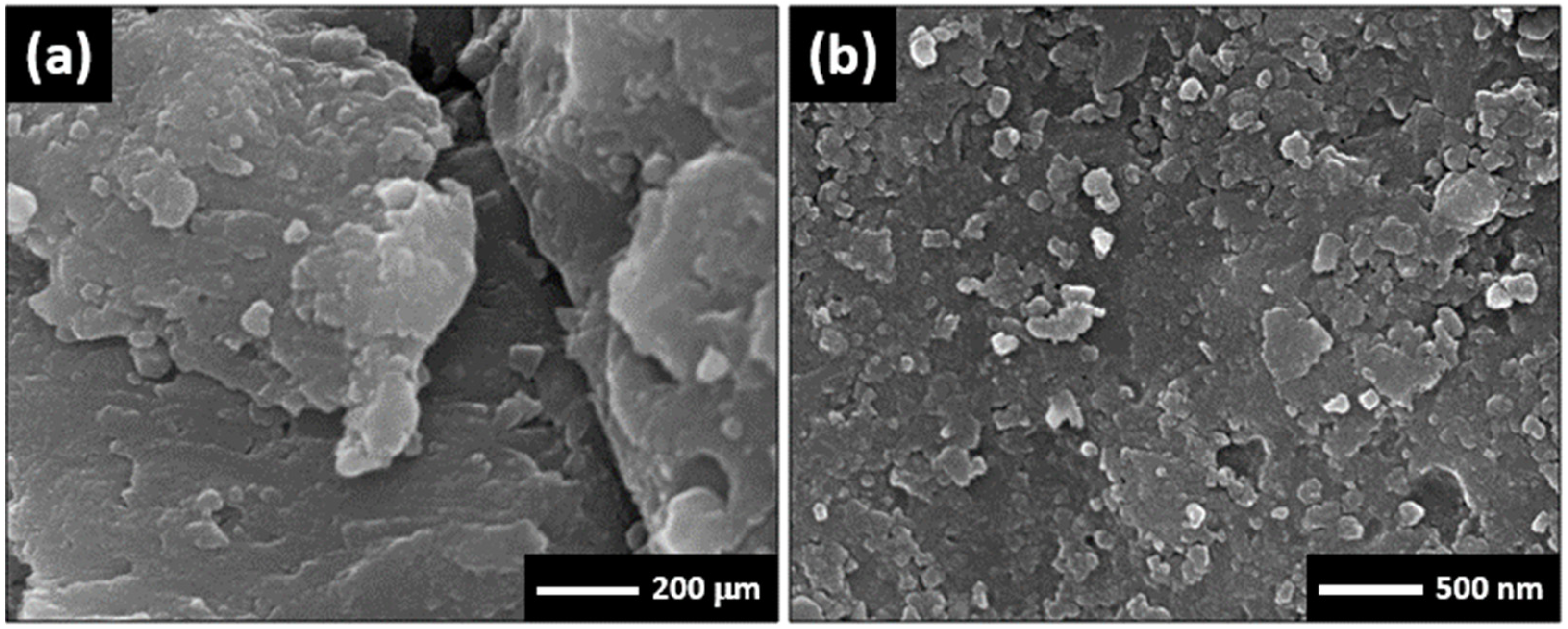
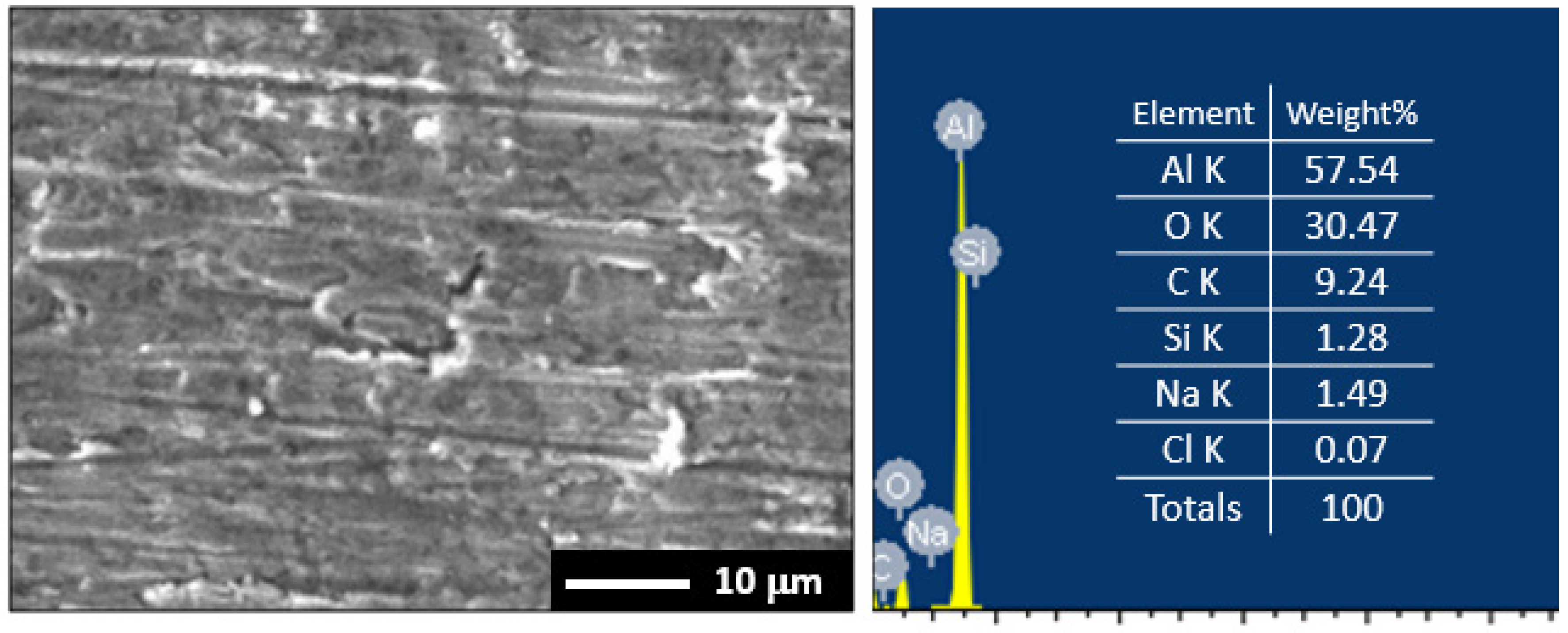
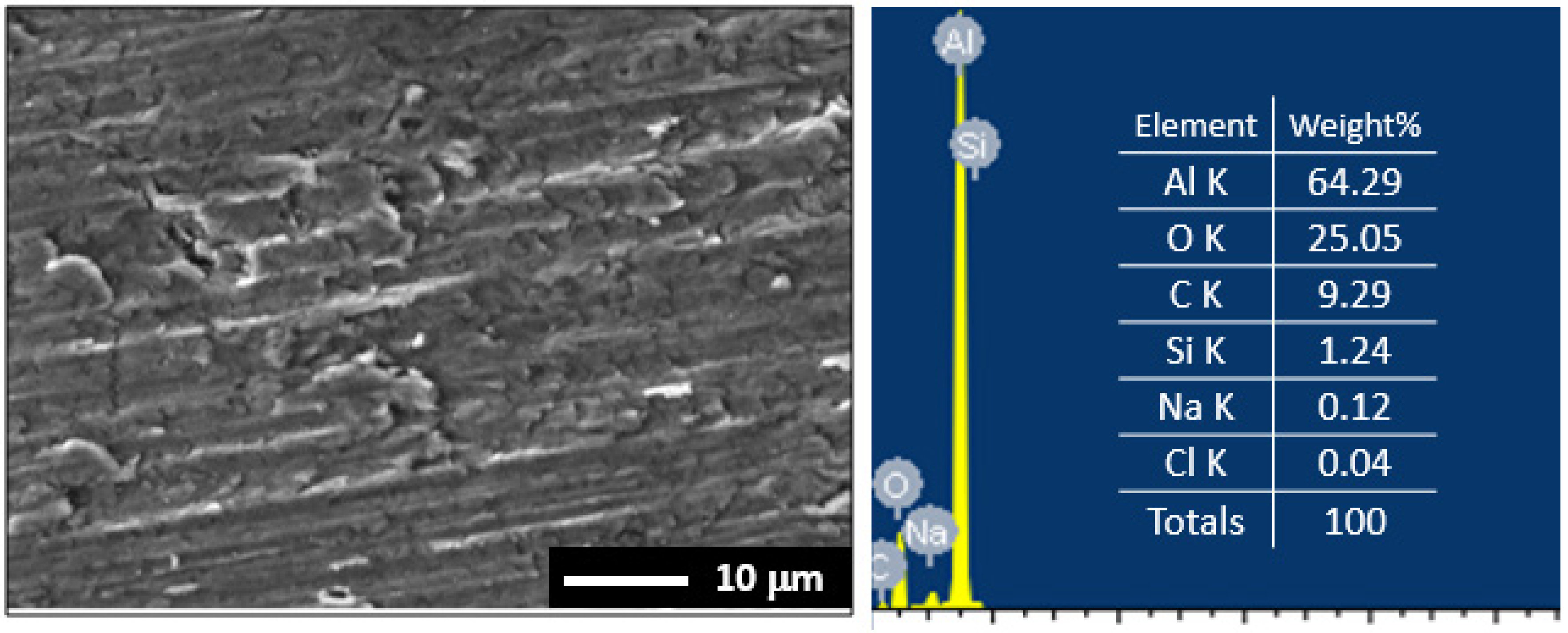
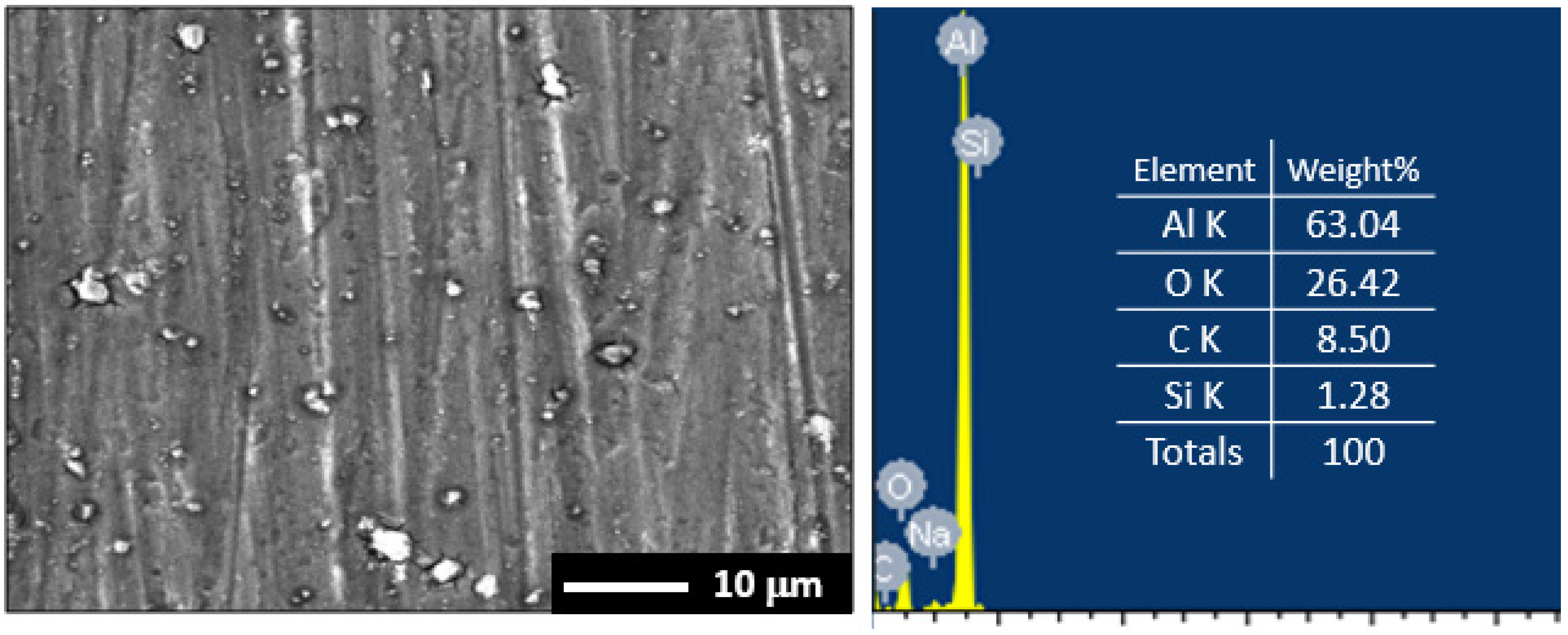
3.1. Morphology and X-ray Investigations
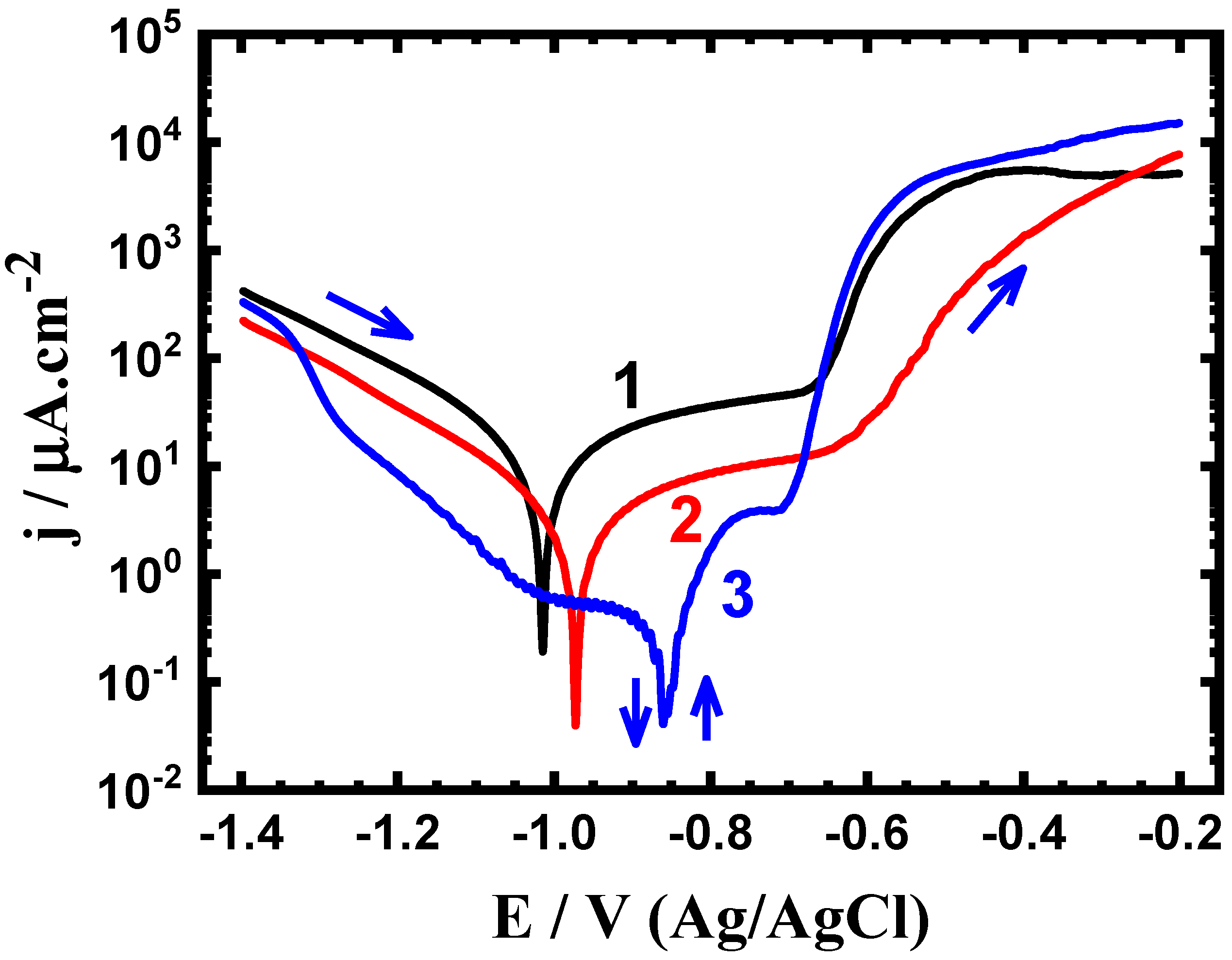
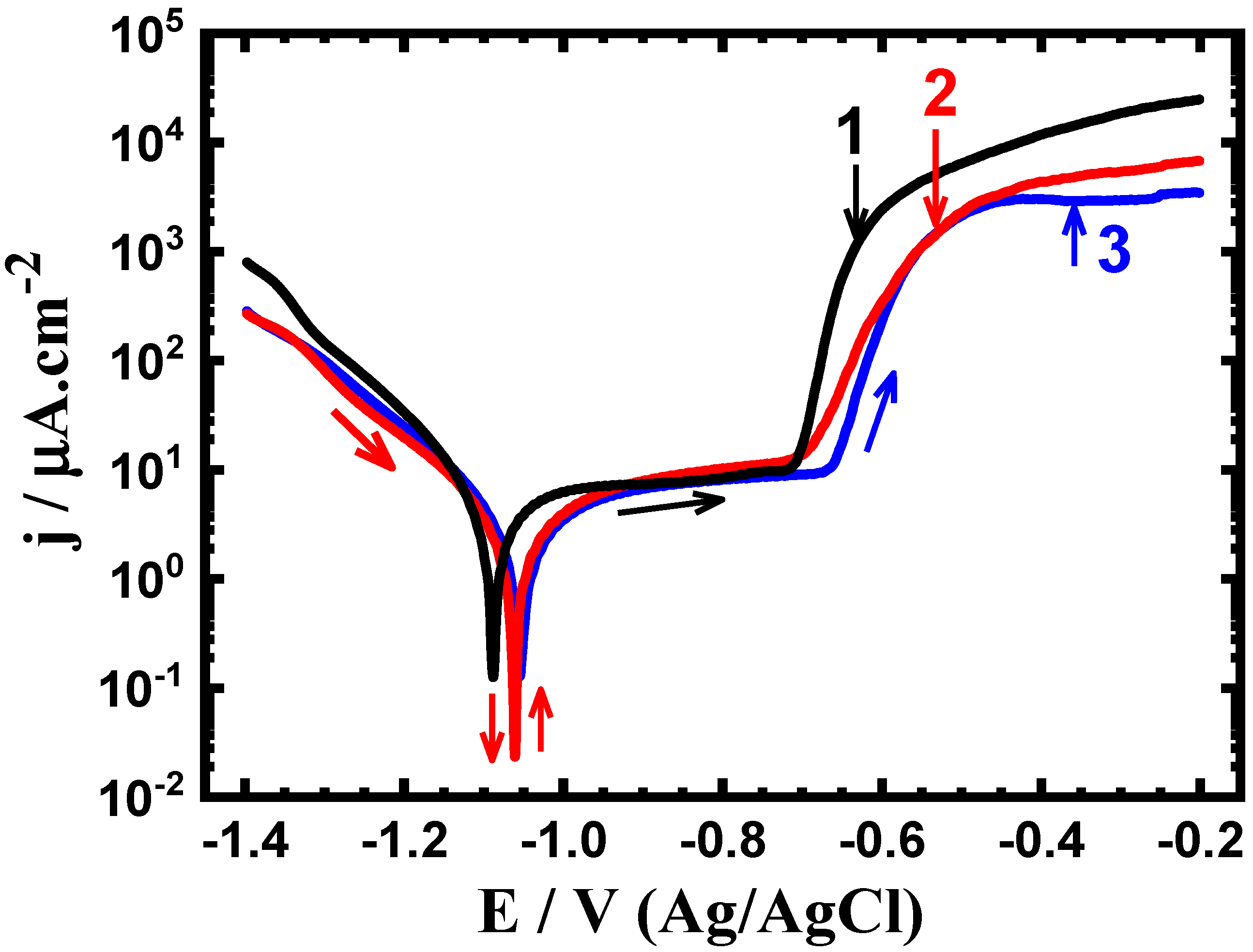
3.2. Potentiodynamic Polarization Data
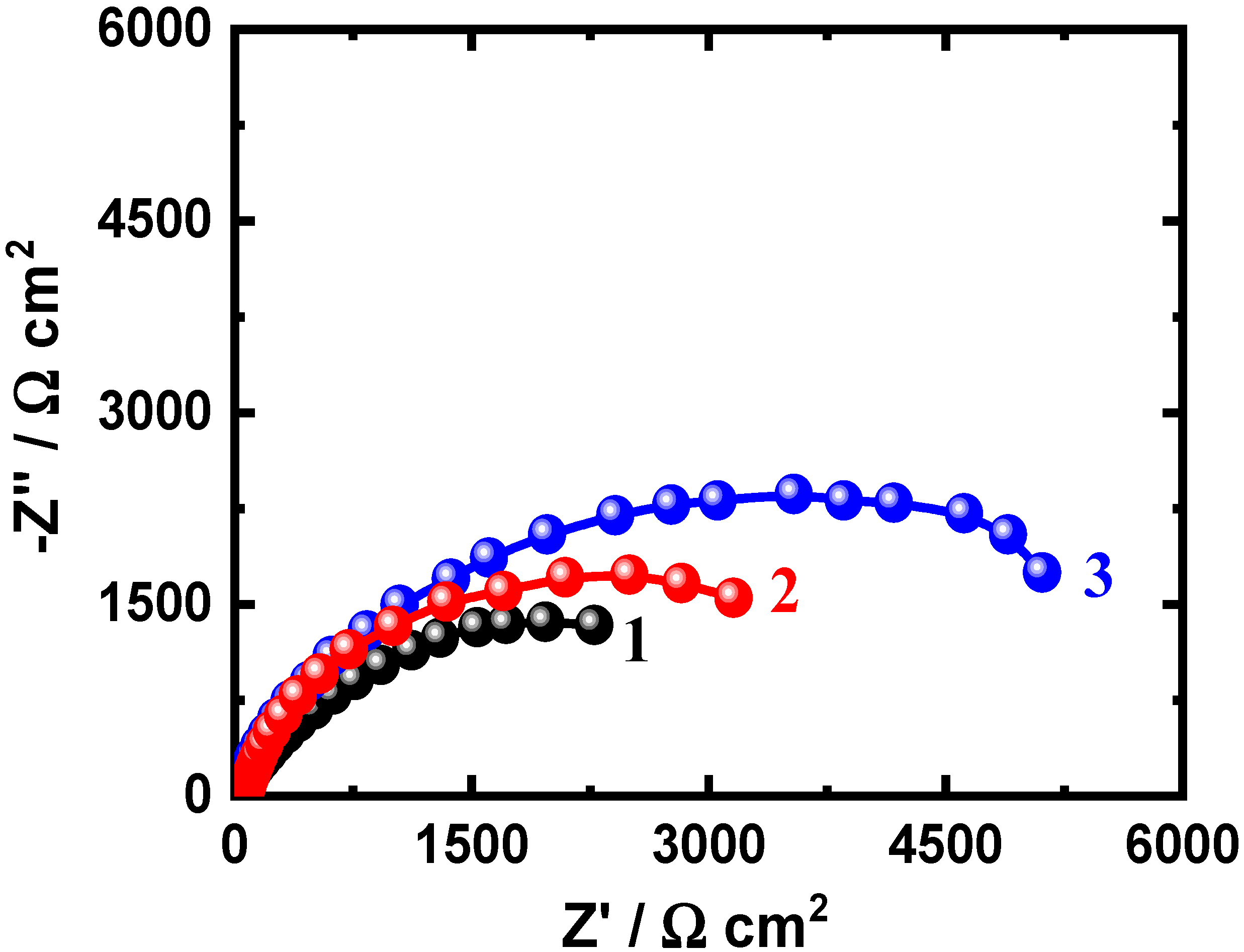
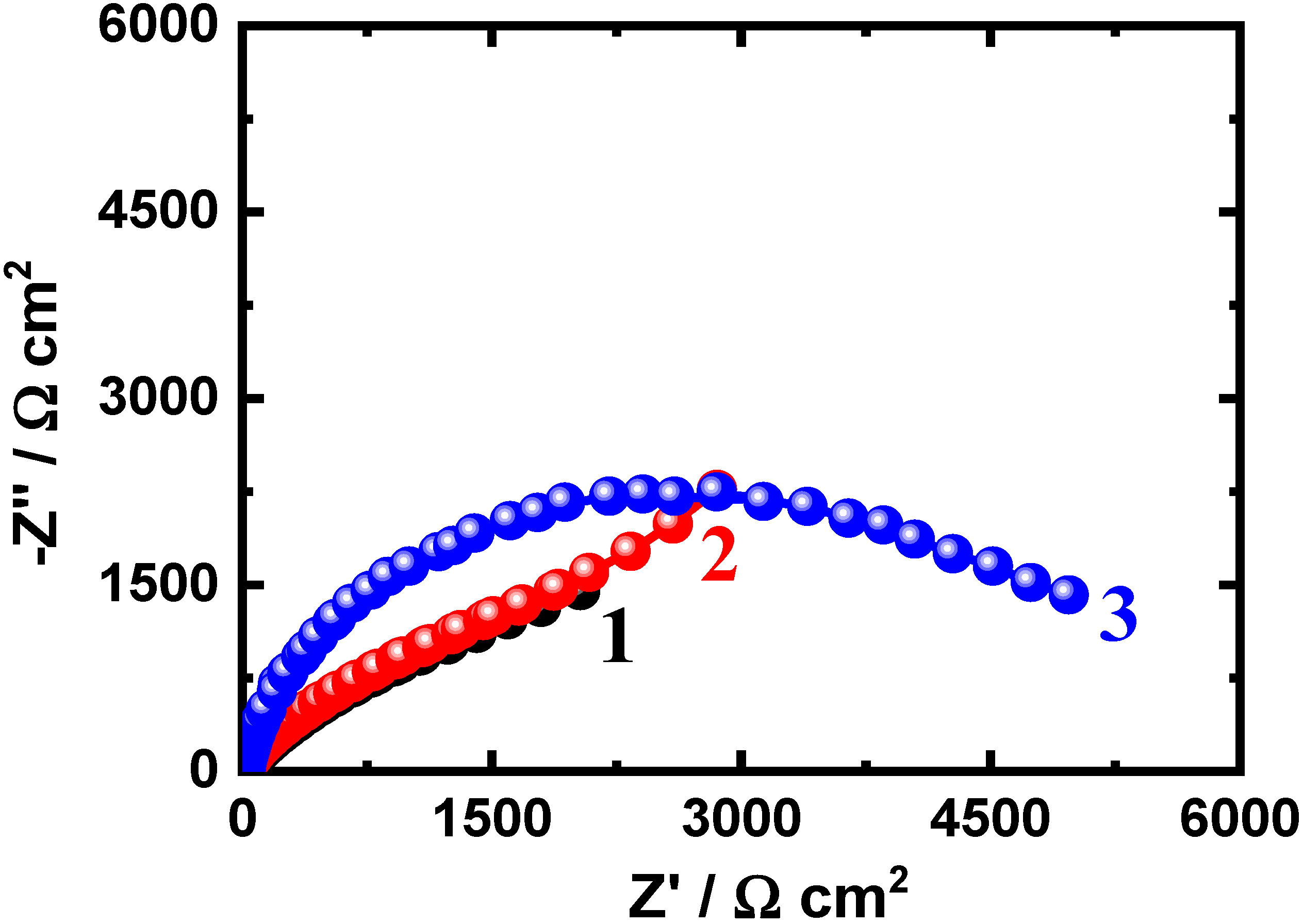
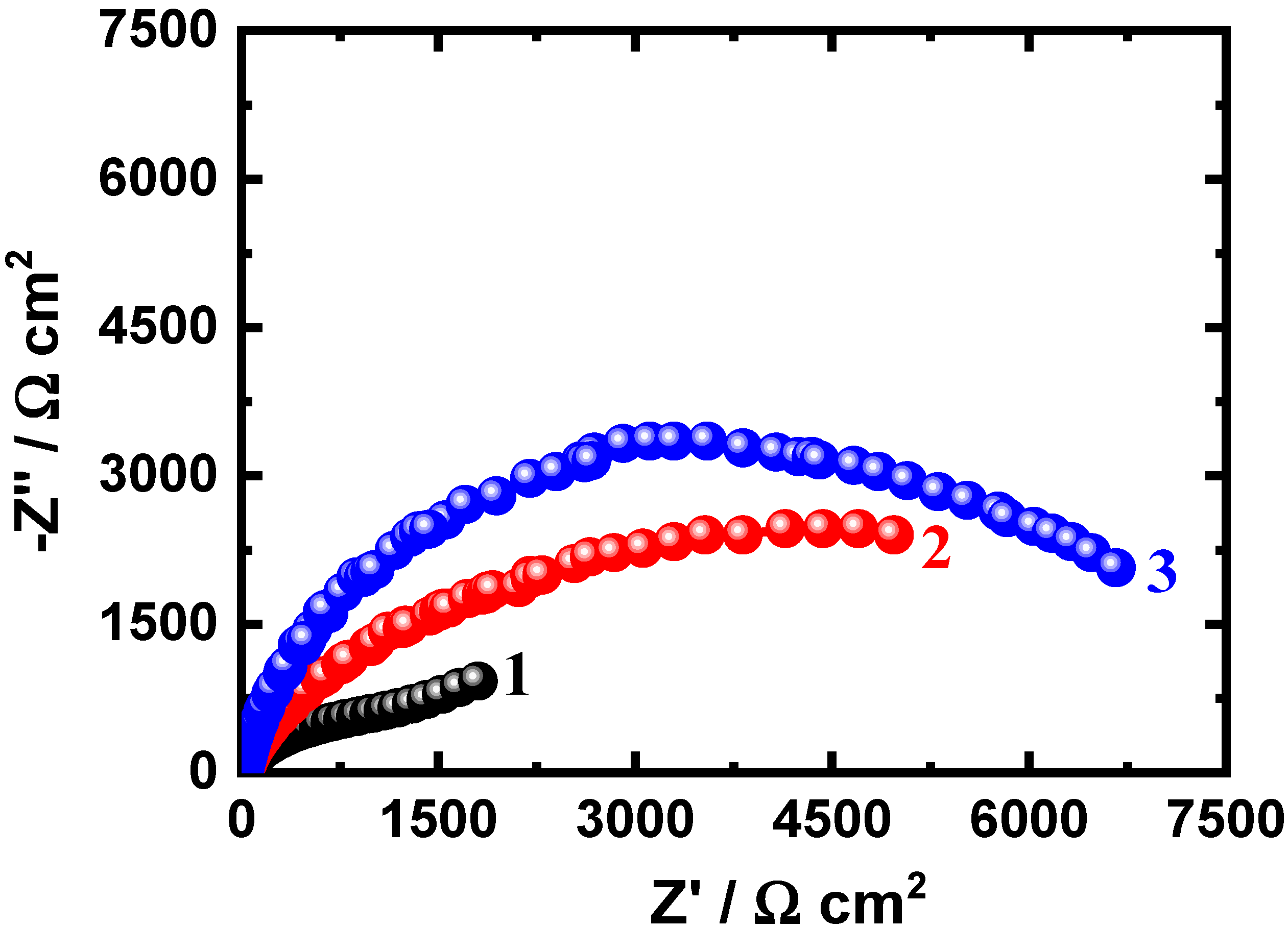
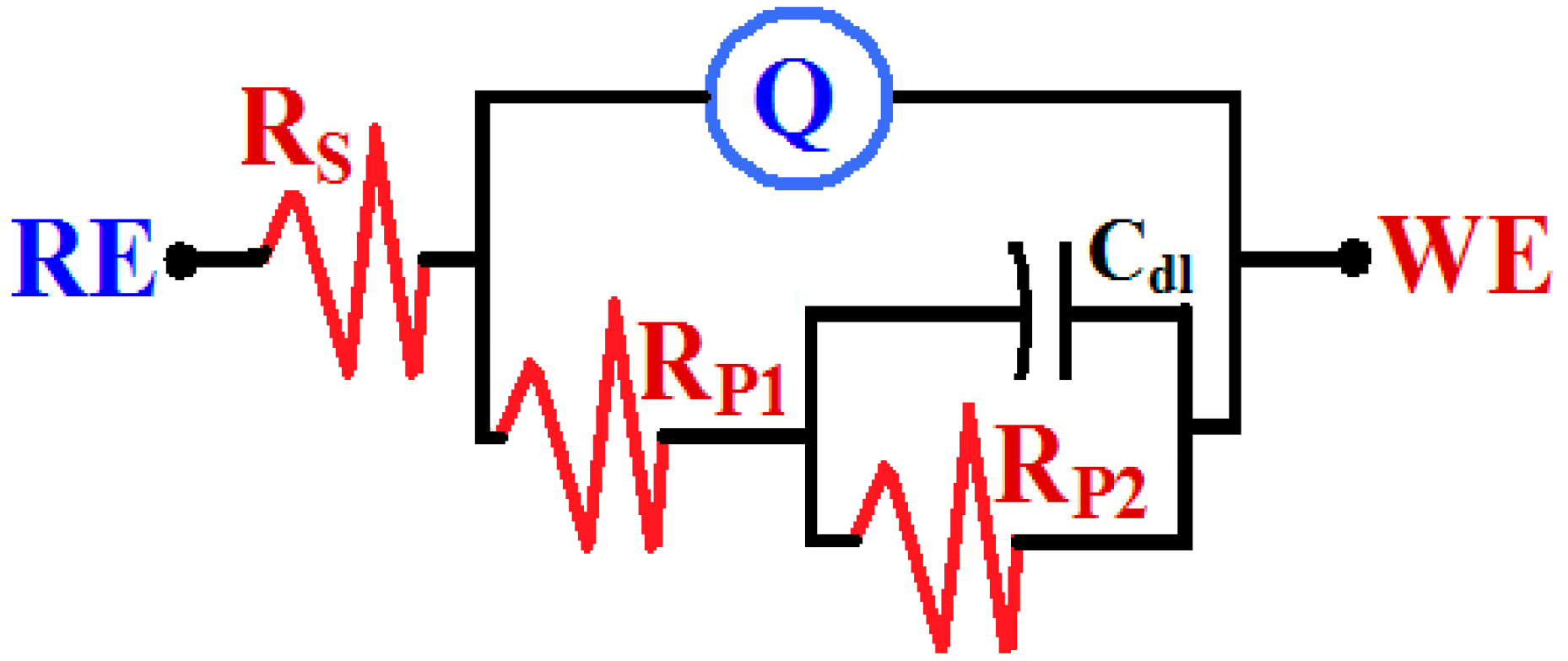
3.3. EIS Measurements
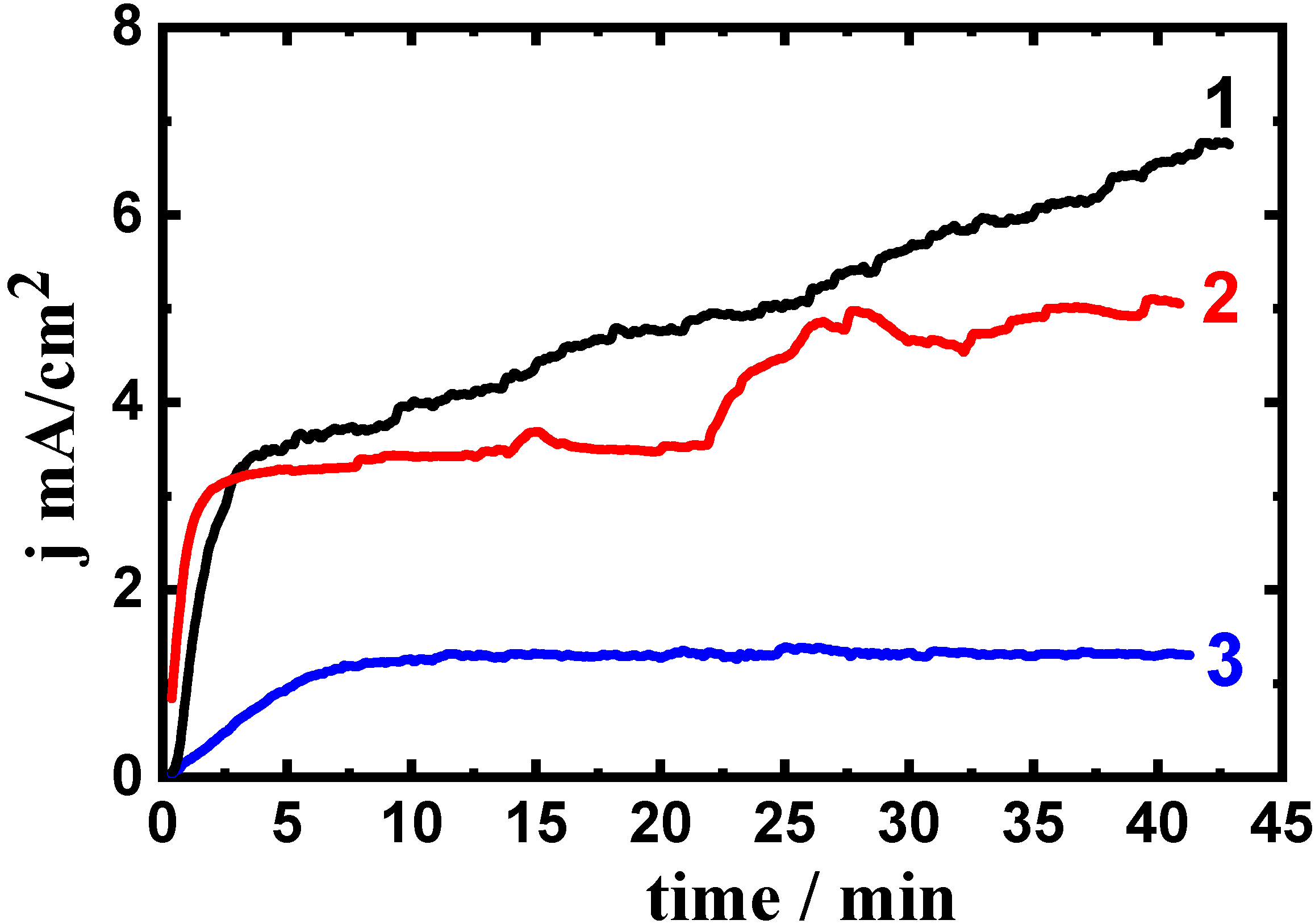
3.4. Current-Time Measurements
3.5. Surface Examinations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miracle, D. Metal matrix composites—From science to technological significance. Compos. Sci. Technol. 2005, 65, 2526–2540. [Google Scholar] [CrossRef]
- Sahin, Y.; Acilar, M. Production and properties of SiCp-reinforced aluminium alloy composites. Compos. Part A 2003, 34, 709–718. [Google Scholar] [CrossRef]
- Hassan, A.M.; Mayyas, A.T.; Alrashdan, A.; Hayajneh, M.T. Wear behavior of Al–Cu and Al–Cu/SiC components produced by powder metallurgy. J. Mater. Sci. 2008, 43, 5368–5375. [Google Scholar] [CrossRef]
- Miracle, D.B.; Donaldson, S.L. ASM Handbook. Composites; ASM International: Cleveland, OH, USA, 2001; Volume 21. [Google Scholar]
- Paciej, R.C.; Agarwala, V.S. Metallurgical variables influencing the corrosion susceptibility of a powder metallurgy aluminum/SiCw composite. Corrosion 1986, 42, 718–729. [Google Scholar] [CrossRef]
- ASM Handbook. Corrosion, 4th ed.; ASM International: Cleveland, OH, USA, 1992; Volume 13.
- Abdo, H.S.; Abdus Samad, U.; Mohammed, J.A.; Ragab, S.A.; Seikh, A.H. Mitigating Corrosion Effects of Ti-48Al-2Cr-2Nb Alloy Fabricated via Electron Beam Melting (EBM) Technique by Regulating the Immersion Conditions. Crystals 2021, 11, 889. [Google Scholar] [CrossRef]
- Abdo, H.S.; Seikh, A.H.; Fouly, A.; Ragab, S.A. Synergistic Strengthening Effect of Reinforcing Spark Plasma Sintered Al-Zn-TiC Nanocomposites with TiC Nanoparticles. Crystals 2021, 11, 842. [Google Scholar] [CrossRef]
- Seah, K.; Krishna, M.; Vijayalakshmi, V.; Uchil, J. Effects of temperature and reinforcement content on corrosion characteristics of LM13/albite composites. Corros. Sci. 2002, 44, 761–772. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nishimura, T.; Matsushima, I. Corrosion resistance of Al-based metal matrix composites. Mater. Sci. Eng. A 1995, 198, 113–118. [Google Scholar] [CrossRef]
- El-Mahallawi, I.; Eigenfeld, K.; Kouta, F.H.; Hussein, A.; Mahmoud, T.S.; Ragaie, R.M.; Shash, A.Y.; Abou-Al-Hassan, W. Synthesis and Characterization of New Cast A356(Al2O3)P Metal Matrix Nano-Composites. In Proceedings of the 2nd Multifunctional Nanocomposites & Nanomaterials: International Conference & Exhibition, Sharm El Sheikh, Egypt, 11–13 January 2008. [Google Scholar] [CrossRef]
- Durai, T.G.; Das, K.; Das, S. Effect of mechanical milling on the corrosion behavior of Al–Zn/Al2O3 composite in NaCl solution. J. Mater. Sci. 2007, 42, 8209–8214. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, B.; Gu, M. Corrosion behavior of Al/AlNp composite in alkaline solution. Mater. Lett. 2006, 60, 2024–2028. [Google Scholar] [CrossRef]
- de Salazar, J.G.; Ureña, A.; Manzanedo, S.; Barrena, M. Corrosion behaviour of AA6061 and AA7005 reinforced with Al2O3 particles in aerated 3.5% chloride solutions: Potentiodynamic measurements and microstructure evaluation. Corros. Sci. 1998, 41, 529–545. [Google Scholar] [CrossRef]
- Kiourtsidis, G.; Skolianos, S.M. Corrosion behavior of squeeze-cast silicon carbide-2024 composites in aerated 3.5 wt.% sodium chloride. Mater. Sci. Eng. A 1998, 248, 165–172. [Google Scholar] [CrossRef]
- Kiourtsidis, G.; Skolianos, S.M. Pitting corrosion of artificially aged T6 AA2024/SiCp composites in 3.5 wt.% NaCl aqueous solution. Corros. Sci. 2007, 49, 2711–2725. [Google Scholar] [CrossRef]
- Abdo, H.; Seikh, A.; Mohammed, J.; Soliman, M. Alloying Elements Effects on Electrical Conductivity and Mechanical Properties of Newly Fabricated Al Based Alloys Produced by Conventional Casting Process. Materials 2021, 14, 3971. [Google Scholar] [CrossRef]
- Trzaskoma, P.P. Pit Morphology of Aluminum Alloy and Silicon Carbide/Aluminum Alloy Metal Matrix Composites. Corrosion 1990, 46, 402–409. [Google Scholar] [CrossRef]
- Zakaria, H. Microstructural and corrosion behavior of Al/SiC metal matrix composites. Ain Shams Eng. J. 2014, 5, 831–838. [Google Scholar] [CrossRef]
- Almotairy, S.M.; Alharthi, N.H.; Alharbi, H.F.; Abdo, H. Superior Mechanical Performance of Inductively Sintered Al/SiC Nanocomposites Processed by Novel Milling Route. Sci. Rep. 2020, 10, 10368. [Google Scholar] [CrossRef] [PubMed]
- Khalil, K.A.; Sherif, E.-S.M.; Nabawy, A.M.; Abdo, H.S.; Marzouk, W.W.; Alharbi, H.F. Titanium Carbide Nanofibers-Reinforced Aluminum Compacts, a New Strategy to Enhance Mechanical Properties. Materials 2016, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Sherif, E.-S.M. Effects of exposure time on the anodic dissolution of Monel-400 in aerated stagnant sodium chloride solutions. J. Solid State Electrochem. 2011, 16, 891–899. [Google Scholar] [CrossRef]
- Latief, F.; Sherif, E.-S.M.; Almajid, A.; Junaedi, H. Fabrication of exfoliated graphite nanoplatelets-reinforced aluminum composites and evaluating their mechanical properties and corrosion behavior. J. Anal. Appl. Pyrolysis 2011, 92, 485–492. [Google Scholar] [CrossRef]
- Alharthi, N.; Sherif, E.-S.M.; Abdo, H.S.; El Abedin, S.Z. Effect of Nickel Content on the Corrosion Resistance of Iron-Nickel Alloys in Concentrated Hydrochloric Acid Pickling Solutions. Adv. Mater. Sci. Eng. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sherif, E.-S.M.; Ragab, S.A.; Abdo, H.S. Role of Vanadium Additions on the Corrosion Mitigation of Ti-6Al-xV Alloy in Simulated Body Fluid. Metals 2020, 10, 903. [Google Scholar] [CrossRef]
- Abdo, H.S.; Sherif, E.-S.M.; El-Serehy, H.A. Manufacturing of Ti-6%Al and Ti-6%Al-4%V Alloys and Their Corrosion in Sodium Chloride Solutions. Crystals 2020, 10, 181. [Google Scholar] [CrossRef] [Green Version]
- Sherif, E.-S.M.; Potgieter, J.; Comins, J.; Cornish, L.; Olubambi, P.; Machio, C. The beneficial effect of ruthenium additions on the passivation of duplex stainless steel corrosion in sodium chloride solutions. Corros. Sci. 2009, 51, 1364–1371. [Google Scholar] [CrossRef]
- Badawy, W.; Al-Kharafi, F.; El-Azab, A. Electrochemical behaviour and corrosion inhibition of Al, Al-6061 and Al–Cu in neutral aqueous solutions. Corros. Sci. 1999, 41, 709–727. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Park, S.-M. Effects of 1,4-naphthoquinone on aluminum corrosion in 0.50 M sodium chloride solutions. Electrochim. Acta 2006, 51, 1313–1321. [Google Scholar] [CrossRef]
- Mazhar, A.; Badawy, W.; Abou-Romia, M. Impedance studies of corrosion resistance of aluminium in chloride media. Surf. Coat. Technol. 1986, 29, 335–345. [Google Scholar] [CrossRef]
- Tomcsányi, L.; Varga, K.; Bartik, I.; Horányi, H.; Maleczki, E. Electrochemical study of the pitting corrosion of aluminium and its alloys—II. Study of the interaction of chloride ions with a passive film on aluminium and initiation of pitting corrosion. Electrochim. Acta 1989, 34, 855–859. [Google Scholar] [CrossRef]
- Vera, M.L.; Linardi, E.; Lanzani, L.; Mendez, C.; Schvezov, C.E.; Ares, A.E. Corrosion resistance of titanium dioxide anodic coatings on Ti-6Al-4V. Mater. Corros. 2015, 66, 1140–1149. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Abdo, H.S.; Alharthi, N.H. Beneficial Effects of Vanadium Additions on the Corrosion of Ti6AlxV Alloys in Chloride Solutions. Metals 2020, 10, 264. [Google Scholar] [CrossRef] [Green Version]
- Diamanti, M.V.; Bolzoni, F.M.; Ormellese, M.; Pérez-Rosales, E.A.; Pedeferri, M. Characterisation of titanium oxide films by potentiodynamic polarisation and electrochemical impedance spectroscopy. Corros. Eng. Sci. Technol. 2010, 45, 428–434. [Google Scholar] [CrossRef]
- Foley, R.T.; Nguyen, T.H. The Chemical Nature of Aluminum Corrosion: V. Energy Transfer in Aluminum Dissolution. J. Electrochem. Soc. 1982, 129, 464–467. [Google Scholar] [CrossRef]
- Hunkeler, F.; Frankel, G.S.; Bohni, H. Technical Note: On the Mechanism of Localized Corrosion. Corrosion 1987, 43, 189–191. [Google Scholar] [CrossRef]
- Sato, N. The stability of localized corrosion. Corros. Sci. 1995, 37, 1947–1967. [Google Scholar] [CrossRef]
- Natishan, P.M.; McCafferty, E.; Hubler, G.K. Surface Charge Considerations in the Pitting of Ion-Implanted Aluminum. J. Electrochem. Soc. 1988, 135, 321–327. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Park, S.-M. Effects of 1,5-Naphthalenediol on Aluminum Corrosion as a Corrosion Inhibitor in 0.50 M NaCl. J. Electrochem. Soc. 2005, 152, B205–B211. [Google Scholar] [CrossRef] [Green Version]




| Sample Code | Phase 1 | Phase 2 | Phase 3 | |||
|---|---|---|---|---|---|---|
| Speed (rpm) | Time (h) | Speed (rpm) | Time (h) | Speed (rpm) | Time (h) | |
| Route (1) | 150 | 8 | 300 | 4 | - | - |
| Route (2) | 150 | 8 | 300 | 4 | 150 | 2 |
| Route (3) | 150 | 8 | 300 | 4 | 450 | 1 |
| Nanocomposite/Time | βc/mV·dec−1 | ECorr/mV | βa/mV·dec−1 | jCorr/µA·cm−2 | EPit/mV | RP/Ω·cm2 | RCorr/mmpy |
|---|---|---|---|---|---|---|---|
| Nanocomposite 1 (1 h) | 85 ± 5 | −1168 ± 2 | 130 ± 2 | 7.5 ± 0.2 | −700 ± 5 | 2980 ± 10 | 0.082 ± 0.003 |
| Nanocomposite 2 (1 h) | 90 ± 5 | −1100 ± 3 | 120 ± 5 | 4.2 ± 0.3 | −680 ± 5 | 5323 ± 12 | 0.046 ± 0.004 |
| Nanocomposite 3 (1 h) | 95 ± 4 | −960 ± 5 | 130 ± 3 | 1.3 ± 0.2 | −695 ± 5 | 18357 ± 10 | 0.014 ± 0.002 |
| Nanocomposite 1 (24 h) | 93 ± 4 | −1118 ± 2 | 123 ± 3 | 5.8 ± 0.2 | −665 ± 5 | 3970 ± 10 | 0.063 ± 0.002 |
| Nanocomposite 2 (24 h) | 91 ± 4 | −970 ± 4 | 125 ± 5 | 3.4 ± 0.1 | −620 ± 5 | 6734 ± 6 | 0.037 ± 0.003 |
| Nanocomposite 3 (24 h) | 120 ± 5 | −875 ± 5 | 110 ± 5 | 0.40 ± 0.1 | −680 ± 5 | 43478 ± 6 | 0.004 ± 0.003 |
| Nanocomposite 1 (48 h) | 93 ± 7 | −1080 ± 5 | 127 ± 3 | 3.7 ± 0.3 | −705 ± 5 | 6309 ± 11 | 0.041 ± 0.004 |
| Nanocomposite 2 (48 h) | 93 ± 7 | −1065 ± 5 | 128 ± 2 | 2.5 ± 0.2 | −680 ± 5 | 9368 ± 2 | 0.027 ± 0.005 |
| Nanocomposite 3 (48 h) | 105 ± 5 | −1045 ± 5 | 160 ± 5 | 2.1 ± 0.2 | −670 ± 5 | 13126 ± 2 | 0.023 ± 0.005 |
| Nanocomposite/Time | Impedance Data | |||||
|---|---|---|---|---|---|---|
| RS/Ω·cm2 | Q | RP1/Ω·cm2 | Cdl/F·cm−2 | RP2/Ω·cm2 | ||
| YQ/F·cm−2 | n | |||||
| Nanocomposite 1 (1 h) | 13.2 ± 0.3 | 0.0658 ± 0.002 | 0.85 ± 0.05 | 220 ± 5 | 0.0485 ± 0.005 | 1632 ± 3 |
| Nanocomposite 2 (1 h) | 15.8 ± 0.2 | 0.0574 ± 0.001 | 0.87 ± 0.03 | 389 ± 6 | 0.0299 ± 0.006 | 4124 ± 6 |
| Nanocomposite 3 (1 h) | 16.2 ± 0.5 | 0.0469 ± 0.003 | 0.88 ± 0.04 | 490 ± 6 | 0.0192 ± 0.003 | 5221 ± 5 |
| Nanocomposite 1 (24 h) | 14.5 ± 0.5 | 0.0587 ± 0.003 | 0.86 ± 0.04 | 314 ± 6 | 0.0367 ± 0.003 | 3745 ± 5 |
| Nanocomposite 2 (24 h) | 16.0 ± 0.6 | 0.0489 ± 0.006 | 0.88 ± 0.02 | 561 ± 4 | 0.0234 ± 0.006 | 5987 ± 8 |
| Nanocomposite 3 (24 h) | 16.8 ± 0.6 | 0.0396 ± 0.006 | 0.89 ± 0.02 | 643 ± 4 | 0.0145 ± 0.006 | 6579 ± 8 |
| Nanocomposite 1 (48 h) | 15.3 ± 0.7 | 0.0421 ± 0.004 | 0.87 ± 0.03 | 524 ± 6 | 0.0347 ± 0.003 | 4589 ± 6 |
| Nanocomposite 1 (48 h) | 16.4 ± 0.7 | 0.0355 ± 0.004 | 0.89 ± 0.03 | 712 ± 6 | 0.0265 ± 0.003 | 5731 ± 6 |
| Nanocomposite 3 (48 h) | 17.1 ± 0.9 | 0.0298 ± 0.002 | 0.91 ± 0.05 | 755 ± 5 | 0.0142 ± 0.008 | 8951 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almotairy, S.M.; Sherif, E.-S.M.; Alharthi, N.H.; Abdo, H.S.; Alharbi, H.F.; Luqman, M. Influence of Milling Route on the Corrosion Passivation of Al-2%SiC Nanocomposites in Chloride Solutions. Crystals 2021, 11, 1231. https://doi.org/10.3390/cryst11101231
Almotairy SM, Sherif E-SM, Alharthi NH, Abdo HS, Alharbi HF, Luqman M. Influence of Milling Route on the Corrosion Passivation of Al-2%SiC Nanocomposites in Chloride Solutions. Crystals. 2021; 11(10):1231. https://doi.org/10.3390/cryst11101231
Chicago/Turabian StyleAlmotairy, Saud M., El-Sayed M. Sherif, Nabeel H. Alharthi, Hany S. Abdo, Hamad F. Alharbi, and Monis Luqman. 2021. "Influence of Milling Route on the Corrosion Passivation of Al-2%SiC Nanocomposites in Chloride Solutions" Crystals 11, no. 10: 1231. https://doi.org/10.3390/cryst11101231








