Kappa Carbide Precipitation in Duplex Fe-Al-Mn-Ni-C Low-Density Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Thermo-Calc Calculations
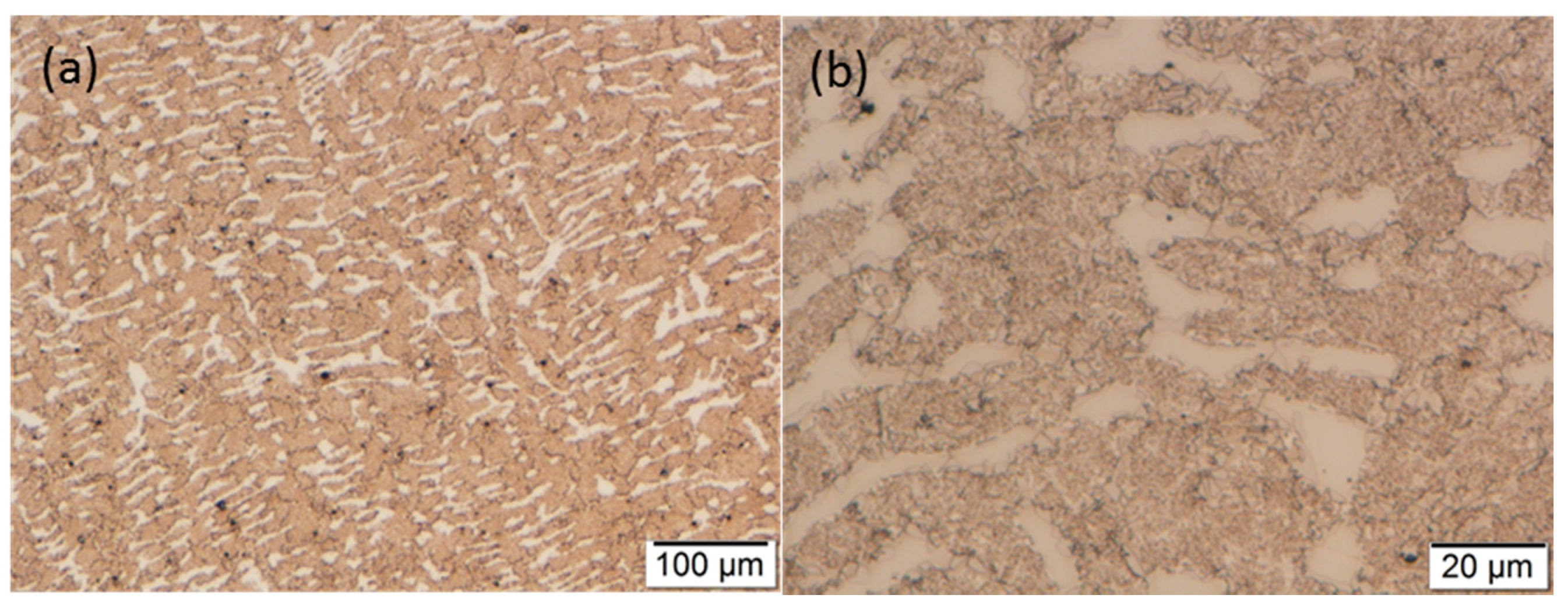
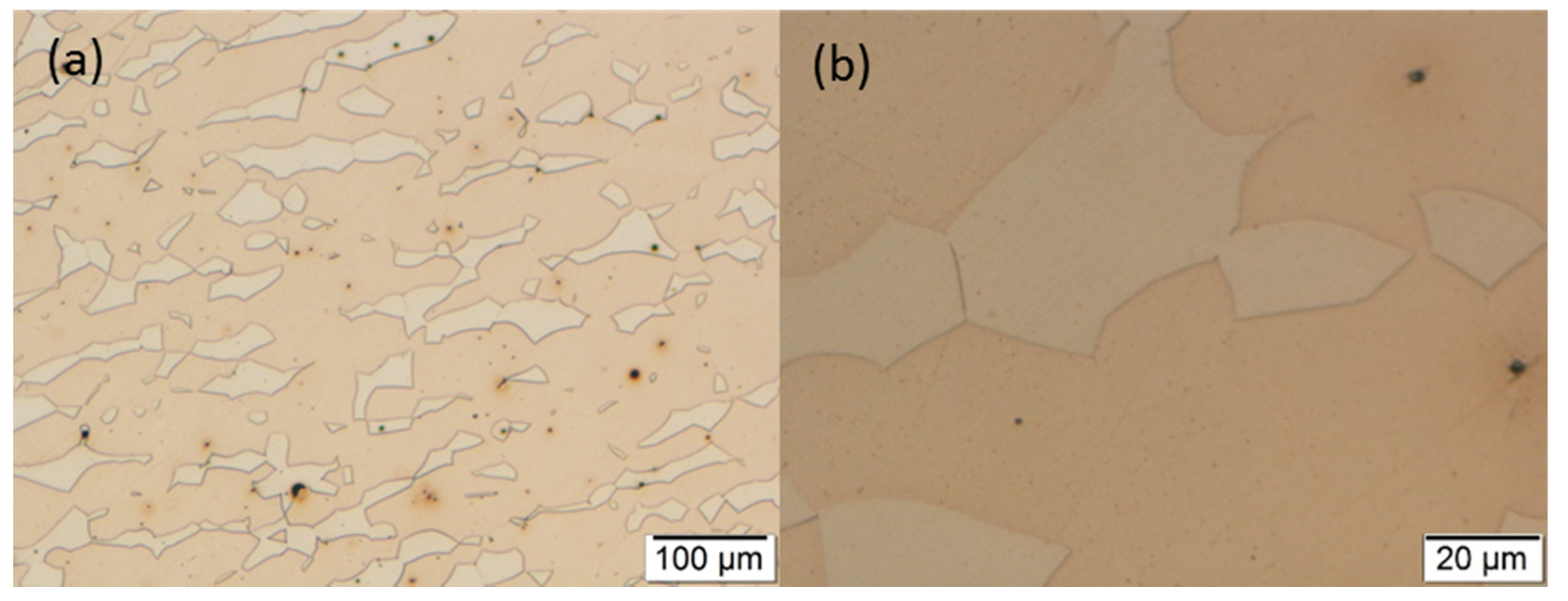
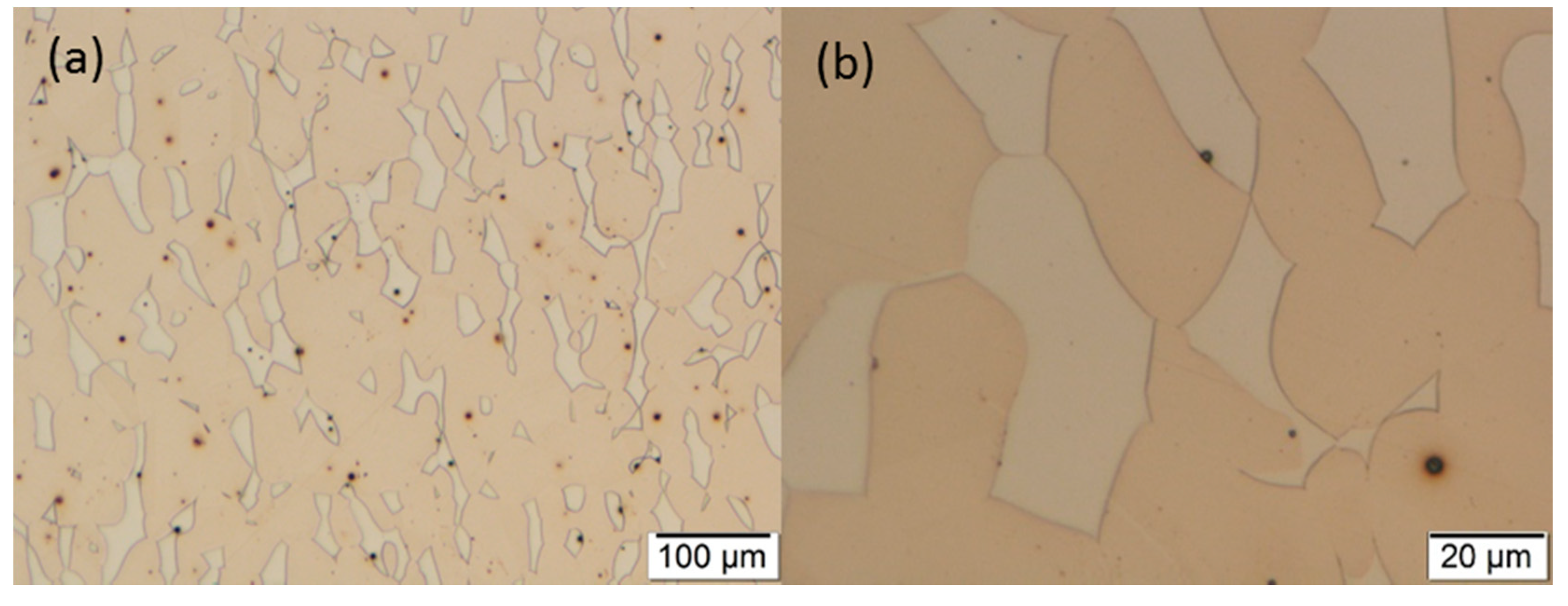
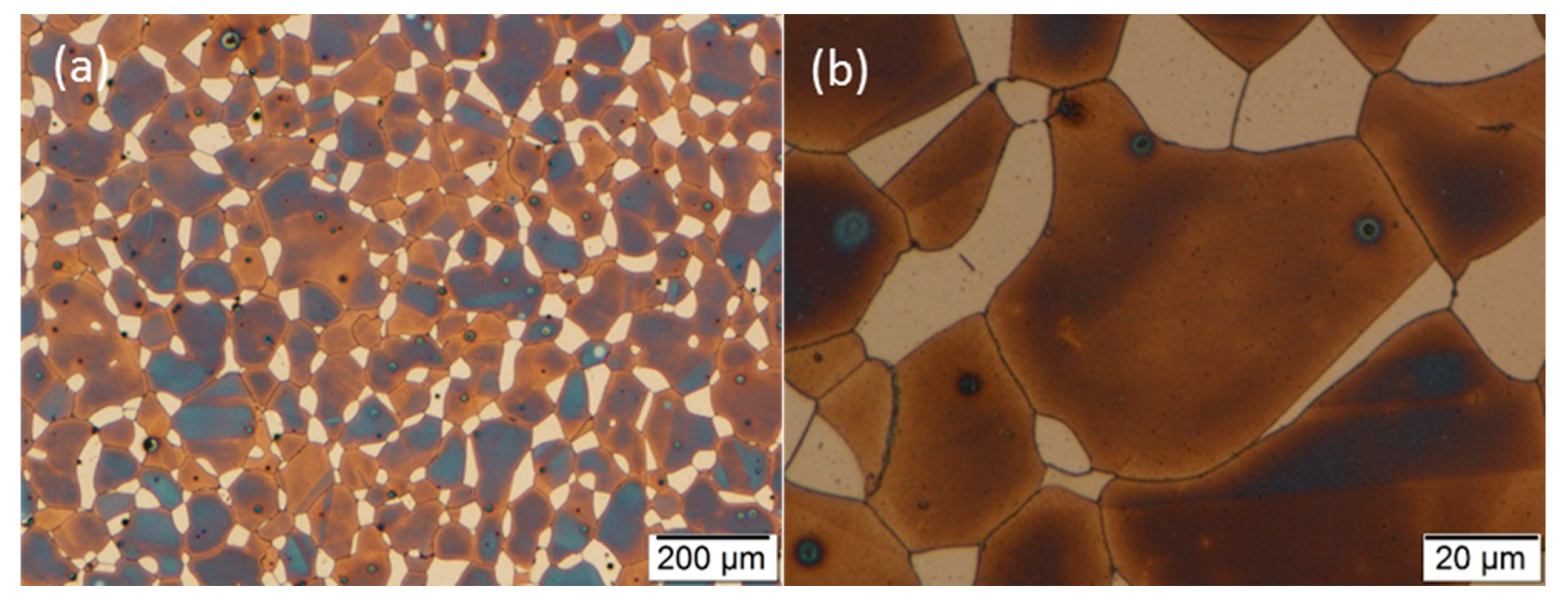
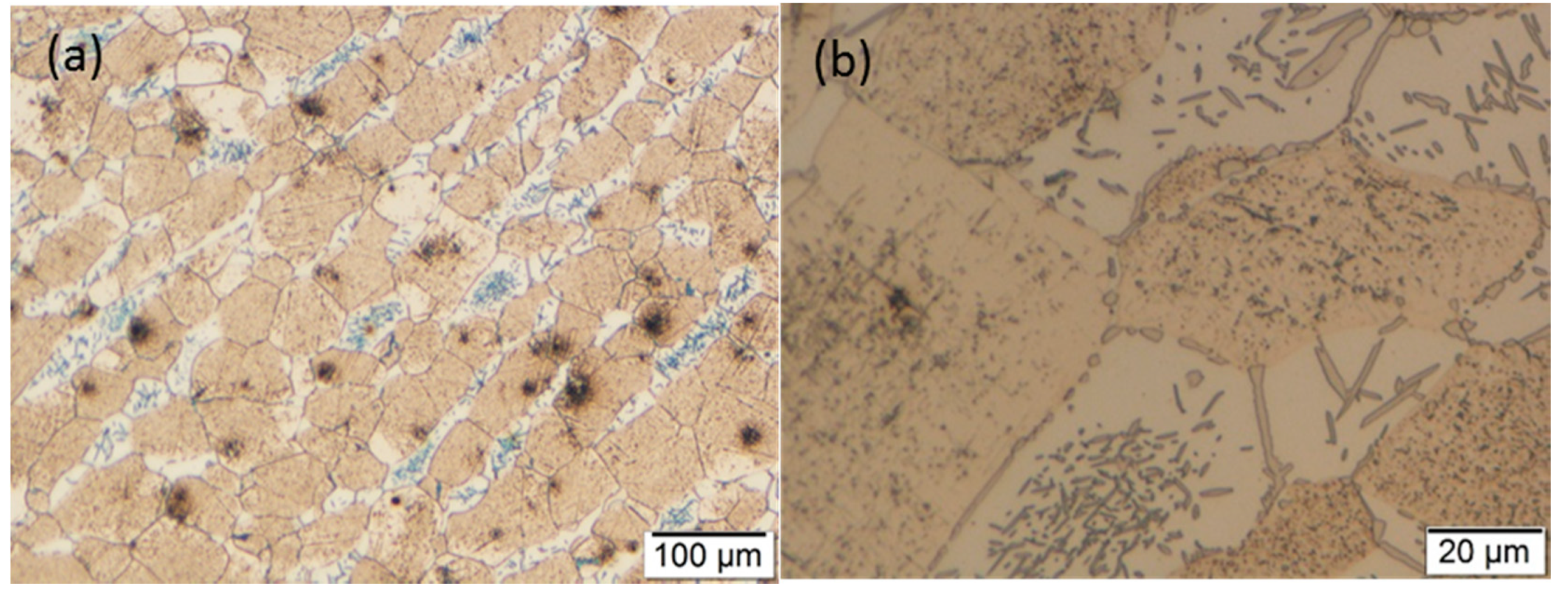
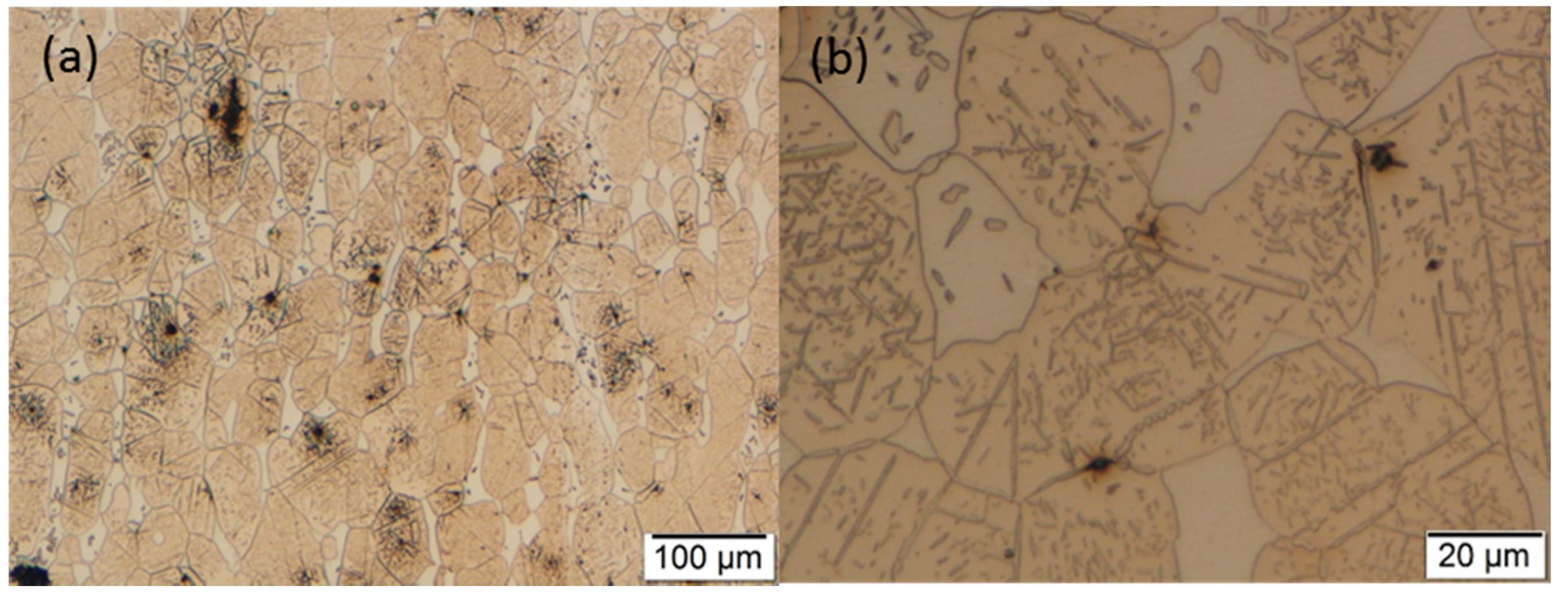
3.2. Optical Microscopy
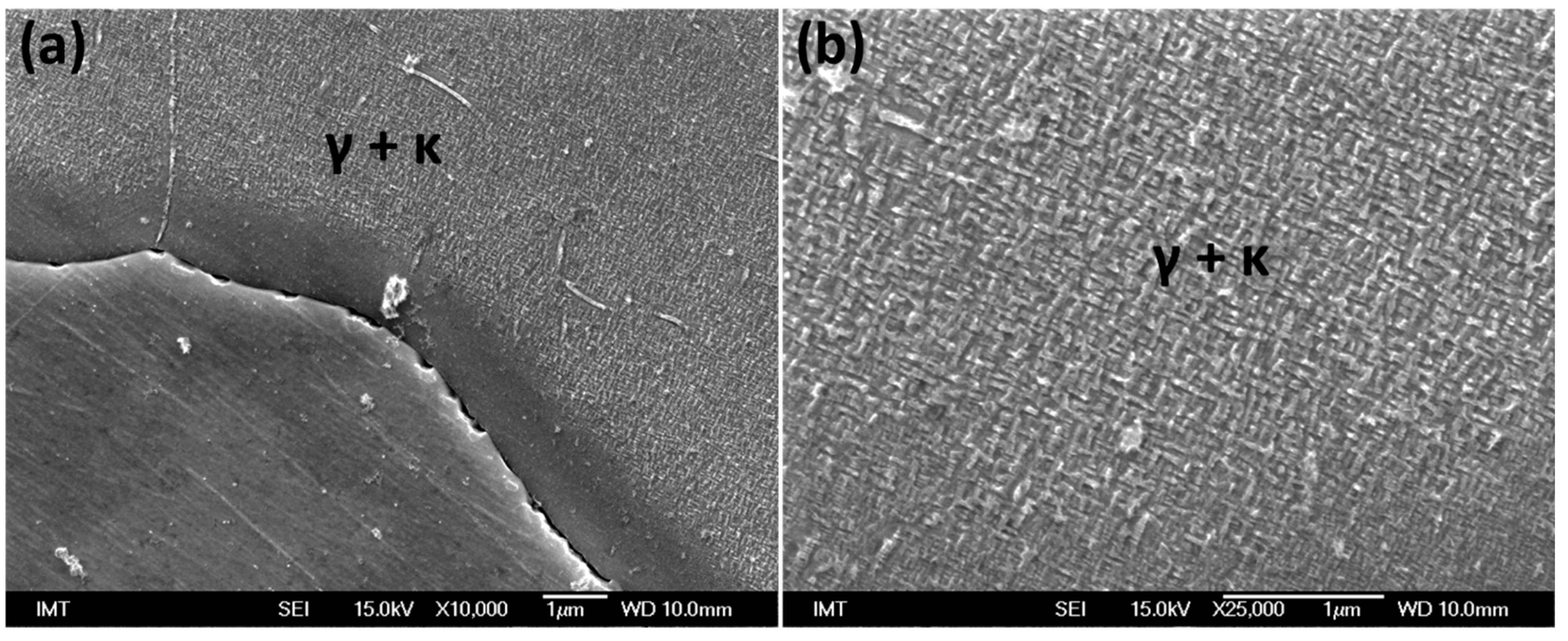
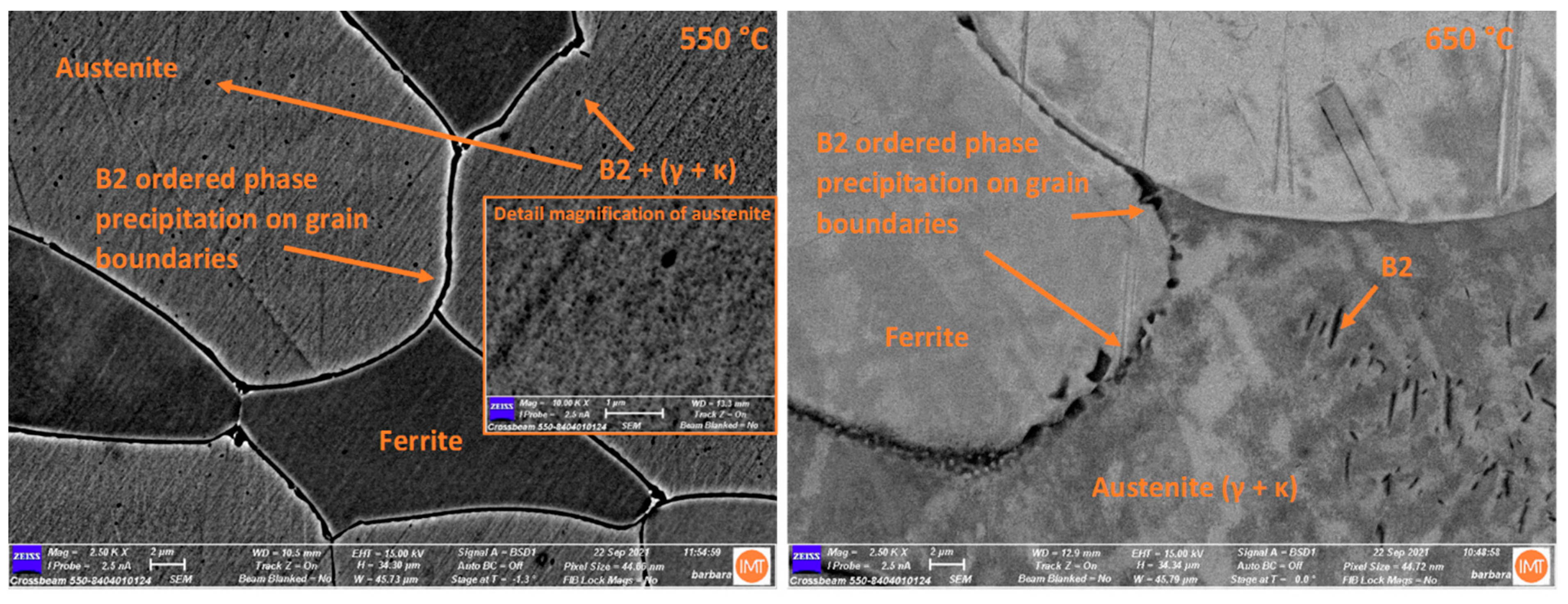
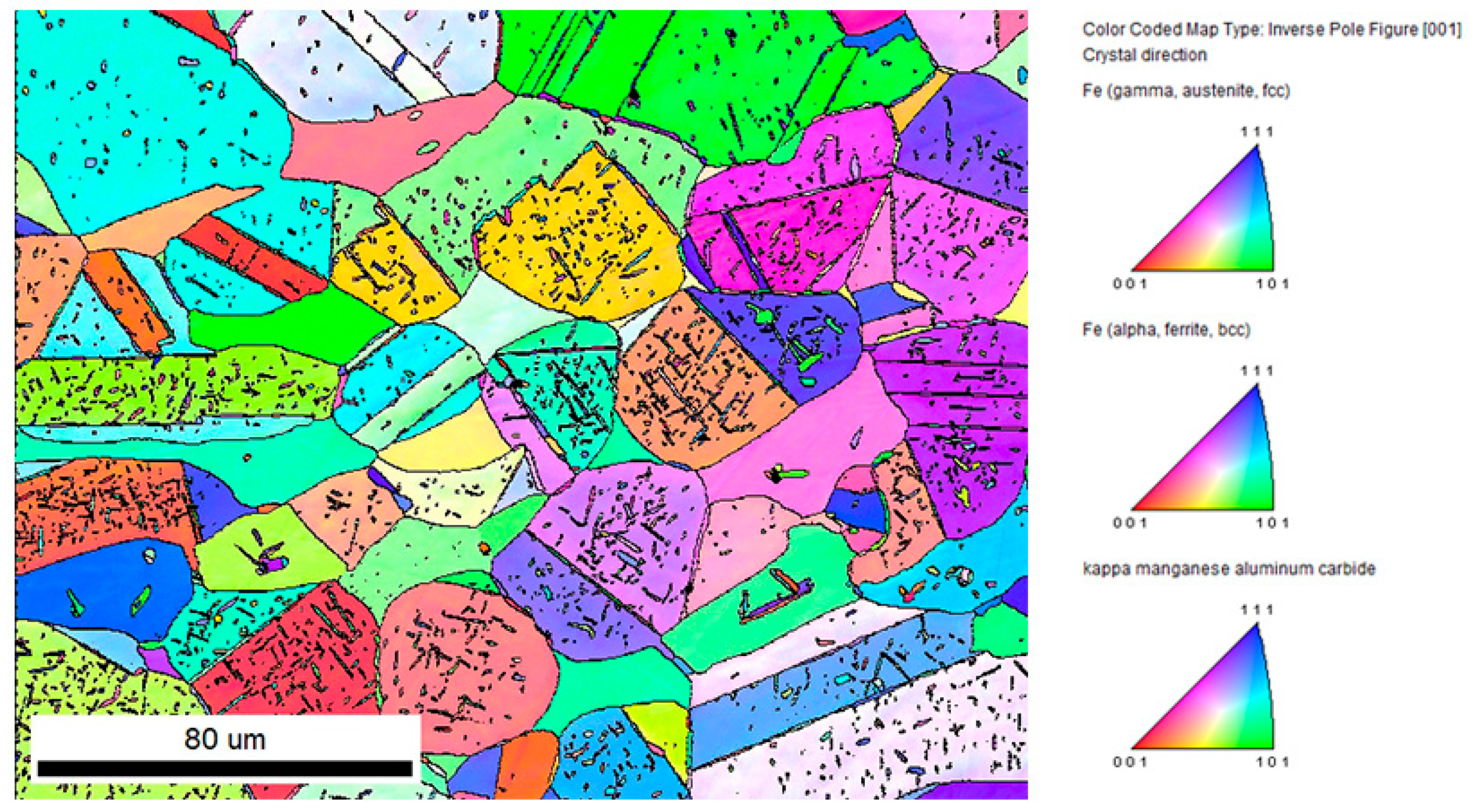
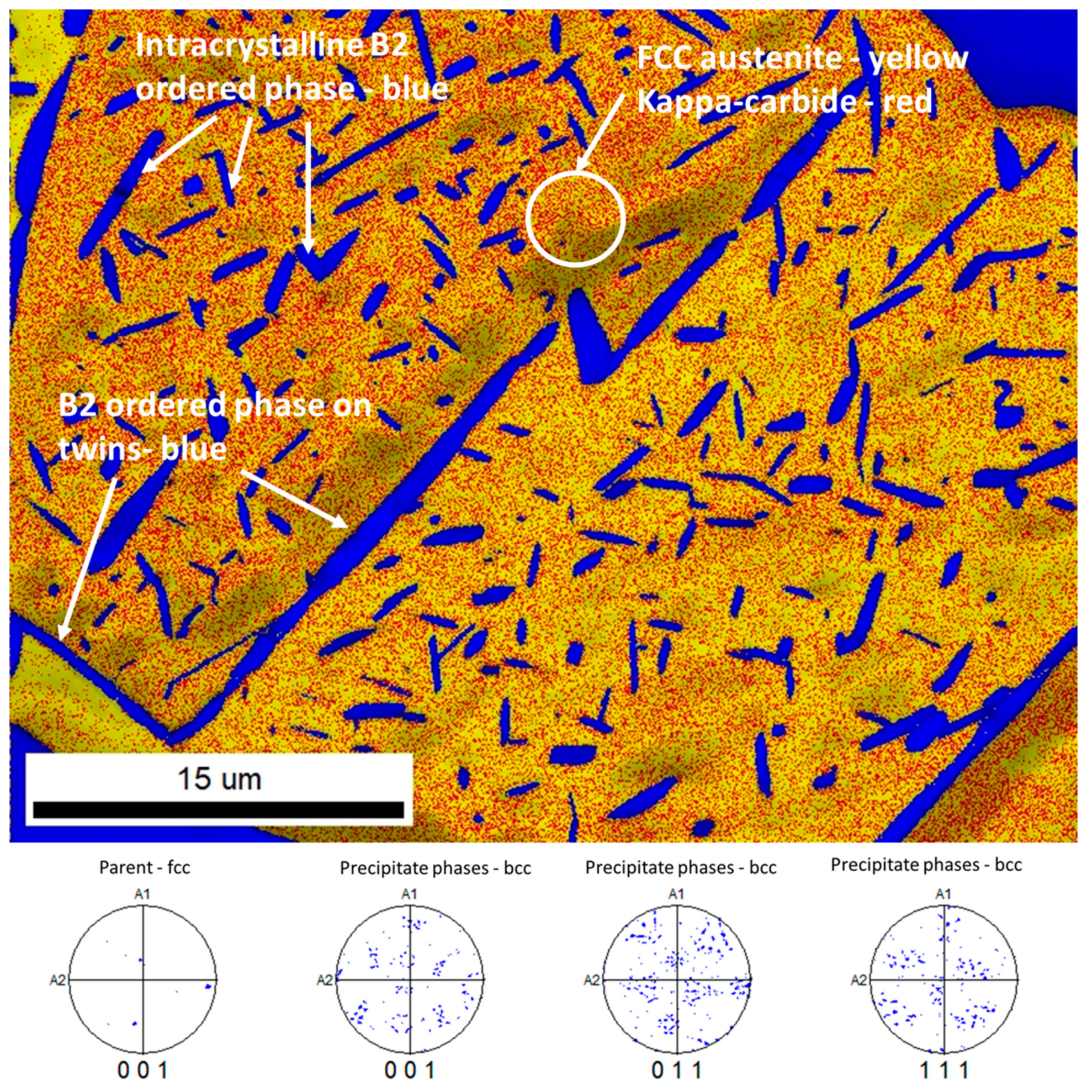
3.3. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, S.; Rana, R.; Haldar, A.; Ray, R.K. Current state of Fe-Mn-Al-C low density steels. Prog. Mater. Sci. 2017, 89, 345–391. [Google Scholar] [CrossRef]
- Howell, R.A.; Van Aken, D.C. A literature review of age hardening Fe-Mn-Al-C alloys. Iron Steel Technol. 2009, 6, 193–212. [Google Scholar]
- Rana, R.; Lahaye, C.; Ray, R.K. Overview of Lightweight Ferrous Materials: Strategies and Promises. JOM 2014, 66, 1734–1746. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, L.; Van Aken, D. High Manganese and Aluminum Steels for the Military and Transportation Industry. JOM 2014, 66, 1770–1784. [Google Scholar] [CrossRef]
- Rana, R. Low-Density Steels. JOM 2014, 66, 1730–1733. [Google Scholar] [CrossRef] [Green Version]
- Frommeyer, G.; Brox, U. Microstructures and Mechanical Properties of High-Strength Fe-Mn-AI-C Light-Weight TRIPLEX Steels. Steel Res. Int. 2006, 77, 627–633. [Google Scholar] [CrossRef]
- Kalashnikov, I.; Acselrad, O.; Shalkevich, A.; Pereira, L.C. Chemical composition optimization for austenitic steels of the Fe-Mn-Al-C system. J. Mater. Eng. Perform. 2000, 9, 597–602. [Google Scholar] [CrossRef]
- Sutou, Y.; Kamiya, N.; Umino, R.; Ohnuma, L.; Ishida, K. High-strength Fe-2OMn-Al-C-based alloys with low density. ISIJ Int. 2010, 50, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Raabe, D.; Springer, H.; Gutierrez-Urrutia, I.; Roters, F.; Bausch, M.; Seol, J.B.; Koyama, M.; Choi, P.P.; Tsuzaki, K. Alloy Design, Combinatorial Synthesis, and Microstructure–Property Relations for Low-Density Fe-Mn-Al-C Austenitic Steels. JOM 2014, 66, 1845–1856. [Google Scholar] [CrossRef]
- Jahn, M.T.; Chang, S.C.; Hsiao, Y.H. Transverse tensile and fatigue properties of Fe-Mn-Al-C alloys. J. Mater. Sci. Lett. 1989, 8, 723–724. [Google Scholar] [CrossRef]
- Kayak, G.L. Fe-Mn-Al precipitation-hardening austenitic alloys. Met. Sci. Heat Treat. 1969, 11, 95–97. [Google Scholar] [CrossRef]
- Zuazo, I.; Hallstedt, B.; Lindahl, B.; Selleby, M.; Soler, M.; Etienne, A.; Perlade, A.; Hasenpouth, D.; Cazottes, S.; Kleber, X.; et al. Low-Density Steels: Complex Metallurgy for Automotive Applications. JOM 2014, 66, 1747–1758. [Google Scholar] [CrossRef]
- Chen, P.; Li, X.; Yi, H. The κ-carbides in low-density fe-mn-al-c steels: A review on their structure, precipitation and deformation mechanism. Metals 2020, 10, 1021. [Google Scholar] [CrossRef]
- Cheng, W.C.; Song, Y.S.; Lin, Y.S.; Chen, K.F.; Pistorius, P.C. On the eutectoid reaction in a quaternary Fe-C-Mn-Al alloy: Austenite → ferrite + kappa-carbide + M23C6 carbide. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 1199–1216. [Google Scholar] [CrossRef]
- Bartlett, L.N.; Van Aken, D.C.; Medvedeva, J.; Isheim, D.; Medvedeva, N.I.; Song, K. An atom probe study of kappa carbide precipitation and the effect of silicon addition. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 2421–2435. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.C.; Cheng, C.Y.; Hsu, C.W.; Laughlin, D.E. Phase transformation of the L12 phase to kappa-carbide after spinodal decomposition and ordering in an Fe-C-Mn-Al austenitic steel. Mater. Sci. Eng. A 2015, 642, 128–135. [Google Scholar] [CrossRef]
- Rahnama, A.; Kotadia, H.; Clark, S.; Janik, V.; Sridhar, S. Nano-mechanical properties of Fe-Mn-Al-C lightweight steels. Sci. Rep. 2018, 8, 9065. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, C.; Barella, S.; Gruttadauria, A.; Mombelli, D.; Bizzozero, M.; Veys, X. γ Decomposition in Fe–Mn–Al–C lightweight steels. J. Mater. Res. Technol. 2020, 9, 4604–4616. [Google Scholar] [CrossRef]
- Kimura, Y.; Handa, K.; Hayashi, K.; Mishima, Y. Microstructure control and ductility improvement of the two-phase γ-Fe/κ-(Fe, Mn)3AlC alloys in the Fe-Mn-Al-C quaternary system. Intermetallics 2004, 12, 607–617. [Google Scholar] [CrossRef]
- Andersson, J.-O.; Thomas, H.; Lars, H.; Pingfang, S.; Bo, S. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Thermo-Calc Software. TCFE10: TCS Steel and Fe-Alloys Database; Thermo-Calc AB: Stockholm, Sweden, 2019. [Google Scholar]





| C | Si | Cu | Ni | Mn | Al | Fe |
|---|---|---|---|---|---|---|
| 0.82 | 0.2 | 0.17 | 5.6 | 14.8 | 10.0 | balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burja, J.; Šetina Batič, B.; Balaško, T. Kappa Carbide Precipitation in Duplex Fe-Al-Mn-Ni-C Low-Density Steel. Crystals 2021, 11, 1261. https://doi.org/10.3390/cryst11101261
Burja J, Šetina Batič B, Balaško T. Kappa Carbide Precipitation in Duplex Fe-Al-Mn-Ni-C Low-Density Steel. Crystals. 2021; 11(10):1261. https://doi.org/10.3390/cryst11101261
Chicago/Turabian StyleBurja, Jaka, Barbara Šetina Batič, and Tilen Balaško. 2021. "Kappa Carbide Precipitation in Duplex Fe-Al-Mn-Ni-C Low-Density Steel" Crystals 11, no. 10: 1261. https://doi.org/10.3390/cryst11101261
APA StyleBurja, J., Šetina Batič, B., & Balaško, T. (2021). Kappa Carbide Precipitation in Duplex Fe-Al-Mn-Ni-C Low-Density Steel. Crystals, 11(10), 1261. https://doi.org/10.3390/cryst11101261








