Preparation of Polyimide Films with Ultra-Low Dielectric Constant by Phase Inversion
Abstract
:1. Introduction
2. Experiments
2.1. Raw Materials
2.2. Preparation Routes of Porous PI Films
2.3. Examination and Characterization
3. Results and Discussion
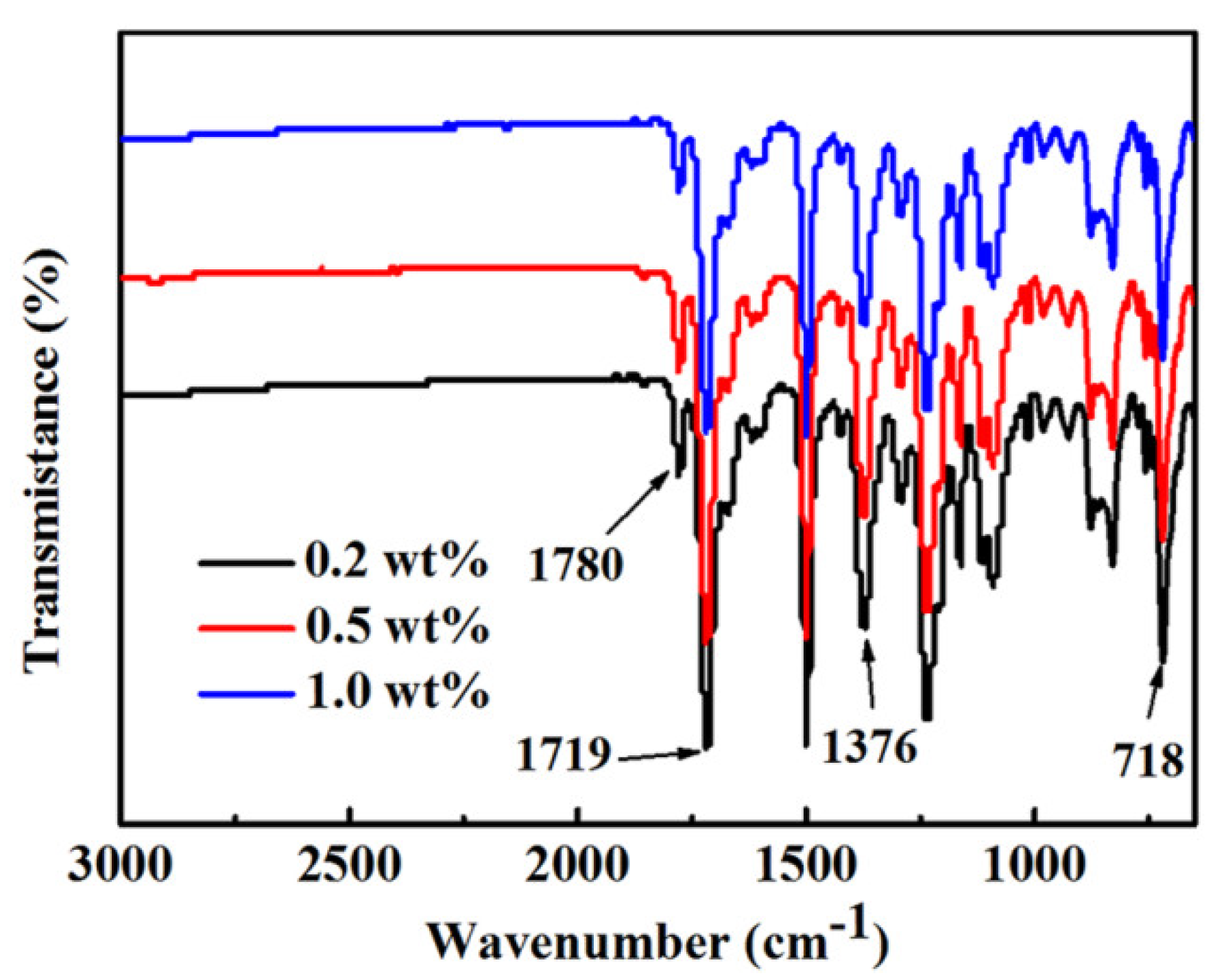
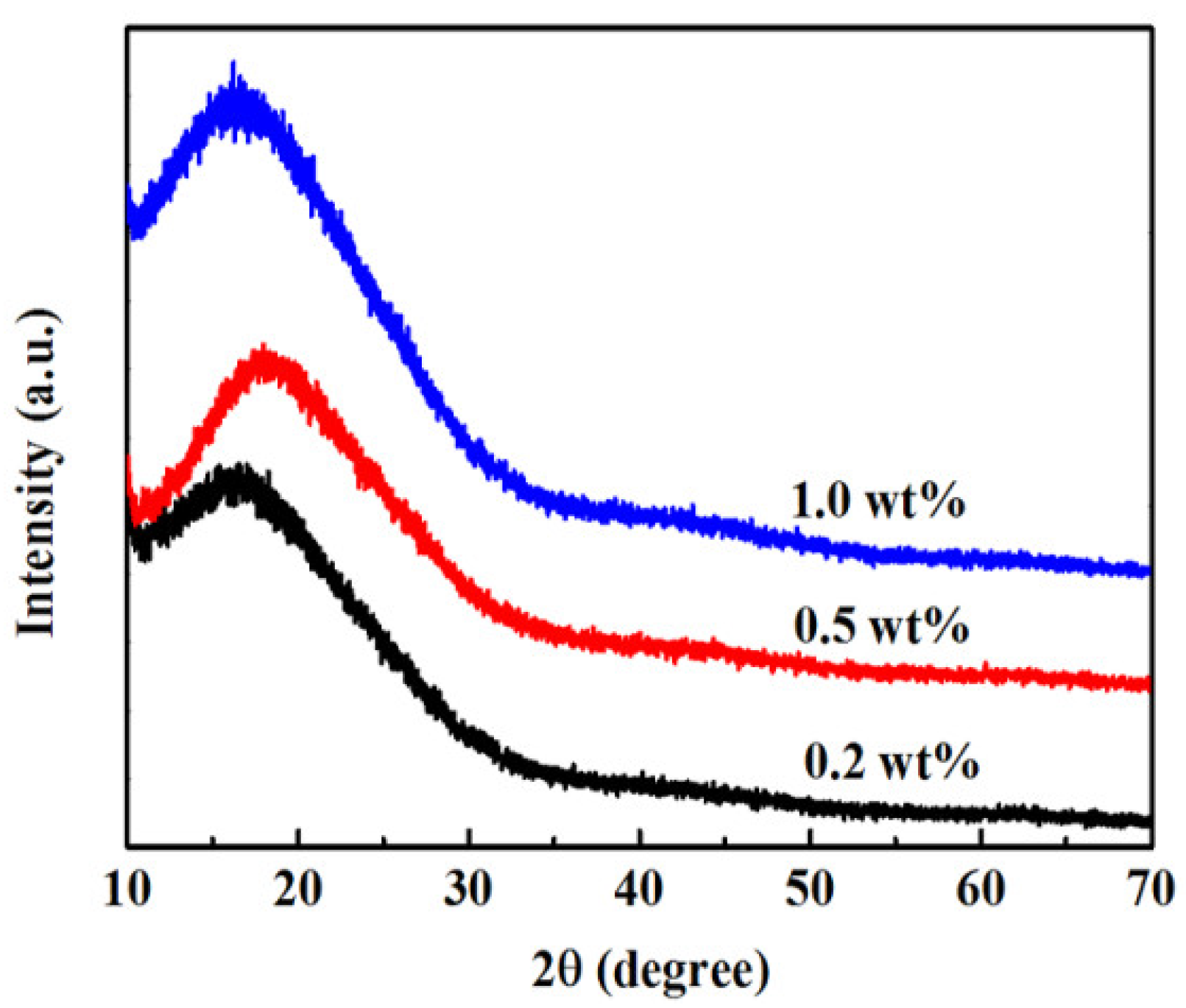
3.1. Structure of PI Films
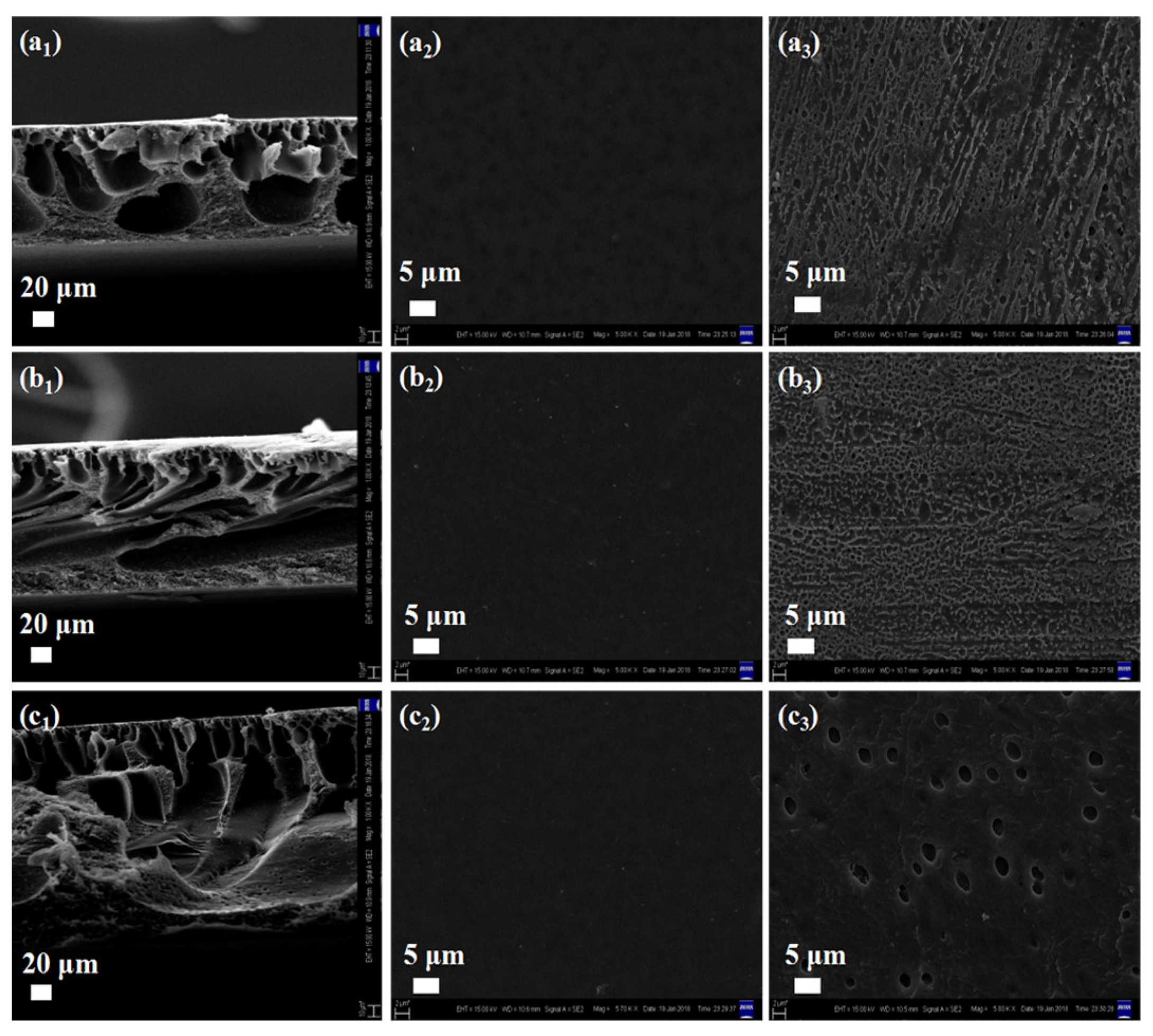
3.2. Morphology of Porous PI Films
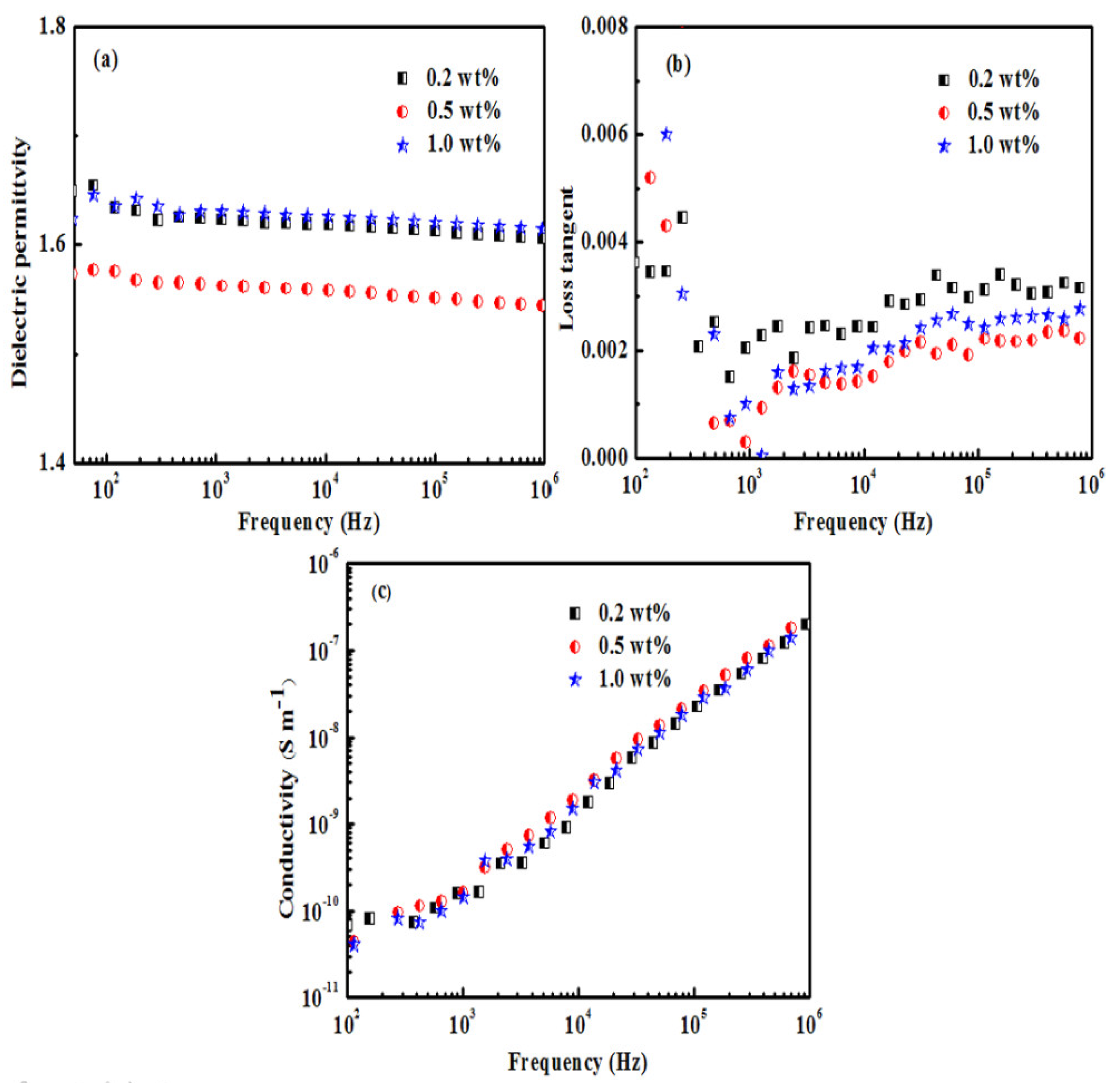
3.3. Analysis of Dielectric Properties
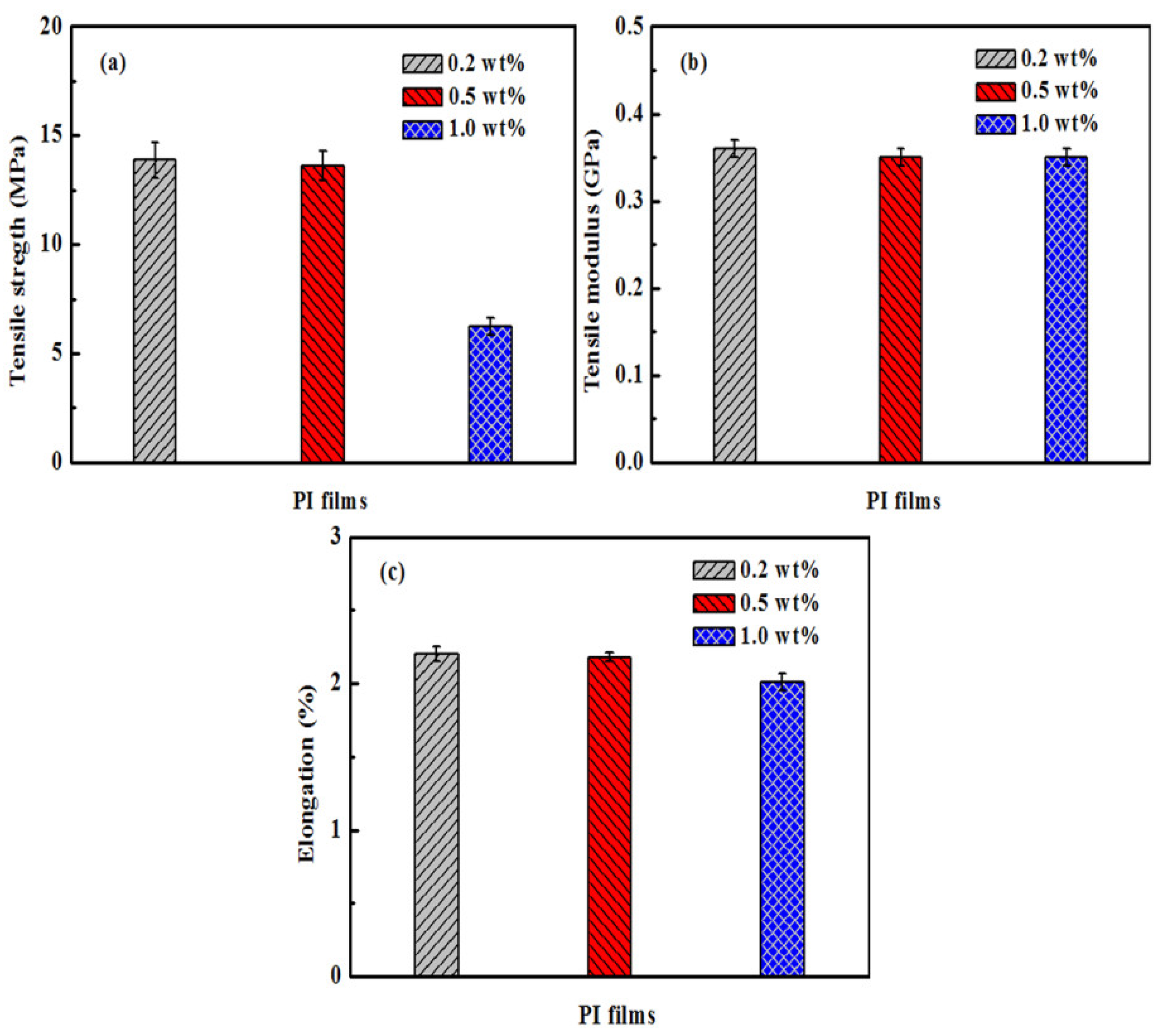
3.4. Analysis of Tensile Properties
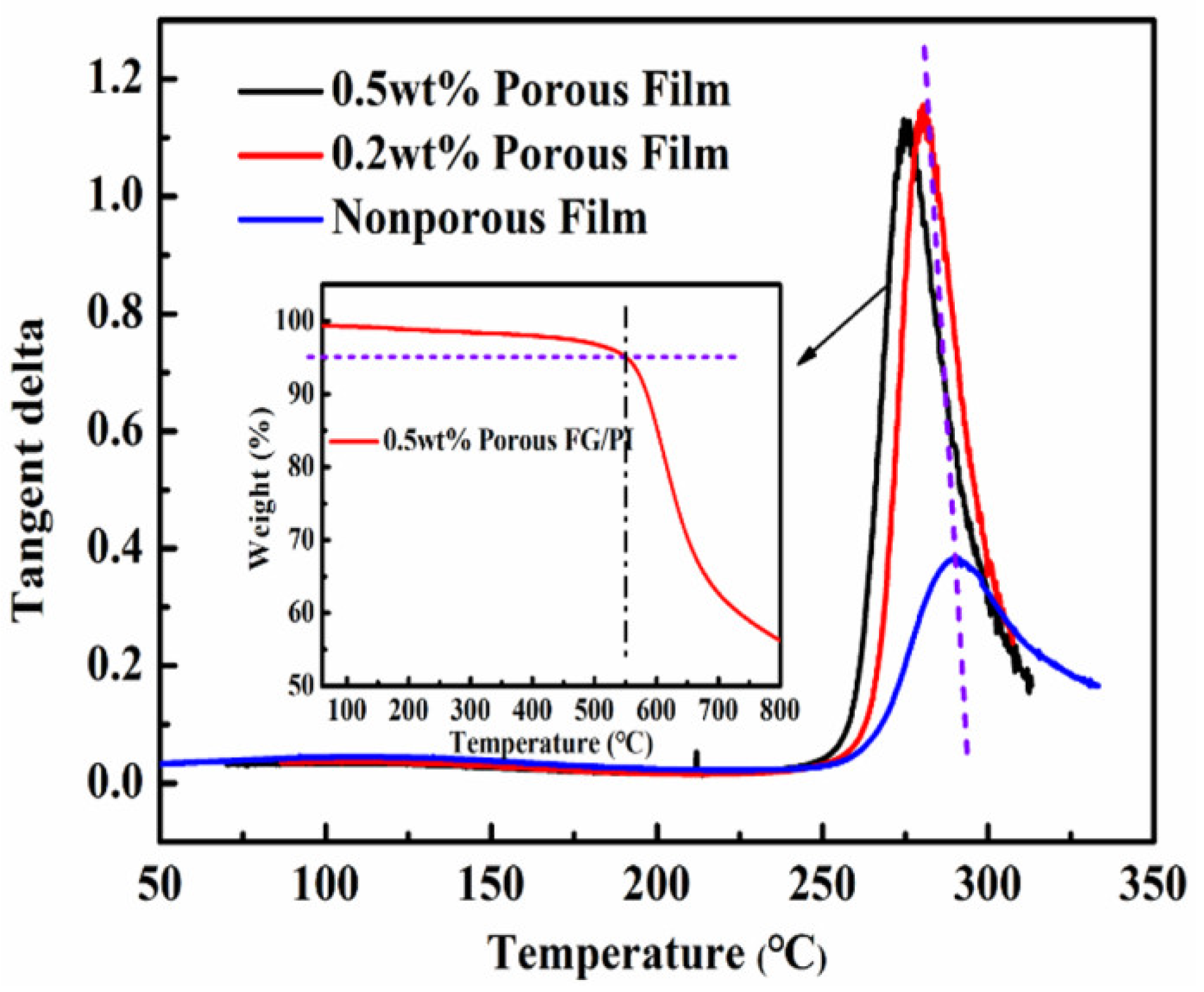
3.5. Analysis of Thermal Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maier, G. Low dielectric constant polymers for microelectronics. Prog. Polym. Sci. 2001, 26, 3–65. [Google Scholar] [CrossRef]
- Yang, M.H. The Dielectrics Characteristics of Base Materials for High Frequency Printed Circuit Boards and the Progress in Modification. Printed. Circuit. Inf. 2009, 4, 27–31. (In Chinese) [Google Scholar]
- Maex, K.; Baklanov, M.R.; Shamiryan, D.; Lacopi, F.; Brongersma, S.H.; Yanovitskaya, Z.S. Low dielectric constant materials for microelectronics. J. Appl. Phys. 2003, 93, 8793–8841. [Google Scholar] [CrossRef]
- Ghosh, M. Polyimides: Fundamentals and Applications; Marcel Dekker Press: New York, NY, USA, 1996; p. 471. ISBN 0-8247-9466-4. [Google Scholar]
- Mittal, K.L. Polyimides: Synthesis, Characterization and Applications; Springer Science & Business Media Press: New York, NY, USA, 1982; p. 537. ISBN 978-1-4615-7639-6. [Google Scholar]
- Stenzenberger, H.D.; Hergenrother, P.M. Polyimides; Springer Science & Business Media Press: New York, NY, USA, 1990; p. 34. ISBN 978-94-010-9663-8. [Google Scholar]
- Volksen, W.; Miller, R.D.; Dubois, G. Low Dielectric Constant Materials. Chem. Rev. 2010, 110, 56–110. [Google Scholar] [CrossRef] [PubMed]
- Meador, M.; Mcmillon, E.; Anna, S.; Barrios, E.; Wilmoth, N.G.; Mueller, C.H.; Miranda, F.A. Dielectric and Other Properties of Polyimide Aerogels Containing Fluorinated Blocks. ACS. Appl. Mater. Interfaces 2014, 6, 6062–6068. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Dong, J.; Gan, F.; Fang, Y.; Zhao, X.; Zhang, Q. Low dielectric constant and moisture-resistant polyimide aerogels containing trifluoromethyl pendent groups. Appl. Surf. Sci. 2018, 440, 595–605. [Google Scholar] [CrossRef]
- Catalin-Paul, C.; Damaceanu, M.; Varganici, C.; Wolinska-Grabczyk, A.; Bruma, M. Dielectric and gas transport properties of highly fluorinated polyimides blends. High. Perform. Polym. 2015, 27, 526–538. [Google Scholar]
- Li, Q.; Wang, Y.; Zhang, S.; Pang, L.; Tong, H.; Li, J.; Xu, Z. Novel fluorinated random co-polyimide/amine-functionalized zeolite MEL50 hybrid films with enhanced thermal and low dielectric properties. J. Mater. Sci. 2017, 52, 5283–5296. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, J.; Zhang, K.; Bai, R.; Wang, Y.; Hua, C.; Liu, X.; Xu, H.; Li, Y. Fluorographene/polyimide composite films: Mechanical, electrical, hydrophobic, thermal and low dielectric properties. Compos. Part A Appl. S. 2016, 84, 428–434. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, G.; He, J.J.; Yang, H.X.; Yang, S.Y. Semi-aromatic thermosetting polyimide resins containing alicyclic units for achieving low melt viscosity and low dielectric constant. React. Funct. Polym. 2020, 146, 104411. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, K.; Chen, X.; Dou, S.; Zhao, J.; Li, Y. Mechanical, dielectric and thermal properties of polyimide films with sandwich structure. Compos. Struct. 2021, 261, 113305. [Google Scholar] [CrossRef]
- Wang, W.C.; Vora, R.; Kang, E.T.; Neoh, K.G.; Ong, C.K.; Chen, L.F. Nanoporous Ultra-Low-κ Films Prepared from Fluorinated Polyimide with Grafted Poly(acrylic acid) Side Chains. Adv. Mater. 2003, 16, 54–57. [Google Scholar] [CrossRef]
- Lv, P.; Dong, Z.; Dai, X.; Wang, H.; Qiu, X. Synthesis and properties of ultralow dielectric porous polyimide films containing adamantan. J. Polym. Sci. Pol. Chem. 2017, 56, 549–559. [Google Scholar] [CrossRef]
- Hong, Z.; Dongyang, W.; Yong, F.; Hao, C.; Yusen, Y.; Jiaojiao, Y.; Liguo, J. Dielectric properties of polyimide/SiO2 hollow spheres composite films with ultralow dielectric constant. Mater. Sci. Eng. B 2016, 203, 13–18. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, J.; Zhang, K.; Wu, Y.; Li, Y. Effect of co-solvent on the structure and dielectric properties of porous polyimide membranes. J. Phys. D Appl. Phys. 2018, 51, 215305. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Z.; Yang, T.; Bei, R.; Zhang, Y.; Liu, S.; Chi, Z.; Chen, X.; Xu, J. Synthesis and properties of highly organosoluble and low dielectric constant polyimides containing non-polar bulky triphenyl methane moiety. React. Funct. Polym. 2016, 108, 71–77. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, K.; Chen, X.; Dou, S.; Zhao, J.; Li, Y. Influence of Coagulation Bath Temperature on the Structure and Dielectric Properties of Porous Polyimide Films in Different Solvent Systems. ACS. Omega. 2020, 5, 29889–29895. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.D. In Search of low-K dielectrics. Science 1999, 286, 421. [Google Scholar] [CrossRef]
- Kawakami, H.; Mikawa, M.; Nagaoka, S. Formation of surface skin layer of asymmetric polyimide membranes and their gas transport properties. J. Membr. Sci. 1997, 137, 241–250. [Google Scholar] [CrossRef]
- Kim, J.H.; Min, B.R.; Won, J.; Park, H.C.; Kang, Y. Phase behavior and mechanism of membrane formation for polyimide/DMSO/water system. J. Membr. Sci. 2001, 187, 47–55. [Google Scholar] [CrossRef]
- Ren, J. Membrane structure control of BTDA-TDI/MDI (P84) co-polyimide asymmetric membranes by wet-phase inversion process. J. Membr. Sci. 2004, 241, 305–314. [Google Scholar] [CrossRef]
- Kim, S.; Jang, K.; Choi, H.D.; Choi, S.H.; Kwon, S.J.; Kim, I.D.; Lim, J.A.; Hong, J.M. Porous Polyimide Membranes Prepared by Wet Phase Inversion for Use in Low Dielectric Applications. Int. J. Mol. Sci. 2013, 14, 8698–8707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Zhang, K.; Dou, S.; Zhao, J.; Yan, X.; Li, Y. Mechanical, Dielectric, and Thermal Attributes of Polyimides Stemmed Out of 4,4’–Diaminodiphenyl Ether. Crystals 2020, 10, 173. [Google Scholar] [CrossRef] [Green Version]


| PI Films | Linear Fitting Results | Weibull Parameters | |||
|---|---|---|---|---|---|
| Slope | ln(−ln(1−P(E)) Intercept | R | β | α/kVmm−1 | |
| 0.2 wt% | 50.09 | −206.71 | 0.9822 | 50.09 | 61.98 |
| 0.5 wt% | 10.84 | −43.71 | 0.9589 | 10.84 | 56.39 |
| 1.0 wt% | 18.00 | −63.97 | 0.9615 | 18.00 | 34.95 |
| Nonporous [26] | 9.68 | −59.74 | 0.9702 | 9.68 | 478.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Zhang, L.; Zhang, K.; Zhao, J.; Li, Y. Preparation of Polyimide Films with Ultra-Low Dielectric Constant by Phase Inversion. Crystals 2021, 11, 1383. https://doi.org/10.3390/cryst11111383
Zhang P, Zhang L, Zhang K, Zhao J, Li Y. Preparation of Polyimide Films with Ultra-Low Dielectric Constant by Phase Inversion. Crystals. 2021; 11(11):1383. https://doi.org/10.3390/cryst11111383
Chicago/Turabian StyleZhang, Panpan, Lize Zhang, Ke Zhang, Jiupeng Zhao, and Yao Li. 2021. "Preparation of Polyimide Films with Ultra-Low Dielectric Constant by Phase Inversion" Crystals 11, no. 11: 1383. https://doi.org/10.3390/cryst11111383
APA StyleZhang, P., Zhang, L., Zhang, K., Zhao, J., & Li, Y. (2021). Preparation of Polyimide Films with Ultra-Low Dielectric Constant by Phase Inversion. Crystals, 11(11), 1383. https://doi.org/10.3390/cryst11111383







