Fabrication of La2O3/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance of Tetracycline Hydrochloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nanocomposites
2.3. Characterization
2.4. Photocatalytic Activity Test
3. Results and Discussion
3.1. XRD Analysis
3.2. FESEM and TEM Analysis
3.3. FTIR Analysis
3.4. XPS Analysis
3.5. UV–Vis Analysis
3.6. PL Analysis
3.7. Photocatalyst Activity
3.8. Stability and Active Species
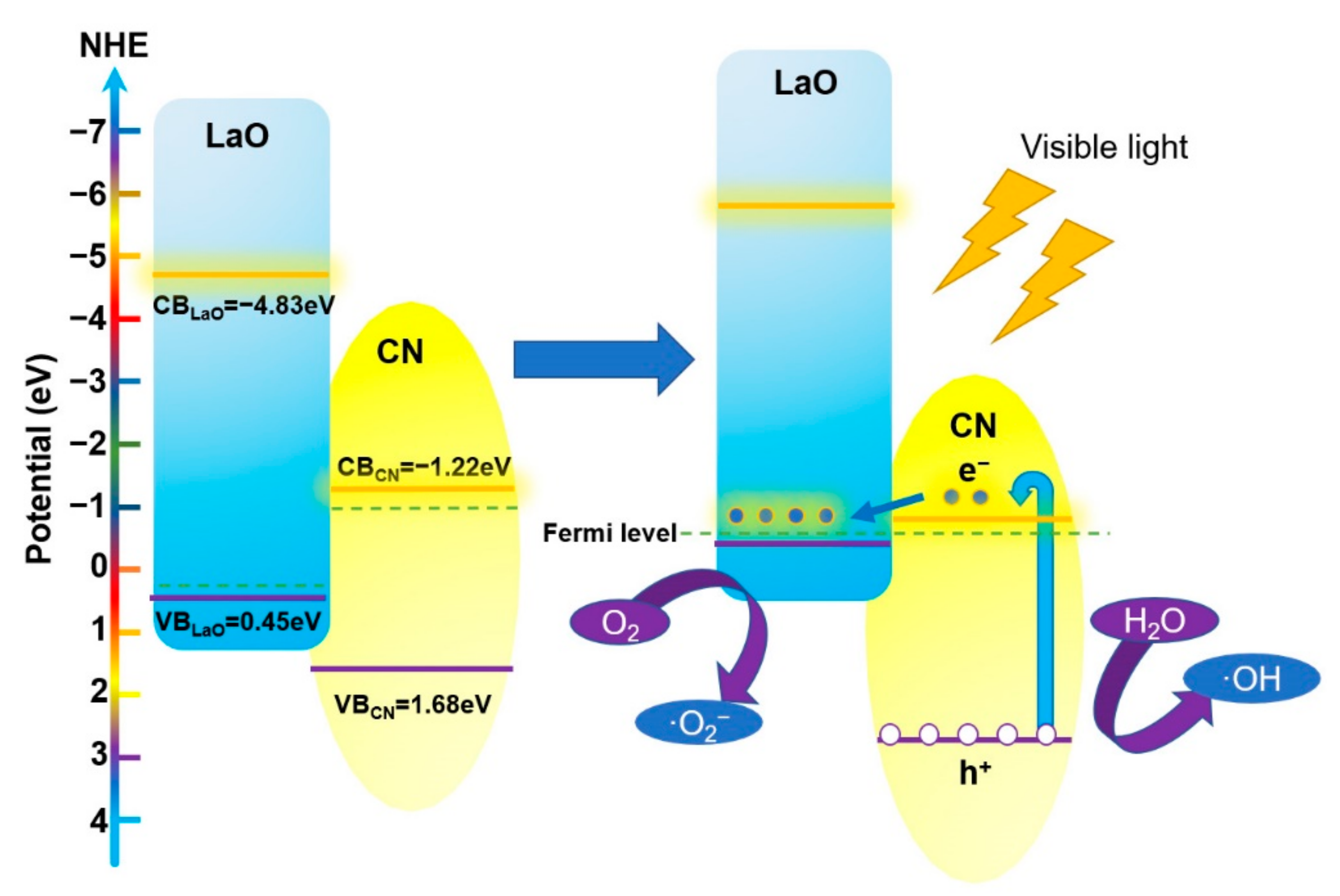
3.9. Photocatalytic Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 1704376. [Google Scholar] [CrossRef]
- Adenuga, D.O.; Tichapondwa, S.M.; Chirwa, E.M. Facile synthesis of a Ag/AgCl/BiOCl composite photocatalyst for visible—light—driven pollutant removal. J. Photochem. Photobiol. A Chem. 2020, 401, 112747. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Mathe, V.L. Oxidative degradation of phthalic acid using TiO2 photocatalyst. J. Mater. Sci. Mater. Electron. 2018, 29, 6183–6187. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, G.; Wang, J. Photo-Fenton degradation of rhodamine B using Fe2O3–Kaolin as heterogeneous catalyst: Characterization, process optimization and mechanism. J. Colloid Interface Sci. 2014, 433, 1–8. [Google Scholar] [CrossRef]
- An, L.; Wang, G.; Zhao, L.; Zhou, Y.; Gao, F.; Cheng, Y. Hexagonal pencil-like CdS nanorods: Facile synthesis and enhanced visible light photocatalytic performance. Russ. J. Phys. Chem. A 2015, 89, 1195–1200. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Sun, S.; Zhou, L.; Zhang, L. Bi2WO6 Nanocrystals with High Photocatalytic Activities under Visible Light. J. Phys. Chem. C 2008, 112, 10407–10411. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2008, 8, 76–80. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, L.; Chang, M.-J.; Yuan, B.; Sun, M.; Fan, S.-M.; Cui, W.-N.; Hui, Q.; Ni, F.-R.; Li, M.-Y.; et al. Fabrication of novel fibrous BiVO4/CdS heterostructures by electrospinning method for efficient visible light photodegradation. Mater. Chem. Phys. 2020, 247, 122858. [Google Scholar] [CrossRef]
- Darvishi-Farash, S.; Afsharpour, M.; Heidarian, J. Novel siligraphene/g-C3N4 composites with enhanced visible light photocatalytic degradations of dyes and drugs. Environ. Sci. Pollut. Res. 2020, 28, 5938–5952. [Google Scholar] [CrossRef]
- Kadam, S.R.; Ghosh, S.; Bar-Ziv, R.; Bar-Sadan, M. Structural Transformation of SnS2 to SnS by Mo Doping Produces Electro/Photocatalyst for Hydrogen Production. Chem.—A Eur. J. 2020, 26, 6679–6685. [Google Scholar] [CrossRef]
- Kaviyarasan, K.; Sivasankar, T.; Ponnusamy, V.K.; Anandan, S. Ni–ZnO nanocomposites assembled under various morphologies like columnar, nanochains, and granular structure for removal of pollutants. Mater. Chem. Phys. 2020, 252, 123299. [Google Scholar] [CrossRef]
- Zhu, Z.; Han, S.; Cao, Y.; Jiang, J. Synthesis of a Novel Photocatalyst MVO4/g-C3N4 (M = La, Gd) with Better Photocatalytic Activity for Tetracycline Hydrochloride Degradation under Visible-Light Irradiation. Crystals 2021, 11, 756. [Google Scholar] [CrossRef]
- Xiong, S.; Liu, X.; Zhu, X.; Liang, G.; Jiang, Z.; Cui, B.; Bai, J. One-step preparation of well-dispersed spindle-like Fe2O3 nanoparticles on g-C3N4 as highly efficient photocatalysts. Ecotoxicol. Environ. Saf. 2020, 208, 111519. [Google Scholar] [CrossRef]
- Sun, J.-X.; Yuan, Y.-P.; Qiu, L.-G.; Jiang, X.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. Fabrication of composite photocatalyst g-C3N4–ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 2012, 41, 6756–6763. [Google Scholar] [CrossRef]
- Panimalar, S.; Uthrakumar, R.; Selvi, E.T.; Gomathy, P.; Inmozhi, C.; Kaviyarasu, K.; Kennedy, J. Studies of MnO2/g-C3N4 hetrostructure efficient of visible light photocatalyst for pollutants degradation by sol-gel technique. Surf. Interfaces 2020, 20, 100512. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Duong, A.T.T.; Nguyen, B.D.; Hai, N.Q.; Chu, V.H.; Nguyen, T.D.; Bach, L.G. Preparation, Characterization and Photocatalytic Activity of La-Doped Zinc Oxide Nanoparticles. Materials 2019, 12, 1195. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Zhang, C.; Cui, S.; Liang, Q.; Xu, S.; Li, Z. In situ Synthesis of p–n LaFeO3/ZnIn2S4 Heterojunctions for Enhanced Photocatalytic Activity. Nano 2019, 14, 1950096. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Li, C.; Hu, R.; Qin, L.; Ding, R.; Li, X.; Wu, H. Enhanced photocatalytic activity of ZnO/La2O3 composite modified by potassium for phenol degradation. Mater. Lett. 2013, 113, 190–194. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Z.; Jiang, J.; Li, H. Fabrication of a novel AgI/LaFeO3/g-C3N4 dual Z-scheme photocatalyst with enhanced photocatalytic performance. Mater. Lett. 2019, 262, 127029. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Wang, X. CeVO4 nanofibers hybridized with g -C3N4 nanosheets with enhanced visible-light-driven photocatalytic activity. Solid State Commun. 2017, 269, 11–15. [Google Scholar] [CrossRef]
- Chuaicham, C.; Karthikeyan, S.; Pawar, R.R.; Xiong, Y.; Dabo, I.; Ohtani, B.; Kim, Y.; Song, J.T.; Ishihara, T.; Sasaki, K. Energy-resolved distribution of electron traps for O/S-doped carbon nitrides by reversed double-beam photoacoustic spectroscopy and the photocatalytic reduction of Cr(vi). Chem. Commun. 2020, 56, 3793–3796. [Google Scholar] [CrossRef]
- Balakumar, V.; Sekar, K.; Chuaicham, C.; Manivannan, R.; Sasaki, K. Synergistic ternary porous CN–PPy–MMt nanocomposite for efficient photocatalytic metronidazole mineralization: Performance, mechanism, and pathways. Environ. Sci. Nano 2021, 8, 2261–2276. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Zhou, H.; Li, T.; Zhang, L. A Z-scheme mechanism of N-ZnO/g-C3N4 for enhanced H2 evolution and photocatalytic degradation. Appl. Surf. Sci. 2018, 466, 133–140. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zeng, J. Ternary heterojunctions catalyst of BiOCl nanosheets with the {001} facets compounded of Pt and reduced graphene oxide for enhancing photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021, 32, 2667–2684. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Al-Saeedi, S.I.; Al-Senani, G.M.; Nafady, A.; Ahamad, T.; Naushad, M.; Stadler, F.J. Fabrication of oxidized graphite supported La2O3/ZrO2 nanocomposite for the photoremediation of toxic fast green dye. J. Mol. Liq. 2018, 277, 738–748. [Google Scholar] [CrossRef]
- Ghosh, U.; Pal, A. Fabrication of a novel Bi2O3 nanoparticle impregnated nitrogen vacant 2D g-C3N4 nanosheet Z scheme photocatalyst for improved degradation of methylene blue dye under LED light illumination. Appl. Surf. Sci. 2019, 507, 144965. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef]
- Cao, S.; Liu, X.-F.; Yuan, Y.-P.; Zhang, Z.; Liao, Y.-S.; Fang, J.; Loo, J.; Sum, T.C.; Xue, C. Solar-to-fuels conversion over In2O3/g-C3N4 hybrid photocatalysts. Appl. Catal. B Environ. 2014, 147, 940–946. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, W.; Guo, Y.; Zhao, Y.; Yuan, X.; Guo, Y. Fabrication of Z-scheme plasmonic photocatalyst Ag@AgBr/g-C3N4 with enhanced visible-light photocatalytic activity. J. Hazard. Mater. 2014, 271, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Chuaicham, C.; Sekar, K.; Xiong, Y.; Balakumar, V.; Mittraphab, Y.; Shimizu, K.; Ohtani, B.; Dabo, I.; Sasaki, K. Single-step synthesis of oxygen-doped hollow porous graphitic carbon nitride for photocatalytic ciprofloxacin decomposition. Chem. Eng. J. 2021, 425, 130502. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and Enhanced Visible-Light Photocatalytic H2-Production Activity of Graphene/C3N4 Composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Magnetic 2D/2D oxygen doped g-C3N4/biochar composite to activate peroxymonosulfate for degradation of emerging organic pollutants. J. Hazard. Mater. 2021, 423, 127207. [Google Scholar] [CrossRef]
- Balakumar, V.; Ramalingam, M.; Sekar, K.; Chuaicham, C.; Sasaki, K. Fabrication and characterization of carbon quantum dots decorated hollow porous graphitic carbon nitride through polyaniline for photocatalysis. Chem. Eng. J. 2021, 426, 131739. [Google Scholar] [CrossRef]
- Wu, T.; He, Q.; Liu, Z.; Shao, B.; Liang, Q.; Pan, Y.; Huang, J.; Peng, Z.; Liu, Y.; Zhao, C.; et al. Tube wall delamination engineering induces photogenerated carrier separation to achieve photocatalytic performance improvement of tubular g-C3N4. J. Hazard. Mater. 2021, 424, 127177. [Google Scholar] [CrossRef]
- Sun, Y.; Hla, S.; Duffy, G.; Cousins, A.; French, D.; Morpeth, L.; Edwards, J.; Roberts, D. Effect of Ce on the structural features and catalytic properties of La(0.9−x)CexFeO3 perovskite-like catalysts for the high temperature water–gas shift reaction. Int. J. Hydrogen Energy 2011, 36, 79–86. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, H.; Xiong, R.; Wei, J. Preparation and Photocatalytic Performance of Double-Shelled Hollow W18O49@C3N4@Ti3C2 Microspheres. J. Wuhan Univ. Technol. Sci. Ed. 2021, 36, 311–317. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Long, J.; Xie, C.; Wang, Y.; Wei, L.; Yang, X. Synthesis and photocatalytic mechanism of visible-light-driven AgBr/g-C3N4 composite. J. Mater. Sci. Mater. Electron. 2021, 32, 6158–6167. [Google Scholar] [CrossRef]
- Wang, Y.; Di, Y.; Antonietti, M.; Li, H.; Chen, X.; Wang, X. Excellent Visible-Light Photocatalysis of Fluorinated Polymeric Carbon Nitride Solids. Chem. Mater. 2010, 22, 5119–5121. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, X.; Chen, Q.; Jin, R.; Lu, Y.; Yu, J.; Wu, Y.; He, Y. Preparation and Photocatalytic Activity of an Inorganic–Organic Hybrid Photocatalyst Ag2WO4/g-C3N4. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1683–1693. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Liu, E.; Fan, J.; Hu, X. Facile strategy to construction Z-scheme ZnCo2O4/g-C3N4 photocatalyst with efficient H2 evolution activity. Appl. Surf. Sci. 2020, 515, 146039. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, C.; Wang, Y.; Chen, Y.; Ma, Y.; Chen, Z.; Yu, J.; Wu, Y.; He, Y. Synthesis of MoS2/YVO4 composite and its high photocatalytic performance in methyl orange degradation and H2 evolution. Sol. Energy 2018, 171, 426–434. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Sun, R.-Q.; Wang, X. A Facile Band Alignment of Polymeric Carbon Nitride Semiconductors to Construct Isotype Heterojunctions. Angew. Chem. Int. Ed. 2012, 51, 10145–10149. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J.; Li, Y. Enhanced visible light photocatalytic activity of novel polymeric g-C3N4 loaded with Ag nanoparticles. Appl. Catal. A Gen. 2011, 409-410, 215–222. [Google Scholar] [CrossRef]
- Cao, G.; Li, Y.; Zhang, Q.; Wang, H. Synthesis and characterization of La2O3/TiO2−xFx and the visible light photocatalytic oxidation of 4-chlorophenol. J. Hazard. Mater. 2010, 178, 440–449. [Google Scholar] [CrossRef]
- Ma, S.; Xue, J.; Zhou, Y.; Zhang, Z. Enhanced visible-light photocatalytic activity of Ag2O/g-C3N4 p–n heterojunctions synthesized via a photochemical route for degradation of tetracycline hydrochloride. RSC Adv. 2015, 5, 40000–40006. [Google Scholar] [CrossRef]
- Mao, W.; Wang, T.; Wang, H.; Zou, S.; Liu, S. Novel Bi2WO6 loaded g-C3N4 composites with enhanced photocatalytic degradation of dye and pharmaceutical wastewater under visible light irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 15174–15182. [Google Scholar] [CrossRef]
- Wu, H.; Li, C.; Che, H.; Hu, H.; Hu, W.; Liu, C.; Ai, J.; Dong, H. Decoration of mesoporous Co3O4 nanospheres assembled by monocrystal nanodots on g-C3N4 to construct Z-scheme system for improving photocatalytic performance. Appl. Surf. Sci. 2018, 440, 308–319. [Google Scholar] [CrossRef]
- Hong, Y.; Li, C.; Zhang, G.; Meng, Y.; Yin, B.; Zhao, Y.; Shi, W. Efficient and stable Nb2O5 modified g-C3N4 photocatalyst for removal of antibiotic pollutant. Chem. Eng. J. 2016, 299, 74–84. [Google Scholar] [CrossRef]
- Li, C.; Yu, S.; Dong, H.; Liu, C.; Wu, H.; Che, H.; Chen, G. Z-scheme mesoporous photocatalyst constructed by modification of Sn3O4 nanoclusters on g-C3N4 nanosheets with improved photocatalytic performance and mechanism insight. Appl. Catal. B Environ. 2018, 238, 284–293. [Google Scholar] [CrossRef]
- Xiao, T.; Tang, Z.; Yang, Y.; Tang, L.; Zhou, Y.; Zou, Z. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics. Appl. Catal. B Environ. 2018, 220, 417–428. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Enhanced visible light photocatalytic activity of g-C3N4 decorated ZrO2−x nanotubes heterostructure for degradation of tetracycline hydrochloride. J. Hazard. Mater. 2020, 384, 121275. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Cai, W.; Cai, J.; Zhou, B.; Chai, X.; Wu, Y. Efficient Photocatalytic Degradation of Phenol over Co3O4/BiVO4 Composite under Visible Light Irradiation. J. Phys. Chem. B 2006, 110, 20211–20216. [Google Scholar] [CrossRef]
- Chang, X.; Huang, J.; Cheng, C.; Sui, Q.; Sha, W.; Ji, G.; Deng, S.; Yu, G. BiOX (X = Cl, Br, I) photocatalysts prepared using NaBiO3 as the Bi source: Characterization and catalytic performance. Catal. Commun. 2010, 11, 460–464. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.-H.; Wen, X.-J.; Fei, Z.-H.; Liu, Z.-T.; Mu, Q.-M. Novel Z-scheme W18O49/CeO2 heterojunction for improved photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2020, 579, 297–306. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, L.; Lv, Y.; Zhao, B.; Ju, X.; Xu, S.; Zhang, J.; Tian, C.; Sun, D.; Tang, X. Ultra-sensitive and fast response formaldehyde sensor based on La2O3-In2O3 beaded nanotubes at low temperature. Chem. Phys. Lett. 2020, 746, 137289. [Google Scholar] [CrossRef]
- Hua, S.; Qu, D.; An, L.; Jiang, W.; Wen, Y.; Wang, X.; Sun, Z. Highly efficient p-type Cu3P/n-type g-C3N4 photocatalyst through Z-scheme charge transfer route. Appl. Catal. B Environ. 2018, 240, 253–261. [Google Scholar] [CrossRef]
- Hu, L.; Hu, H.; Lu, W.; Lu, Y.; Wang, S. Novel composite BiFeO3/ZrO2 and its high photocatalytic performance under white LED visible-light irradiation. Mater. Res. Bull. 2019, 120, 110605. [Google Scholar] [CrossRef]
- Ye, L.; Chen, J.; Tian, L.; Liu, J.; Peng, T.; Deng, K.; Zan, L. BiOI thin film via chemical vapor transport: Photocatalytic activity, durability, selectivity and mechanism. Appl. Catal. B Environ. 2013, 130-131, 1–7. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Lin, H.; Nong, Q.; Wu, Y.; Wu, T.; He, Y. Synthesis and characterization of a ZrO2/g-C3N4 composite with enhanced visible-light photoactivity for rhodamine degradation. RSC Adv. 2014, 4, 40029–40035. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Z.; Jiang, J.; Li, H. Ultrasound-Assisted Hydrothermal Fabrication of AgI/MFeO3/g-C3N4 (M = Y, Gd, La) Nano Sheet–Sphere–Sheet Photocatalysts with Enhanced Photodegradation Activities for Norfloxacin. Catalysts 2020, 10, 373. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Shi, F.; Jiang, D.; Chen, M.; Shi, W. CdIn2S4/g-C3N4 heterojunction photocatalysts: Enhanced photocatalytic performance and charge transfer mechanism. RSC Adv. 2016, 7, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kong, J.; Yuan, J.; Zhao, W.; Zhu, X.; Sun, C.; Xie, J. Enhanced photocatalytic activity over flower-like sphere Ag/Ag2CO3/BiVO4 plasmonic heterojunction photocatalyst for tetracycline degradation. Chem. Eng. J. 2017, 331, 242–254. [Google Scholar] [CrossRef]



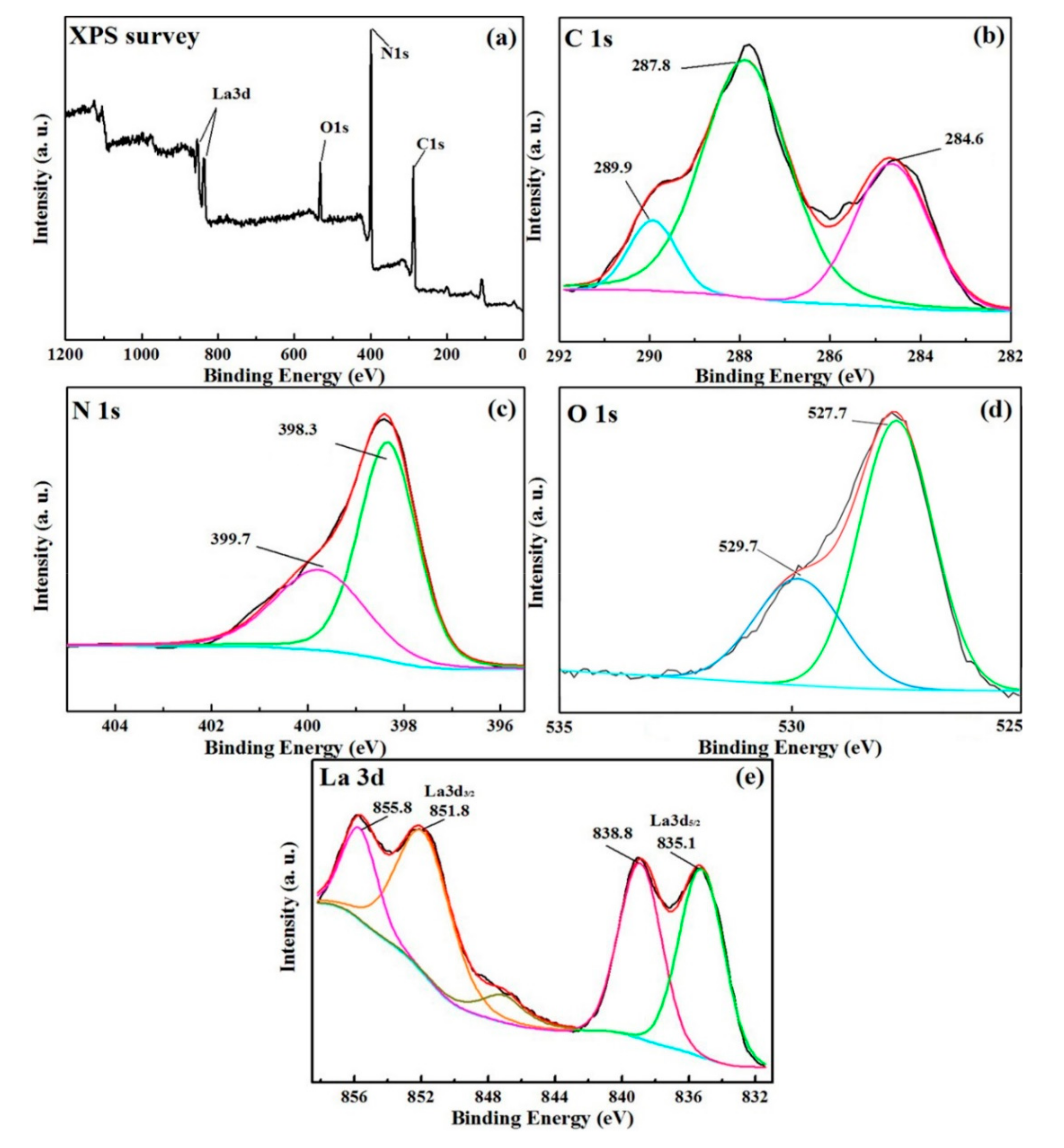
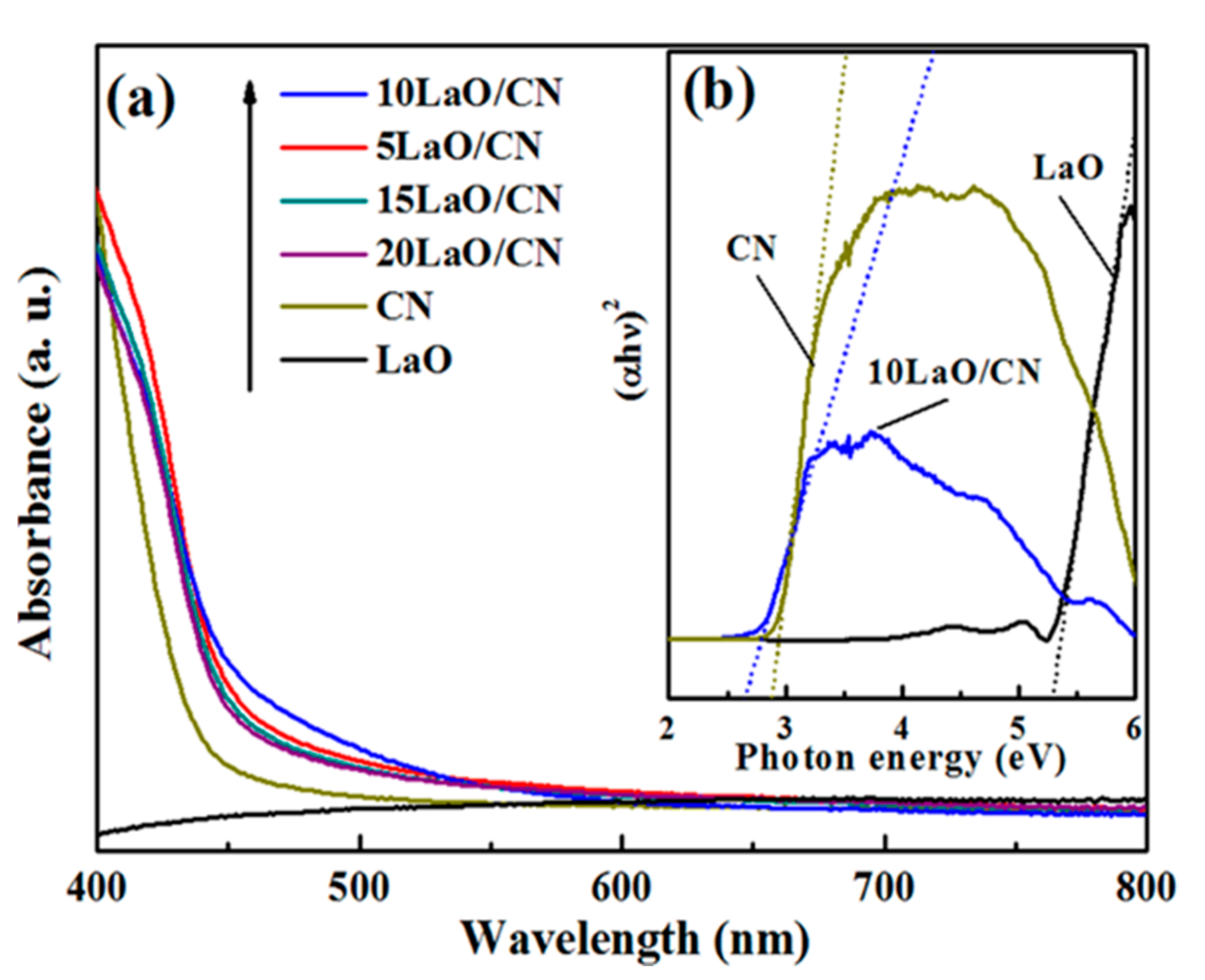
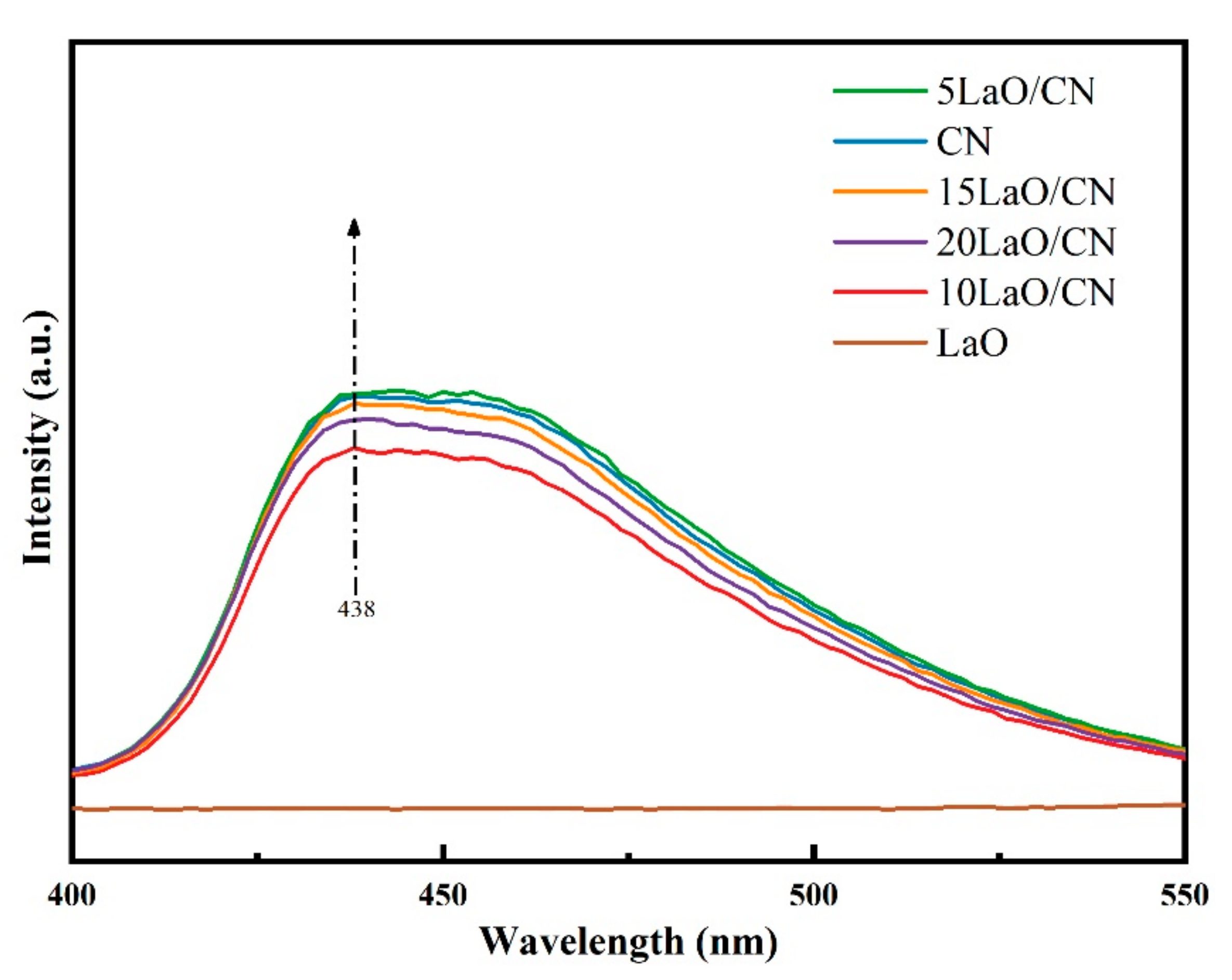
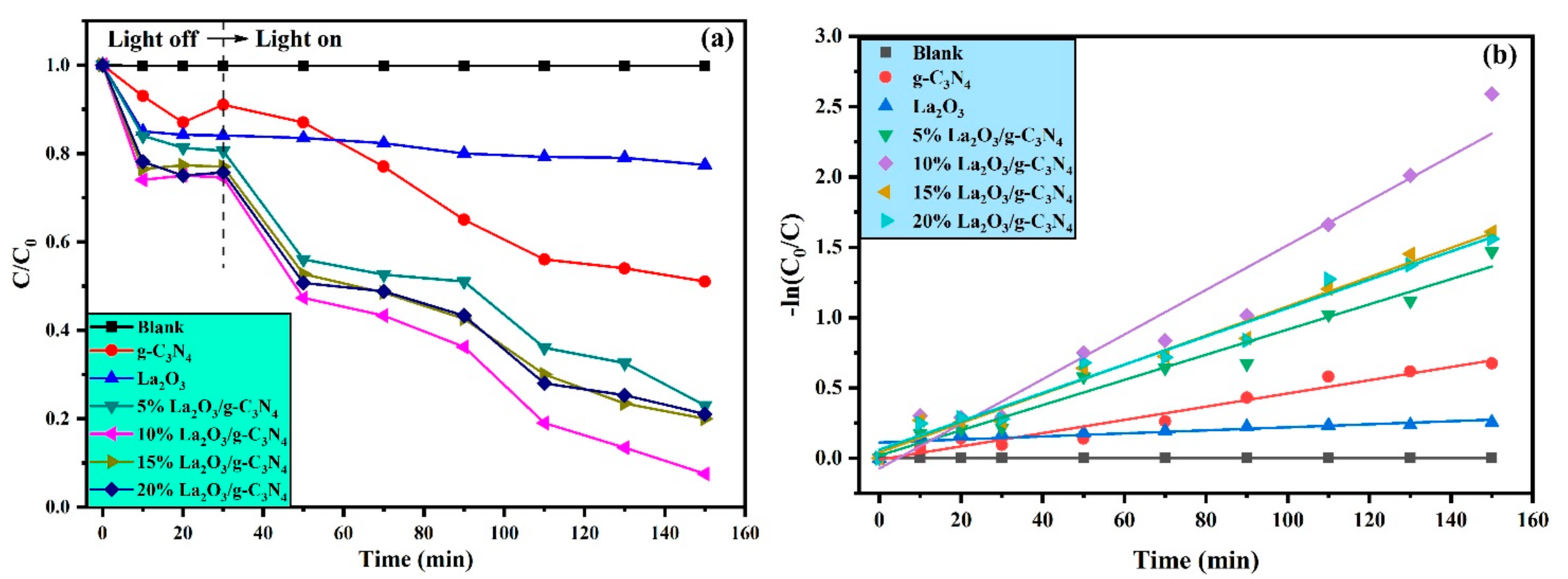


| Composition | Binding Energy (eV) | FWHM (eV) |
|---|---|---|
| C 1s | 289.9 eV | 1.18 eV |
| 287.8 eV | 2.20 eV | |
| 284.6 eV | 1.87 eV | |
| O 1s | 529.7 eV | 2.21 eV |
| 527.7 eV | 1.80 eV | |
| N 1s | 399.7 eV | 1.47 eV |
| 398.3 eV | 2.01 eV | |
| La 3d5/2 | 838.8 eV | 3.42 eV |
| 835.1 eV | ||
| La 3d3/2 | 855.8 eV | 3.42 eV |
| 851.8 eV |
| Sample Name | Degradation (%) | Time (Min) | Light Source | Reference |
|---|---|---|---|---|
| Ag2O/g-C3N4 | 89% | 180 min | UV light | [47] |
| Bi2WO6/g-C3N4 | 73% | 105 min | visible light | [48] |
| Co3O4/g-C3N4 | 73.8% | 120 min | visible light | [49] |
| Nb2O5/g-C3N4 | 73.7% | 60 min | visible light | [50] |
| Sn3O4/g-C3N4 | 72.2% | 120 min | visible light | [51] |
| WO3/g-C3N4 | 82% | 120 min | visible light | [52] |
| ZrO2/g-C3N4 | 90.6% | 60 min | visible light | [53] |
| La2O3/g-C3N4 | 93% | 180 min | visible light | This work |
| Sample Name | Degradation (%) | K (Min−1) | R2 |
|---|---|---|---|
| g-C3N4 | 49% | 0.00470 | 0.96022 |
| 5% La2O3/g-C3N4 | 77% | 0.00896 | 0.97010 |
| 10% La2O3/g-C3N4 | 93% | 0.01588 | 0.95393 |
| 15% La2O3/g-C3N4 | 80% | 0.01039 | 0.98064 |
| 20% La2O3/g-C3N4 | 79% | 0.01007 | 0.97583 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Xia, H.; Wu, R.; Cao, Y.; Li, H. Fabrication of La2O3/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance of Tetracycline Hydrochloride. Crystals 2021, 11, 1349. https://doi.org/10.3390/cryst11111349
Zhu Z, Xia H, Wu R, Cao Y, Li H. Fabrication of La2O3/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance of Tetracycline Hydrochloride. Crystals. 2021; 11(11):1349. https://doi.org/10.3390/cryst11111349
Chicago/Turabian StyleZhu, Zhengru, Haiwen Xia, Rina Wu, Yongqiang Cao, and Hong Li. 2021. "Fabrication of La2O3/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance of Tetracycline Hydrochloride" Crystals 11, no. 11: 1349. https://doi.org/10.3390/cryst11111349
APA StyleZhu, Z., Xia, H., Wu, R., Cao, Y., & Li, H. (2021). Fabrication of La2O3/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance of Tetracycline Hydrochloride. Crystals, 11(11), 1349. https://doi.org/10.3390/cryst11111349






