The Use of Recycled Aggregate Sludge for the Preparation of GGBFS and Fly Ash Based Geopolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Geopolymer Mortar
2.3. Preparation of Geopolymer Mortar
3. Results and Discussion
3.1. Characteristics of Geopolymer Mortar Prepared with AS and GGBFS
3.1.1. Effect of L/S Ratio on the Compressive Strength of Geopolymer Mortar
3.1.2. Effect of NaOH Concentration on the Compressive Strength of Geopolymer Mortar
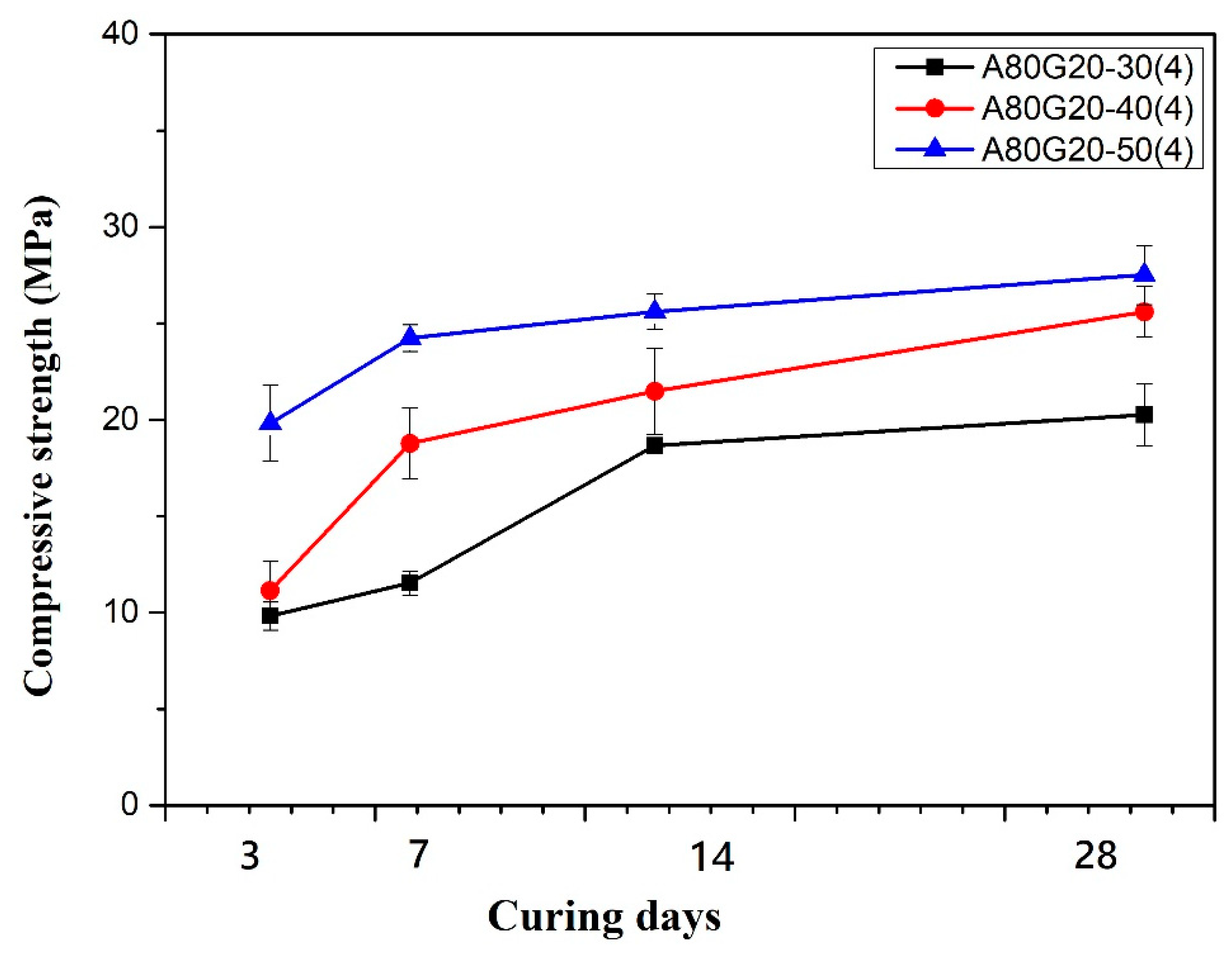
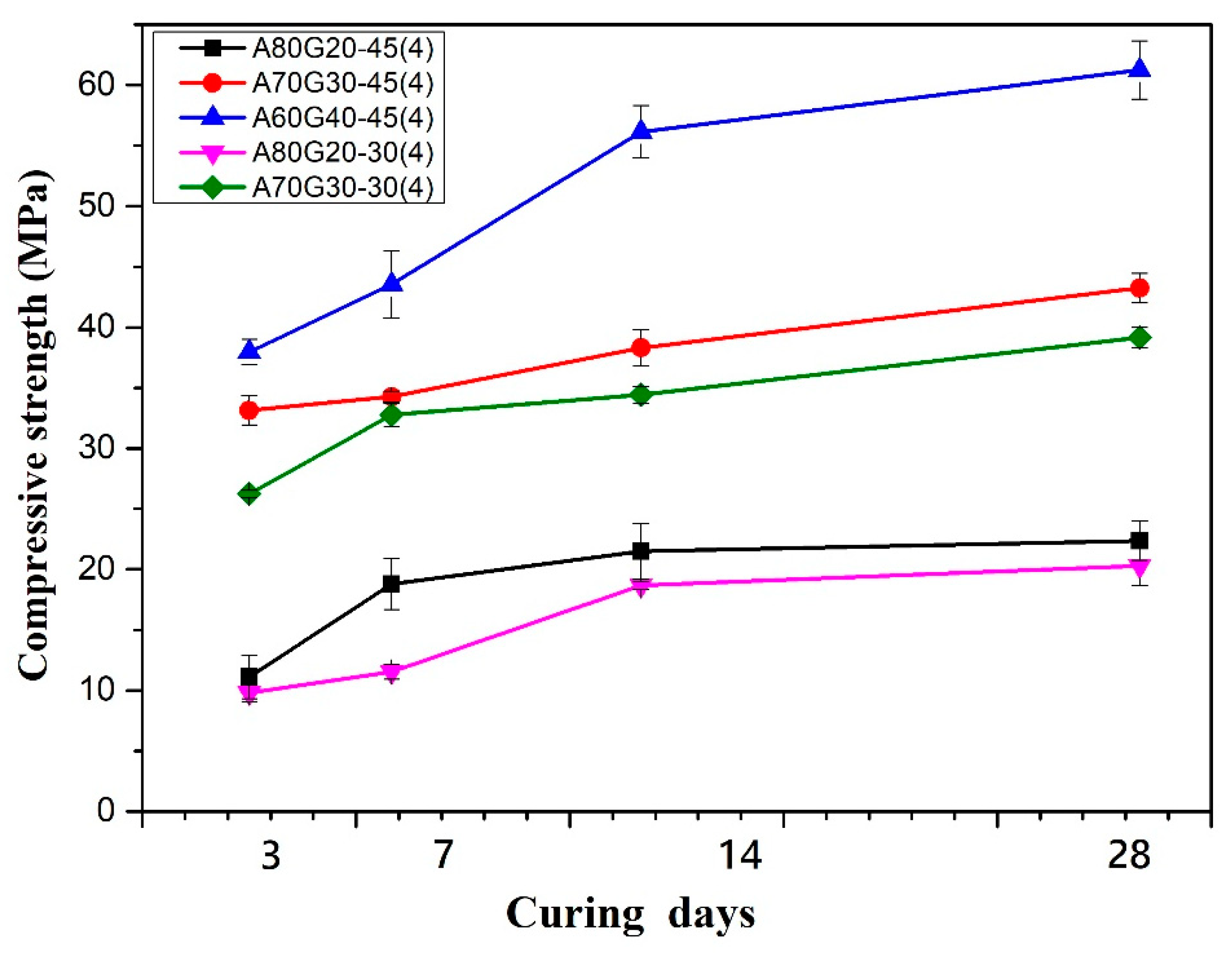
3.1.3. AS/GGBFS Ratios on Compressive Strength of Geopolymer Mortar
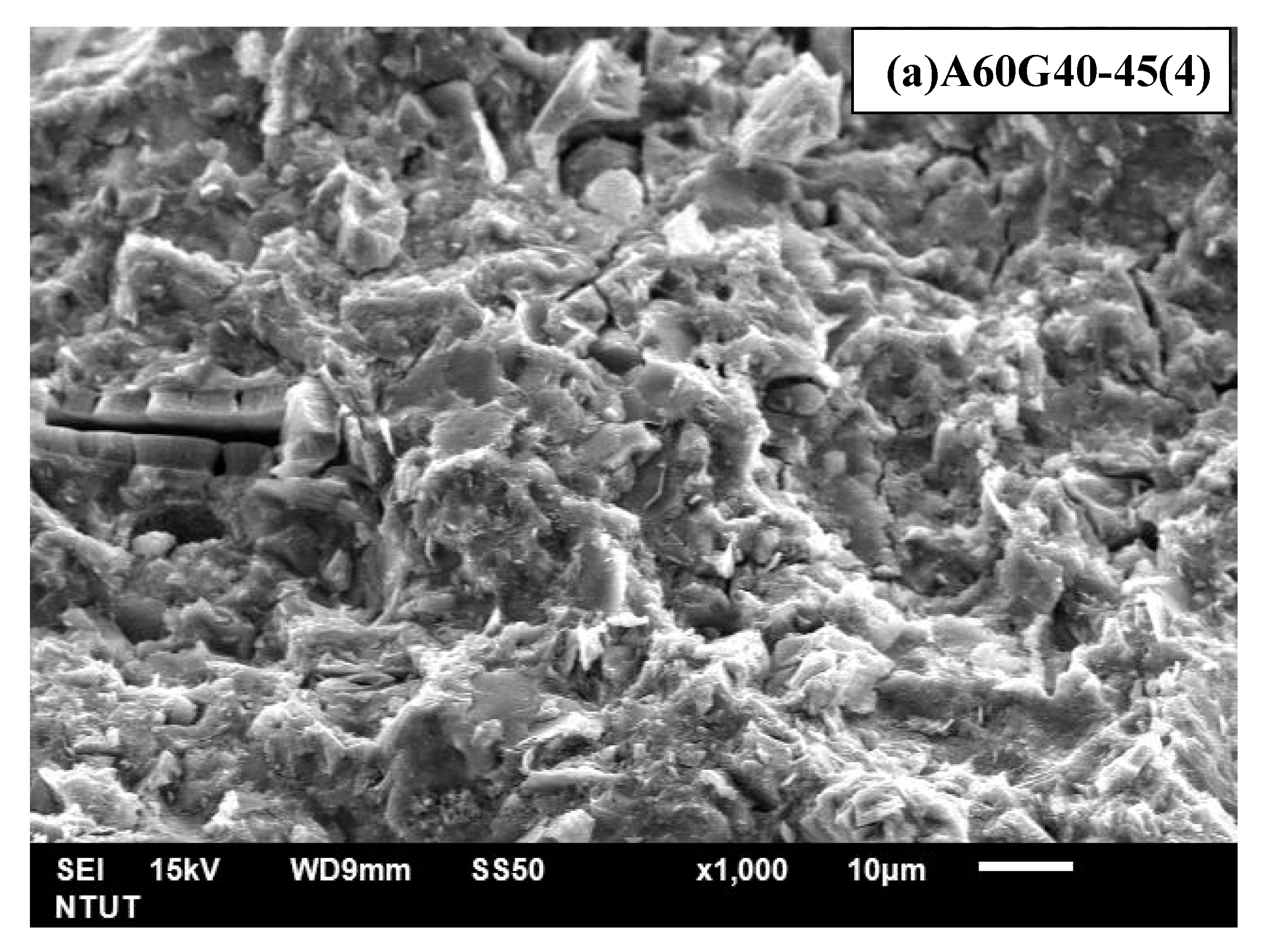
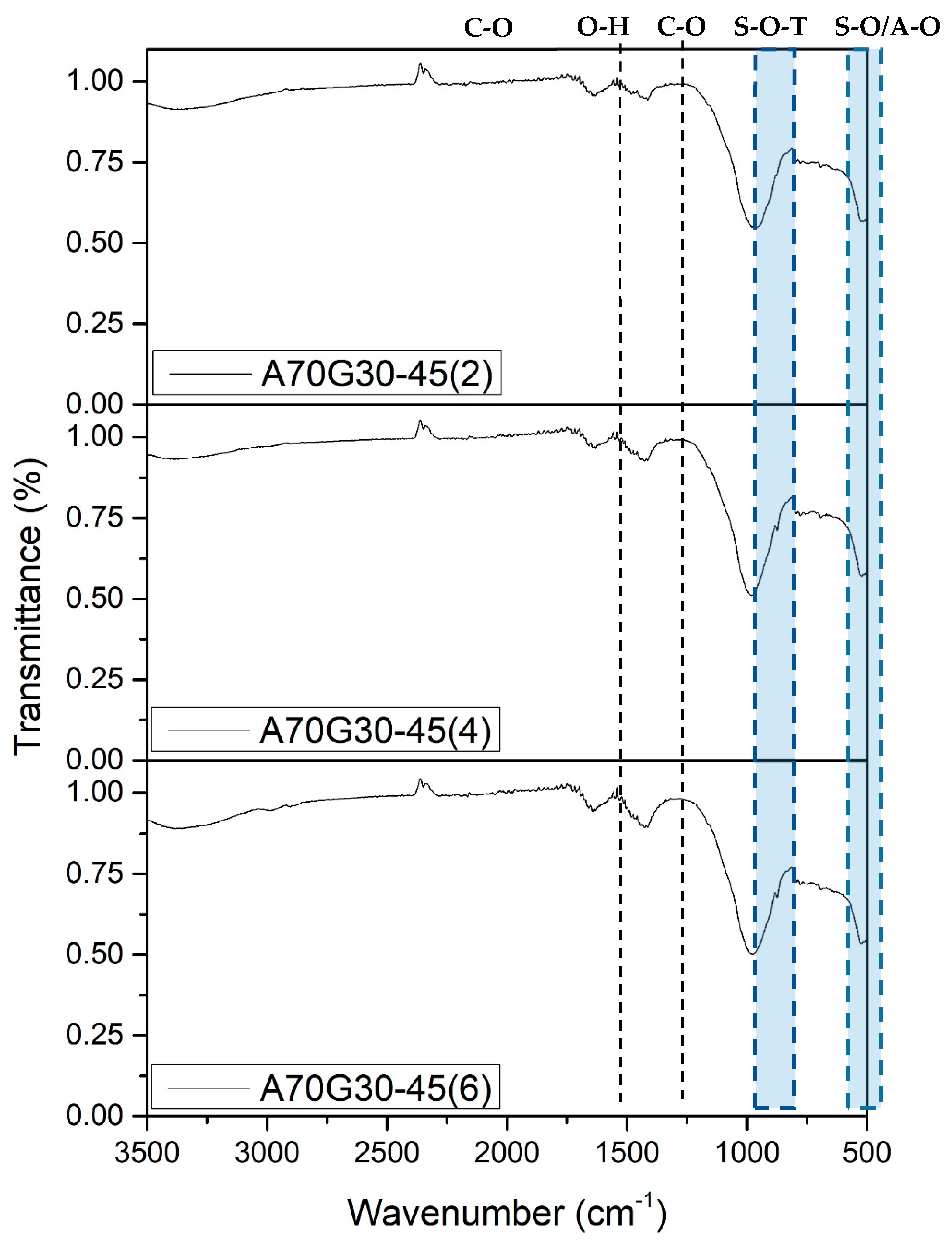
3.1.4. Physical Properties of AS/GGBFS Geopolymer Mortar
3.2. Characteristics of Geopolymer Mortar Prepared with AS, GGBFS and FA
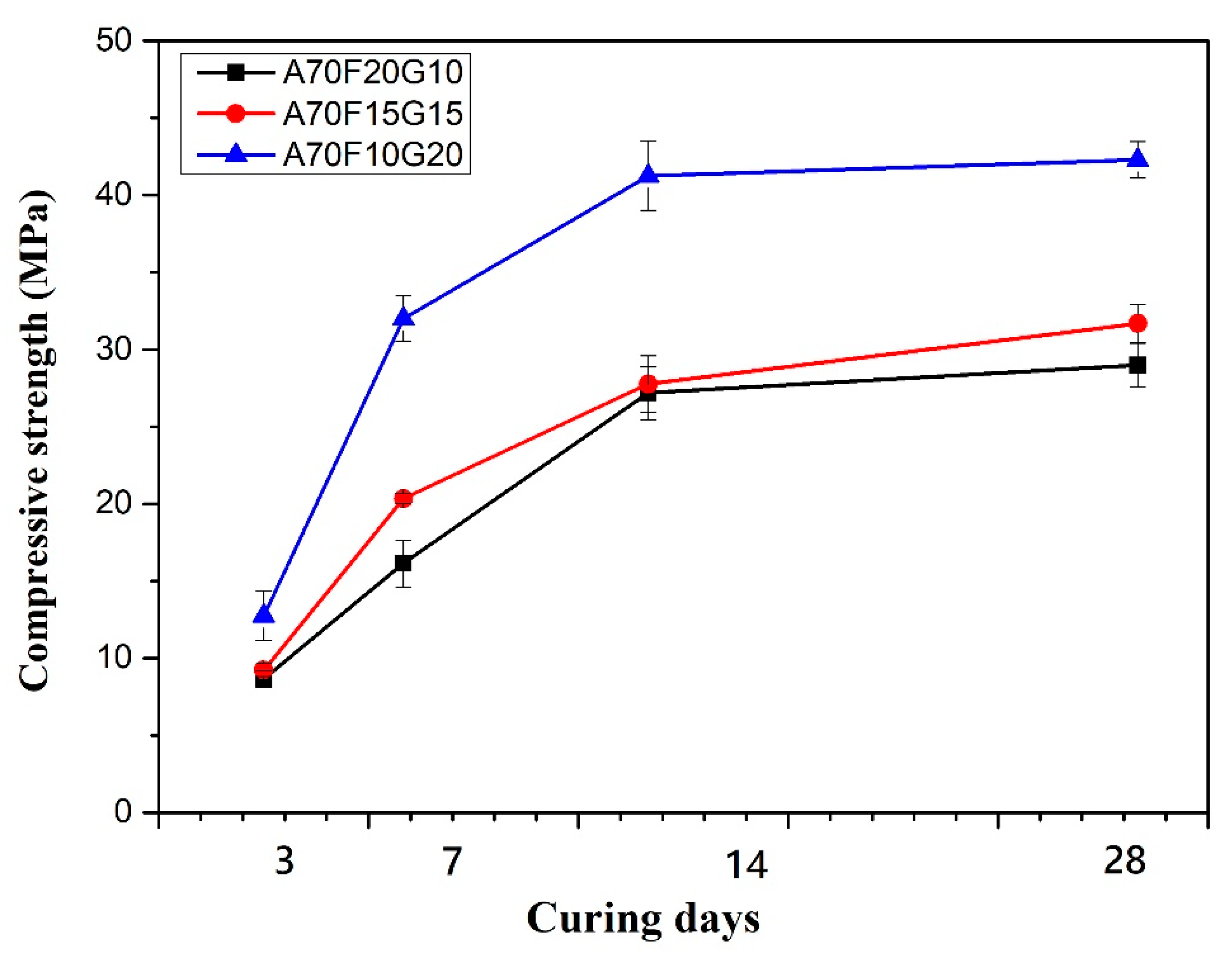
3.2.1. Effect of FA/GGBFS Ratios on the Compressive Strength of Geopolymer Mortar
3.2.2. Physical Properties of AS/GGBFS/FA Geopolymer Mortar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Suksiripattanapong, C.; Horpibulsuk, S.; Chanprasert, P.; Sukmak, P.; Arulrajah, A. Compressive strength development in fly ash geopolymer masonry units manufactured from water treatment sludge. Constr. Build. Mater. 2015, 82, 20–30. [Google Scholar] [CrossRef]
- Weng, C.H.; Lin, D.F.; Chiang, P.C. Utilization of sludge as brick materials. Adv. Environ. Res. 2003, 7, 679–985. [Google Scholar] [CrossRef]
- Chen, J.H.; Huang, J.S.; Chang, Y.W. Use of reservoir sludge as a partial replacement of metakaolin in the production of geopolymers. Cem. Concr. Compos. 2011, 33, 602–610. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Thamal Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Bhogayata, A.; Dave, S.V.; Arora, N.K. Utilization of expanded clay aggregates in sustainable lightweight geopolymer concrete. J. Mater. Cycles Waste Manag. 2020, 22, 1780–1792. [Google Scholar] [CrossRef]
- Kim, Y.; Hanif, A.; Usman, M.; Munir, M.J.; Kazmi, S.M.S.; Kim, S. Slag waste incorporation in high early strength concrete as cement replacement: Environmental impact and influence on hydration; durability attributes. J. Clean. Prod. 2018, 172, 3056–3065. [Google Scholar] [CrossRef]
- Mishra, J.; Das, S.K.; Krishna, R.S.; Nanda, B. Utilization of ferrochrome ash as a source material for production of geopolymer concrete for a cleaner sustainable environment. Indian Concr. J. Waste Manag. Res. 2020, 94, 40–49. [Google Scholar]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2021, 29, 84–104. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Cheng, T.W.; Chiu, J.P. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Cheng, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl. Clay Sci. 2012, 56, 90–96. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 3rd ed.; Institut Gèopolymère: Saint-Quentin, France, 2011. [Google Scholar]
- Criado, M.; Fernández-Jiménez, A.; Torre, A.G.d.; Aranda, M.A.G.; Palomo, A. An XRD study of the effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Cem. Concr. Res. 2007, 37, 671–679. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary Portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Olivia, M.; Nikraz, H. Properties of fly ash geopolymer concrete designed by Taguchi method. Mater. Des. 2012, 36, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, N.A.; Rangan, B.V. Geopolymer concrete with fly ash. In Proceedings of the Second International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; Volume 3, pp. 1493–1504. [Google Scholar]
- Pan, Z.; Sanjayan, J.G. Factors influencing softening temperature and hotstrength of geopolymers. Cem. Concr. Compos. 2012, 34, 261–264. [Google Scholar] [CrossRef]
- Jambunathan, N.; Sanjayan, J.G.; Pan, Z.; Li, G.; Liu, Y.; Korayem, A.H. The role of alumina on performance of alkali-activated slag paste exposed to 50 °C. Cem. Concr. Res. 2013, 54, 143–150. [Google Scholar] [CrossRef]
- Hester, D.; McNally, C.; Richardson, M. A study of the influence of slag alkali level on the alkali–silica reactivity of slag concrete. Constr. Build. Mater. 2005, 19, 661–665. [Google Scholar] [CrossRef]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash Based Geopolymer Concrete; Curtin University of Technology: Perth, Australia, 2005. [Google Scholar]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Reseffarch 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Allouche, E.N. Impact of alkali silica reaction on fly ash-based geopolymer concrete. J. Mater. Civ. Eng. 2013, 25, 131–139. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag(Si + Ca) and metakaolin (Si + Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.; Lukey, G.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of green concrete. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Granulated (Water Quenched) Blast-Furnace Slag. CNS 12223:2013. Available online: http://cns-standards.org/CNS_standard_cn.asp?code=CNS%2012223 (accessed on 29 June 2021).
- Method of Test for Apparent Porosity, Water Absorption and Specific Gravity of Refractory Bricks. CNS 619-1986. Available online: http://www.gbstandards.org/CNS/CNS_standard_chinese.asp?word=CNS%20619 (accessed on 29 June 2021).
- Method of Test for Compressive Strength of Cylindrical Concrete Specimens. CNS 1232-2002. Available online: http://www.gbstandards.org/CNS/CNS_standard_chinese.asp?word=CNS%201232 (accessed on 29 June 2021).
- Provis, J.L.; Young, C.Z.; Duxson, P.; van Deventer, J.S.J. Correlating mechanical and thermal properties of sodium silicate-fly ash geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2009, 336, 57–63. [Google Scholar] [CrossRef]
- Cho, Y.K.; Yoo, S.W.; Jung, S.H.; Lee, K.M.; Kwon, S.J. Effect of Na2O content, SiO2/Na2O molar ratio, and curing conditions on the compressive strength of FA-based geopolymer. Constr. Build. Mater. 2017, 145, 253–260. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Usman, M.; Park, C.; Hanif, A. Durability of slag waste incorporated steel fiber-reinforced concrete in marine environment. J. Build. Eng. 2021, 33, 101641. [Google Scholar] [CrossRef]
- Limbachiya, V.; Ganjian, E.; Claisse, P. Strength, durability and leaching properties of concrete paving blocksincorporating GGBS and SF. Constr. Build. Mater. 2016, 113, 273–279. [Google Scholar] [CrossRef]
- Lee, W.H. Effect of Microstructure on Mechanical Properties of Geopolymer. Ph.D. Dissertation, National Taipei University of Technology, Taiwan, 2018. [Google Scholar]
- Innocenzi, P.; Falcaro, P.; Grosso, D.; Babonneau, F. Order-Disorder Transitions and Evolution of Silica Structure in Self-Assembled Mesostructured Silica Films Studied through FTIR Spectroscopy. J. Phys. Chem. B 2003, 107, 4711–4717. [Google Scholar] [CrossRef]
- Reesm, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. In Situ ATR-FTIR Study of the Early Stages of Fly Ash Geopolymer Gel Formation. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef]
- Nergis, D.D.B.; Vizureanu, P.; Ardelean, I.; Sandu, A.V.; Corbu, O.C.; Matei, E. Revealing the Influence of Microparticles on Geopolymers’ Synthesis and Porosity. Materials 2020, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Uddin, I.; Moeez, S.; Ahmad, A. Fungus-Mediated Preferential Bioleaching of Waste Material Such as Fly-Ash as a Means of Producing Extracellular, Protein Capped, Fluorescent and Water Soluble Silica Nanoparticles. PLoS ONE 2014, 9, e107597. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, G.R.; Wang, J.; Chen, Q. Effect of polyacrylic resin on mechanical properties of granulated blast furnace slag based geopolymer. J. Non-Cryst. Solids 2018, 481, 4–9. [Google Scholar] [CrossRef]
- Jean, N.Y.D.; Antoine, E.; Herve, K.; Sanjay, K. Reactivity of volcanic ash in alkaline medium, microstructural and strength characteristics of resulting geopolymers under different synthesis conditions. J. Mater. Sci. 2016, 51, 10301–10317. [Google Scholar]
- Walkley, B.; Provis, J.; Nicolas, R.S.; Gregory, J.R.; Sani, M.A.; Hanna, J.V.; van Deventer, J.S.J. Stoichiometrically controlled C-(A)-S-H/N-A-S-H gel blends via alkali activation of synthetic precursors. Adv. Appl. Ceram. 2015, 114, 372–377. [Google Scholar] [CrossRef]
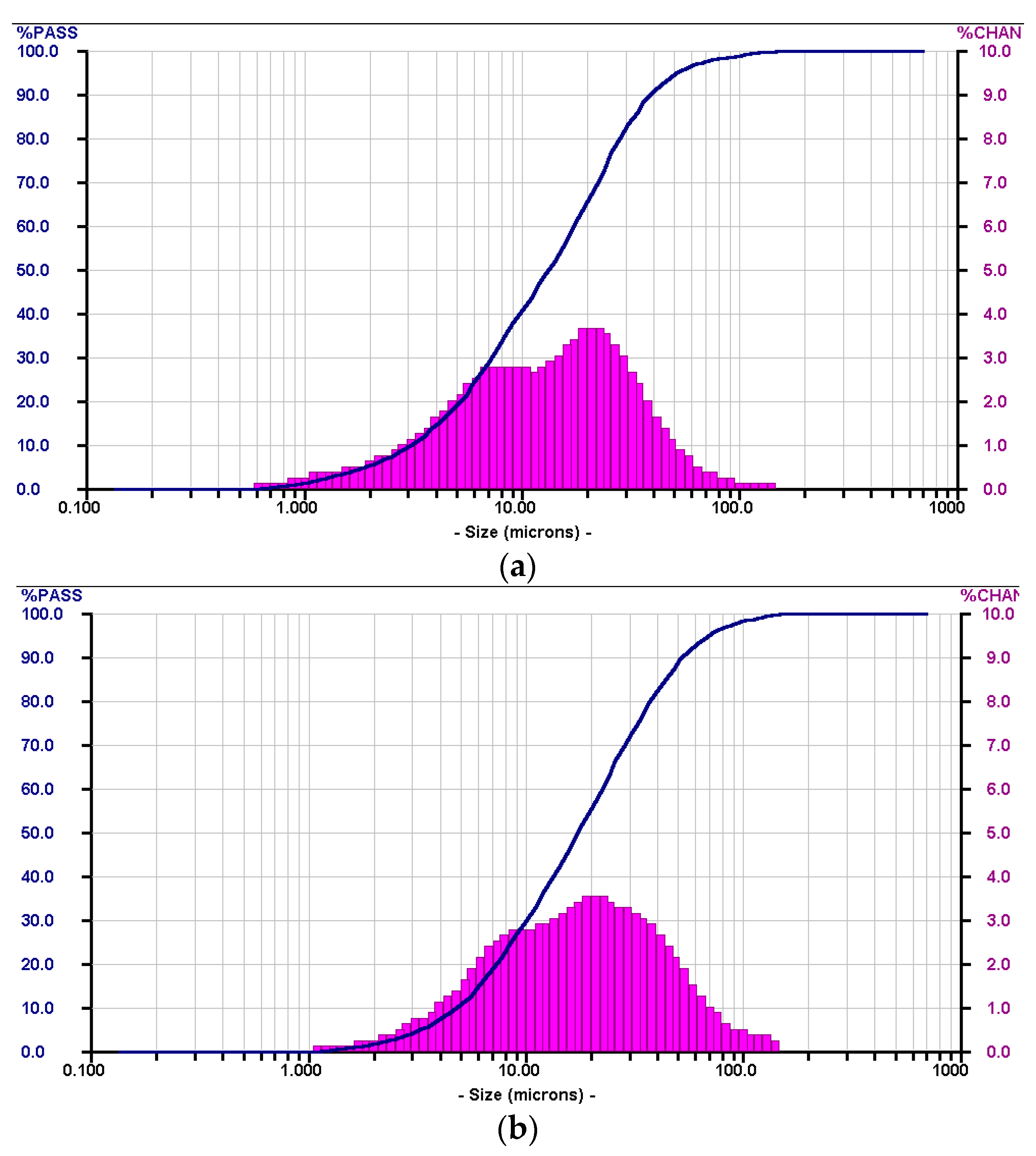
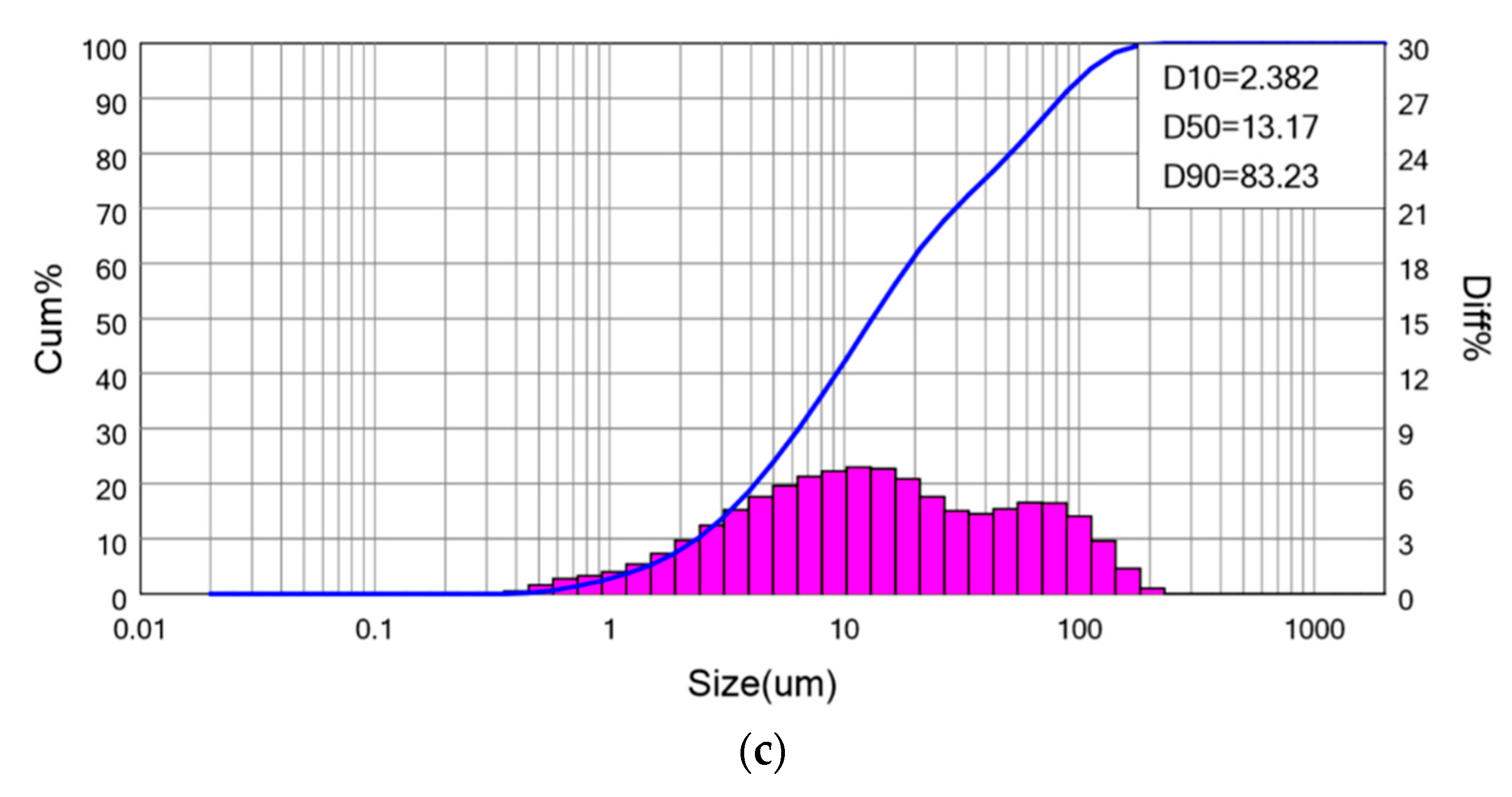

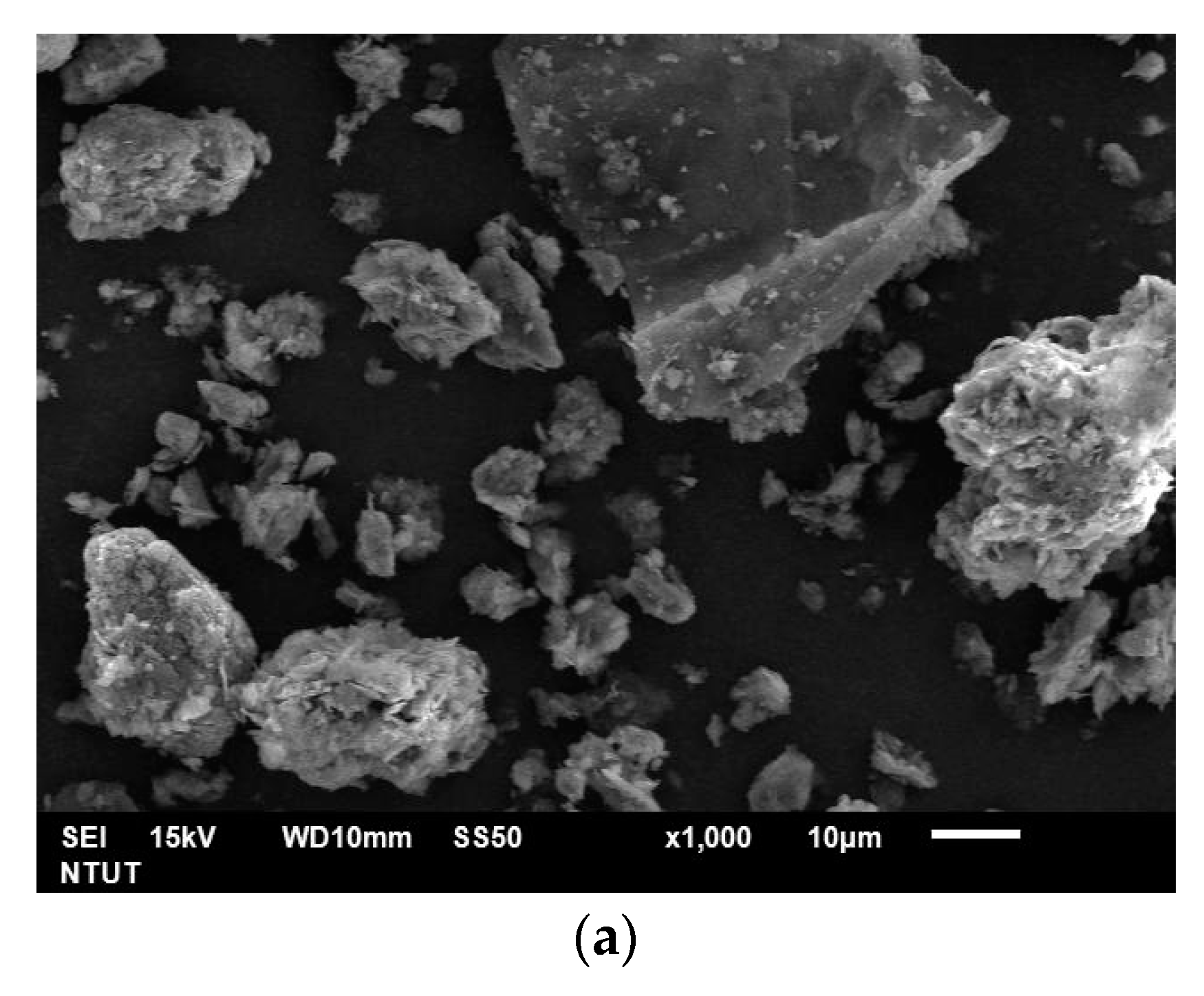
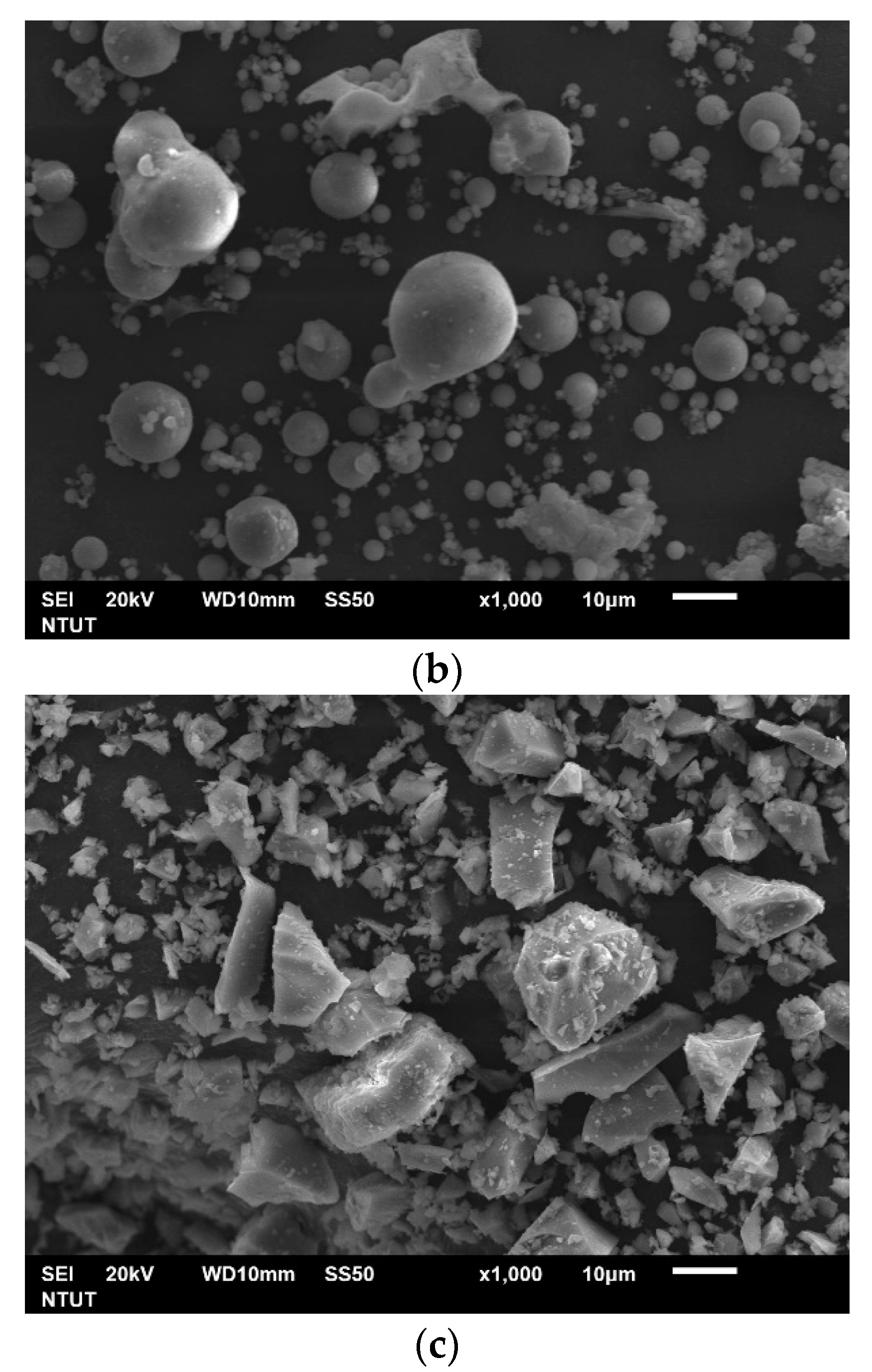






| Elements (wt%) | SiO2 | Al2O3 | CaO | Fe2O3 | Others |
|---|---|---|---|---|---|
| AS | 60.03 | 17.11 | 2.37 | 10.89 | 9.60 |
| FA | 67.64 | 12.58 | 4.31 | 7.56 | 7.91 |
| GGBFS | 27.56 | 10.85 | 57.62 | 0.57 | 3.40 |
| Particle Size Distribution | d10 (μm) | d50 (μm) | d90 (μm) |
|---|---|---|---|
| AS | 2.38 | 13.17 | 83.23 |
| FA | 6.74 | 26.70 | 93.48 |
| GGBFS | 4.61 | 12.50 | 53.85 |
| SiO2/Na2O Molar Ratio | SiO2/Al2O3 Molar Ratio | |
|---|---|---|
| Alkali solutions | 1.28 | 50 |
| AS (wt%) | GGBFS (wt%) | NaOH Molar Raio | SiO2/Na2O Molar Ratio | SiO2/Al2O3 Molar Raio | L/S Ratio |
|---|---|---|---|---|---|
| 60–80 | 20–40 | 2 M–4 M | 1.28 | 50 | 0.30–0.50 |
| AS (wt%) | FA (wt%) | GGBFS (wt%) | NaOH Molar Ratio | SiO2/Na2O Molar Ratio | SiO2/Al2O3 Molar Ratio | L/S Ratio |
|---|---|---|---|---|---|---|
| 60–80 | 10–25 | 10–20 | 4 M | 1.28 | 50 | 0.25–0.50 |
| Specimens | AS (wt%) | GGBFS (wt%) | NaOH Molar Ratio | SiO2/Na2O Molar Ratio | SiO2/Al2O3 Molar Raio | Liquid/Solid Ratio |
|---|---|---|---|---|---|---|
| A80G20-30(4) | 80 | 20 | 4 M | 1.28 | 50 | 0.30 |
| A80G20-45(4) | 0.45 | |||||
| A80G20-50(4) | 0.50 | |||||
| A70G30-30(4) | 70 | 30 | 4 M | 1.28 | 50 | 0.30 |
| A70G30-40(4) | 0.40 | |||||
| A70G30-45(4) | 0.45 | |||||
| A70G30-45(2) | 70 | 30 | 2 M | 1.28 | 50 | 0.45 |
| A70G30-45(4) | 4 M | |||||
| A70G30-45(6) | 6 M | |||||
| A60G40-45(4) | 60 | 40 | 4 M | 1.28 | 50 | 0.45 |
| Specimens | Curing Time (Days) | Bulk Density (g/cm3) | Apparent Specific Gravity | Porosity (%) | Water Absorption (%) |
|---|---|---|---|---|---|
| A70G30-45(2) | 3 | 1.35 | 2.30 | 38 | 31 |
| 7 | 1.35 | 2.29 | 36 | 31 | |
| 14 | 1.38 | 2.29 | 36 | 29 | |
| 28 | 1.37 | 2.30 | 35 | 28 | |
| A70G30-45(4) | 3 | 1.36 | 2.28 | 36 | 29 |
| 7 | 1.38 | 2.25 | 36 | 28 | |
| 14 | 1.37 | 2.25 | 34 | 27 | |
| 28 | 1.36 | 2.24 | 31 | 26 | |
| A70G30-45(6) | 3 | 1.36 | 2.28 | 35 | 29 |
| 7 | 1.36 | 2.22 | 33 | 27 | |
| 14 | 1.35 | 2.22 | 32 | 27 | |
| 28 | 1.36 | 2.20 | 32 | 27 |
| Specimens | AS (wt%) | FA (wt%) | GGBFS (wt%) | SiO2/Na2O Molar Ratio | SiO2/Al2O3 Molar Rati | L/S Ratio | NaOH Molar Ratio |
|---|---|---|---|---|---|---|---|
| A70F10G20 | 70 | 10 | 20 | 1.28 | 50 | 0.40 | 4 M |
| A70F15G15 | 15 | 15 | |||||
| A70F20G10 | 20 | 10 | |||||
| A80F10G10 | 80 | 10 | 10 | ||||
| A70F15G15 | 70 | 15 | 15 | ||||
| A60F25G15 | 60 | 25 |
| Specimens | Curing Time (Days) | Bulk Density (g/cm3) | Apparent Specific Gravity | Porosity (%) | Water Absorption (%) |
|---|---|---|---|---|---|
| A70F20G10 | 3 | 1.36 | 2.22 | 38 | 31 |
| 7 | 1.36 | 2.24 | 36 | 31 | |
| 14 | 1.39 | 2.23 | 36 | 29 | |
| 28 | 1.36 | 2.20 | 35 | 28 | |
| A70F15G15 | 3 | 1.37 | 2.24 | 35 | 29 |
| 7 | 1.37 | 2.22 | 33 | 27 | |
| 14 | 1.39 | 2.23 | 32 | 27 | |
| 28 | 1.36 | 2.23 | 32 | 27 | |
| A70F10G20 | 3 | 1.37 | 2.24 | 34 | 29 |
| 7 | 1.34 | 2.23 | 32 | 28 | |
| 14 | 1.39 | 2.23 | 31 | 27 | |
| 28 | 1.35 | 2.23 | 31 | 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Lee, W.-H.; Ding, Y.-C. The Use of Recycled Aggregate Sludge for the Preparation of GGBFS and Fly Ash Based Geopolymer. Crystals 2021, 11, 1486. https://doi.org/10.3390/cryst11121486
Chen Y-C, Lee W-H, Ding Y-C. The Use of Recycled Aggregate Sludge for the Preparation of GGBFS and Fly Ash Based Geopolymer. Crystals. 2021; 11(12):1486. https://doi.org/10.3390/cryst11121486
Chicago/Turabian StyleChen, Yi-Chen, Wei-Hao Lee, and Yung-Chin Ding. 2021. "The Use of Recycled Aggregate Sludge for the Preparation of GGBFS and Fly Ash Based Geopolymer" Crystals 11, no. 12: 1486. https://doi.org/10.3390/cryst11121486
APA StyleChen, Y.-C., Lee, W.-H., & Ding, Y.-C. (2021). The Use of Recycled Aggregate Sludge for the Preparation of GGBFS and Fly Ash Based Geopolymer. Crystals, 11(12), 1486. https://doi.org/10.3390/cryst11121486







