Carbonation of EAF Stainless Steel Slag and Its Effect on Chromium Leaching Characteristics
Abstract
:1. Introduction
2. Experiment
2.1. Raw Materials
2.2. Mineral Phase Analysis
2.3. Slurry-Phase Carbonation
2.4. Sequential Leaching
3. Results and Discussion
3.1. Mineral Composition of the Original EAF Slag
3.2. Carbonation Thermodynamic Analysis
3.3. Carbonation Products of EAF Slag
3.4. TG-DTG Analysis
3.5. Effect of Carbonation on Leaching Characteristics of EAF Slag
3.5.1. Effect of Carbonation on the Leachate’s pH
3.5.2. Effect of Carbonation on the Leachate’s Redox Potential (Eh)
3.5.3. Effect of Carbonation on the Leachate’s Conductivity
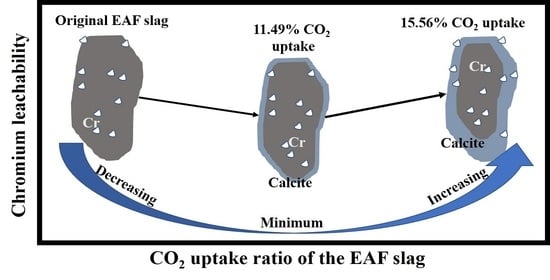
3.5.4. Effect of Carbonation on Chromium Leachability in EAF Slag
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, D.X.; Xu, A.J.; He, D.F.; Tian, N.Y.; Yang, Q. Beneficial Reuse of EAF Slag and Its Leaching Behavior of Cr. Iron Steel 2012, 47, 92–96. [Google Scholar]
- Wang, Z.J.; Yang, J.; Pan, D.A.; Liu, B.; Tian, J.J.; Zhang, S.G. Present state of stainless steel slag resources disposal technique. J. Iron Steel Res. 2015, 27, 1–6. [Google Scholar]
- Lv, Y.; Yang, L.B.; Lin, L.; Liang, Q. Leaching characteristics and affecting factors of total chromium and chromium VI in chromium-containing special steel slag. Iron Steel 2019, 54, 103–108. [Google Scholar]
- Rosales, J.; Cabrera, M.; Agrela, F. Effect of stainless steel slag waste as a replacement for cement in mortars. Mechanical and statistical study. Constr. Build. Mater. 2017, 142, 444–458. [Google Scholar] [CrossRef]
- Lan, S.W.; Zhang, Z.W.; Hong, A.Y.; Wang, X.; Xu, H. Research Status of Comprehensive Utilization of Stainless Steel Slag. Metall. Eng. 2019, 6, 34–39. [Google Scholar] [CrossRef]
- Zhao, L.; Li, J.G.; Zeng, Y.N.; Wang, Y.J. Effect of Dissolved Oxygen on Static Leaching of EAF Slag. Ind. Saf. Environ. Prot. 2019, 45, 93–97. [Google Scholar]
- Zhao, J.H.; Wang, D.M.; Yan, P.Y. Design and experimental study of a ternary blended cement containing high volume steel slag and blast-furnace slag based on Fuller distribution model. Constr. Build. Mater. 2017, 140, 248–256. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, J.S.; Yuan, T.F.; Yoon, Y.S.; Mitchell, D. Comparing Properties of Concrete Containing Electric Arc Furnace Slag and Granulated Blast Furnace Slag. Materials 2019, 12, 1371. [Google Scholar] [CrossRef] [Green Version]
- Pillay, K.; Blottnitz, H.V.; Petersen, J. Ageing of chromium(III)-bearing slag and its relation to the atmospheric oxidation of solid chromium(III)-oxide in the presence of calcium oxide. Chemosphere 2003, 52, 1771–1779. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zeng, Y.-N.; Li, J.-G.; Zhang, Y.-Z.; Zhang, Y.-J.; Zhao, Q.-Z. Carbonation of argon oxygen decarburization stainless steel slag and its effect on chromium leachability. J. Clean. Prod. 2020, 256, 120377. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zeng, Y.-N.; Li, J.-G.; Gao, Z.-Y. Leaching characteristics and mineralogical control of chromium in electric-arc-furnace stainless-steel slag. Mater. Tehnol. 2021, 55, 127–133. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Bartolomeo, E.D.; Polettini, A.; Pomi, R. Carbonation of Stainless Steel Slag as a Process for CO2 Storage and Slag Valorization. Waste Biomass Valorization 2010, 1, 467–477. [Google Scholar] [CrossRef]
- Chen, Q.; Johnson, D.C.; Zhu, L.; Yuan, M.; Hills, C.D. Accelerated carbonation and leaching behavior of the slag from iron and steel making industry. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2007, 14, 297–301. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R. Effects of thin-film accelerated carbonation on steel slag leaching. J. Hazard. Mater. 2015, 286, 369–378. [Google Scholar] [CrossRef]
- Kim, E.; Spooren, J.; Broos, K.; Nielsen, P.; Horckmans, L.; Vrancken, K.C.; Quaghebeur, M. New method for selective Cr recovery from stainless steel slag by NaOCl assisted alkaline leaching and consecutive BaCrO4 precipitation. Chem. Eng. J. 2016, 295, 542–551. [Google Scholar] [CrossRef]
- Li, L.; Ling, T.-C.; Pan, S.-Y. Environmental benefit assessment of steel slag utilization and carbonation: A systematic review. Sci. Total Environ. 2022, 806, 150280. [Google Scholar] [CrossRef]
- Luo, Y.; He, D. Research status and future challenge for CO2 sequestration by mineral carbonation strategy using iron and steel slag. Environ. Sci. Pollut. Res. 2021, 28, 49383–49409. [Google Scholar] [CrossRef]
- Costa, G.; Polettini, A.; Pomi, R.; Stramazzo, A. Leaching modelling of slurry-phase carbonated steel slag. J. Hazard. Mater. 2016, 302, 415–425. [Google Scholar] [CrossRef]
- Wang, C.Y.; Bao, W.J.; Xu, D.H.; Guo, Z.C.; Li, H.Q.; Pan, K. Reaction characteristics of steelmaking slag carbonation in dilute alkali medium. Iron Steel 2016, 51, 87–93. [Google Scholar]
- Santos, R.M.; Bouwel, J.V.; Vandevelde, E.; Mertens, G.; Elsen, J.; Gerven, T.V. Accelerated mineral carbonation of stainless steel slags for CO2 storage and waste valorization: Effect of process parameters on geochemical properties. Int. J. Greenh. Gas Control 2013, 17, 32–45. [Google Scholar] [CrossRef] [Green Version]
- BS EN 12457-2:2002. Characterisation of Waste-Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludges; Technical Specification; British Standards Institution: London, UK, 2002. [Google Scholar]
- Wang, Z.M.; Li, J.G.; Liu, B.; Zeng, Y.N.; Gao, Z.Y. Study on mineralogical phase composition and quantitative analysis of argon oxygen decarburization slag. Metall. Anal. 2017, 37, 15–20. [Google Scholar]
- Tang, H.Y.; Sun, S.H.; Meng, W.J.; Liu, H.; Wang, S.; Li, J.S. Progress in carbon dioxide sequestration by mineral carbonation. China Metall. 2013, 23, 2–8. [Google Scholar]
- Zhao, W.Y.; Zhang, Q.J.; Peng, C.Q. FTIR spectra for molecular structure of wollastonite. J. Chin. Ceram. Soc. 2006, 34, 1137–1139. [Google Scholar]
- Xu, H.C.; Xu, X.J.; Wang, K.; Huang, J.G. An analysis of factors affecting the oxidation reduction potential in drinking water. J. Univ. Sci. Technol. Suzhou (Eng. Technol.) 2007, 20, 63–66. [Google Scholar]









| Oxides | CaO | SiO2 | MgO | Al2O3 | Fe2O3 | TiO2 | Cr2O3 | Other |
|---|---|---|---|---|---|---|---|---|
| Content | 38.64 | 24.01 | 12.63 | 9.55 | 4.32 | 1.61 | 6.15 | 3.09 |
| Mineral Phase | Carbonation Reaction Equation | Standard Gibbs Free Energy (ΔGθ) | Entropy (Q) |
|---|---|---|---|
| Ca3Mg(SiO4)2 | Ca3MgSi2O8 + 4CO2 = 3CaCO3 + MgCO3 + 2SiO2 | -412,023 J/mol | −4 |
| Ca2SiO4 | Ca2SiO4 + 2CO2 = 2CaCO3 + SiO2 | -211,878 J/mol | −2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-J.; Tao, M.-J.; Li, J.-G.; Zeng, Y.-N.; Qin, S.; Liu, S.-H. Carbonation of EAF Stainless Steel Slag and Its Effect on Chromium Leaching Characteristics. Crystals 2021, 11, 1498. https://doi.org/10.3390/cryst11121498
Wang Y-J, Tao M-J, Li J-G, Zeng Y-N, Qin S, Liu S-H. Carbonation of EAF Stainless Steel Slag and Its Effect on Chromium Leaching Characteristics. Crystals. 2021; 11(12):1498. https://doi.org/10.3390/cryst11121498
Chicago/Turabian StyleWang, Ya-Jun, Meng-Jie Tao, Jun-Guo Li, Ya-Nan Zeng, Song Qin, and Shao-Hua Liu. 2021. "Carbonation of EAF Stainless Steel Slag and Its Effect on Chromium Leaching Characteristics" Crystals 11, no. 12: 1498. https://doi.org/10.3390/cryst11121498
APA StyleWang, Y.-J., Tao, M.-J., Li, J.-G., Zeng, Y.-N., Qin, S., & Liu, S.-H. (2021). Carbonation of EAF Stainless Steel Slag and Its Effect on Chromium Leaching Characteristics. Crystals, 11(12), 1498. https://doi.org/10.3390/cryst11121498