Correlation between Ion Composition of Oligomineral Water and Calcium Oxalate Crystal Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CaOx Precipitation Protocol
- Solution A, containing calcium chloride (1 mmol L−1), sodium chloride (0.15 mol L−1), and sodium cacodylate (10 mmol L−1);
- Solution B, containing sodium oxalate (0.2 mmol L−1), sodium chloride (0.15 mol L−1), and sodium cacodylate (10 mmol L−1).
2.3. Gravimetric Measurements
2.4. Optical Microscopy
2.5. Statistical Analysis
3. Results
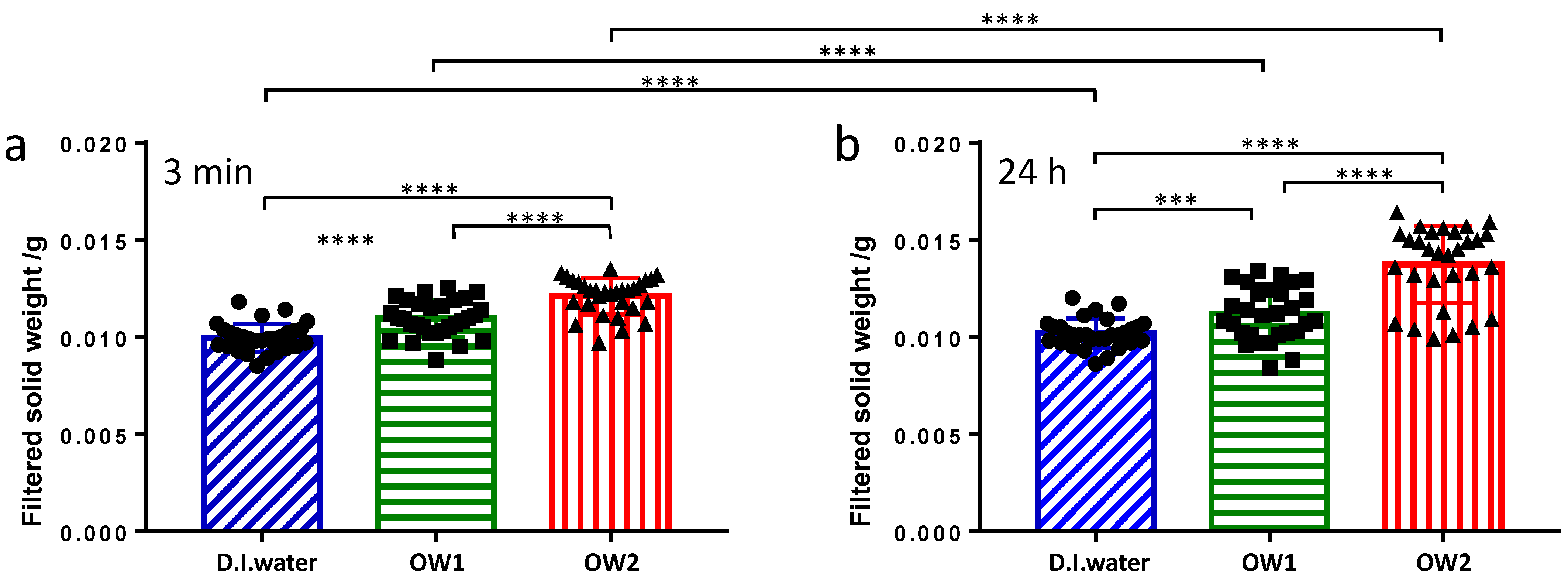
3.1. CaOx Precipitation and Gravimetric Analysis
3.2. Optical Microscopy Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Edvardsson, V.O.; Indridason, O.S.; Haraldsson, G.; Kjartansson, O.; Palsson, R. Temporal trends in the incidence of kidney stone disease. Kidney Int. 2013, 83, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Sorokin, I.; Mamoulakis, C.; Miyazawa, K.; Rodgers, A.; Talati, J.; Lotan, Y. Epidemiology of stone disease across the world. World J. Urol. 2017, 35, 1301–1320. [Google Scholar] [CrossRef]
- Pak, C.Y.C. Kidney stones. Lancet 1998, 351, 1797–1801. [Google Scholar] [CrossRef]
- Coe, F.L.; Evan, A.; Worcester, E. Kidney stone disease. J. Clin. Investig. 2005, 115, 2598–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbar, F.; Asif, M.; Dutani, H.; Hussain, A.; Malik, A.; Kamal, M.A.; Rasool, M. Assessment of the role of general, biochemical and family history characteristics in kidney stone formation. Saudi J. Biol. Sci. 2015, 22, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Baatiah, N.Y.; Alhazmi, R.B.; Albathi, F.A.; Albogami, E.G.; Mohammedkhalil, A.K.; Alsaywid, B.S. Urolithiasis: Prevalence, risk factors, and public awareness regarding dietary and lifestyle habits in Jeddah, Saudi Arabia in 2017. Urol. Ann. 2020, 12, 57–62. [Google Scholar] [CrossRef]
- Daudon, M.; Jungers, P. Drug-induced renal calculi: Epidemiology, prevention and management. Drugs 2004, 64, 245–275. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, C.; Wang, X.-L.; Liu, T.-Z.; Zeng, X.-T.; Li, S.; Duan, X.-W. Self-fluid management in prevention of kidney stones: A PRISMA-compliant systematic review and dose–response meta-analysis of observational studies. Medicine 2015, 94, e1042. [Google Scholar] [CrossRef]
- Borghi, L.; Meschi, T.; Schianchi, T.; Briganti, A.; Guerra, A.; Allegri, F.; Novarini, A. Urine volume: Stone risk factor and preventive measure. Nephron 1999, 81, 31–37. [Google Scholar] [CrossRef]
- Mirzazadeh, M.; Nouran, M.G.; Richards, K.A.; Zare, M. Effects of drinking water quality on urinary parameters in men with and without urinary tract stones. Urology 2012, 79, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Haddock, R.L.; Olson, D.R.; Backer, L.; Malilay, J. Urolithiasis, urinary Cancer, and home drinking water source in the united states territory of Guam, 2006–2010. Int. J. Environ. Res. Public Health 2016, 13, 523. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, D.; Verma, V.; Amiti; Rohil, S.; Arunai Nambi Raj, N.; Vidya, R. Demographic study of water hardness with potential to predict formation of renal crystals. Adv. Mater. Res. 2012, 584, 504–508. [Google Scholar] [CrossRef]
- Panhwar, A.H.; Kazi, T.G.; Afridi, H.I.; Shaikh, H.R.; Arain, S.A.; Arain, S.S.; Brahman, K.D. Evaluation of calcium and magnesium in scalp hair samples of population consuming different drinking water: Risk of kidney stone. Biol. Trace Elem. Res. 2013, 156, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Domrongkitchaiporn, S.; Stitchantrakul, W.; Kochakarn, W. Causes of hypocitraturia in recurrent calcium stone formers: Focusing on urinary potassium excretion. Am. J. Kidney Dis. 2006, 48, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Davydova, N.K.; Sergeev, V.N.; Girbul, E. The role of humous acids in Acqua di Fiuggi mineral water in degrading stones formed in the urinary tract. Pharm. Chem. J. 2014, 48, 589–594. [Google Scholar] [CrossRef]
- Kershaw, K.N.; Klikuszowian, E.; Schrader, L.; Siddique, J.; Van Horn, L.; Womack, V.Y.; Zenk, S.N. Assessment of the influence of food attributes on meal choice selection by socioeconomic status and race/ethnicity among women living in Chicago, USA: A discrete choice experiment. Appetite 2019, 139, 19–25. [Google Scholar] [CrossRef]
- Ryal, R.L.; Bagley, C.J.; Marshall, V.R. Independent assessment of the growth and aggregation of calcium oxalate crystals using the coulter counter. Investig. Urol. 1981, 18, 401–405. [Google Scholar]
- Gardner, G.L. Nucleation and crystal growth of calcium oxalate trihydrate. J. Cryst. Growth 1975, 30, 158–168. [Google Scholar] [CrossRef]
- Bramley, A.S.; Hounslow, M.J.; Ryall, R.L. Aggregation during precipitation from solution. Kinetics for calcium oxalate monohydrate. Chem. Eng. Sci. 1997, 52, 747–757. [Google Scholar] [CrossRef]
- Finlayson, B. Calcium stones: Some physical and clinical aspects, Chapter 10. In Calcium Metabolism in Renal Failure and Nephrolithiasis; David, D.S., Ed.; JohnWiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Bashar, A.; Townshend, A. Comparative study of methods for precipitating calcium oxalate from homogeneous solution. Talanta 1966, 13, 1123–1128. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Lin, Y.-H.; Shiau, L.-D. Effects of various inhibitors on the nucleation of calcium oxalate in synthetic urine. Crystals 2020, 10, 333. [Google Scholar] [CrossRef] [Green Version]
- Kuliasha, C.A.; Rodriguez, D.; Lovett, A.; Gower, L.B. In situ flow cell platform for examining calcium. Oxalate and calcium phosphate crystallization on films of basement membrane extract in the presence of urinary ‘inhibitors’. CrystEngComm 2020, 22, 1448–1458. [Google Scholar] [CrossRef]
- Stanković, A.; Šafranko, S.; Jurišić, K.; Balić, I.; Bijelić, J.; Jokić, S.; Medvidović-Kosanović, M. Investigation of system complexity and addition of vitamin C on calcium oxalate precipitation. Chem. Pap. 2020, 74, 3279–3291. [Google Scholar] [CrossRef]
- Stanković, A.; Šafranko, S.; Kontrec, J.; Njegić DžakulaJurišić, B.; Lyons, D.M.; Marković, B.; Kralj, D. Calcium oxalate precipitation in model systems mimicking the conditions of hyperoxaluria. Cryst. Res. Technol. 2019, 54, 1800210. [Google Scholar] [CrossRef]
- Benalia, H.; Djeridane, A.; Bensafieddine, F.; Yousfi, M. High in vitro antiurolithiatic effect of Pituranthos scoparius roots extracts. Pharmacologyonline 2016, 1, 31–43. [Google Scholar]
- Bensatal, A.; Rahmoun, D.; Ardja, S.A.; Cheikh, M.; Kahouadji, A.; Bekhit, M. In vitro antilithiasic activity of saponins rich fraction from the leaves of Zizyphus lotus. Int. J. Green Pharm. 2020, 14, 280–285. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, H.; Liu, X.; Qiao, L.; Guo, R. Calcium oxalate crystallization in the presence of amphiphilic phosphoproteins. CrystEngComm 2014, 16, 8841–8851. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.R.; Gilani, A.H. Studies on the in vitro and in vivo antiurolithic activity of Holarrhena antidysenterica. Urol. Res. 2012, 40, 671–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, W.A.; Shahzad, M.; Shabbir, A.; Ahmad, A. Evaluation of anti-urolithiatic and diuretic activities of watermelon (Citrullus lanatus) using in vivo and in vitro experiments. Biomed. Pharmacother. 2018, 97, 1212–1221. [Google Scholar] [CrossRef]
- Marangella, M.; Vitale, C.; Petrarulo, M.; Rovera, L.; Dutto, F. Effects of mineral composition of drinking water on risk for stone formation and bone metabolism in idiopathic calcium nephrolithiasis. Clin. Sci. 1996, 91, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.-C.; Pan, L.-C.; Shiau, L.-D. A Photomicroscopic study on the growth rates of calcium oxalate crystals in a new synthetic urine without inhibitors and with various inhibitors. Crystals 2021, 11, 223. [Google Scholar] [CrossRef]
- Mohamaden, W.I.; Wang, H.; Guan, H.; Meng, X.; Li, J. Osteopontin and Tamma-Horsefall proteins–Macromolecules of myriad values. J. Basic Appl. Zool. 2014, 67, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Turudic, D.; Batinic, D.; Golubic, A.T.; Lovric, M.; Milosevic, D. Calcium oxalate urolithiasis in children: Urinary promoters/inhibitors and role of their ratios. Eur. J. Pediatr. 2016, 175, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Rimer, J.D.; Asplin, J.R. Hydroxycitrate: A potential new therapy for calcium urolithiasis. Urolithiasis 2019, 47, 311–320. [Google Scholar] [CrossRef]
- Rodgers, A.; Gauvin, D.; Edeh, S.; Allie-Hamdulay, S.; Jackson, G.; Lieske, J.C. Sulfate but not thiosulfate reduces calculated and measured urinary ionized calcium and supersaturation: Implications for the treatment of calcium renal stones. PLoS ONE 2014, 9, e103602. [Google Scholar] [CrossRef] [Green Version]
- Landry, G.M.; Hirata, T.; Anderson, J.B.; Cabrero, P.; Gallo, C.J.R.; Dow, J.A.T.; Romero, M.F. Sulfate and thiosulfate inhibit oxalate transport via a dPrestin (Slc26a6)-dependent mechanism in an insect model of calcium oxalate nephrolithiasis. Am. J. Physiol. Renal. Physiol. 2016, 310, F152–F159. [Google Scholar] [CrossRef] [Green Version]
- Queau, Y.; Bijsmans, E.S.; Feugier, A.; Biourge, V.C. Increasing dietary sodium chloride promotes urine dilution and decreases struvite and calcium oxalate relative supersaturation in healthy dogs and cats. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1524–1530. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, P.M.; Baccaro, R.; Baroni, S.; D’Alessandri, L.; Carpenito, C.; Di Daniele, N.; Urbani, A.; Gambaro, G. Effect of water composition and timing of ingestion on urinary lithogenic profile in healthy volunteers: A randomized crossover trial. J. Nephrol. 2021, 34, 875–881. [Google Scholar] [CrossRef]
- Prezioso, D.; Di Domenico, D.; Pane, M.; Ciccarelli, D.; D’Errico, G. Ion specificity in determining physico-chemical properties of drinking water. Food Sci. Technol. 2019, 39, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Tobias, D.J.; Hemminger, J.C. Getting specific about specific ion effects. Science 2008, 319, 1197–1198. [Google Scholar] [CrossRef]
- Du, H.; Liu, J.; Ozdemir, O.; Nguyen, A.V.; Miller, J.D. Molecular features of the air/carbonate solution interface. J. Colloid Interface Sci. 2008, 318, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Gerard, D.E.; Nancollas, G.H. Nucleation at surfaces: The importance of interfacial energy. J. Am. Soc. Nephrol. 1999, 10, S355–S358. [Google Scholar] [PubMed]
- Goujon, F.; Dequidt, A.; Ghoufi, A.; Malfreyt, P. How does the surface tension depend on the surface area with coarse-grained models? J. Chem. Theory Comput. 2018, 14, 2644–2651. [Google Scholar] [CrossRef]
- Li, Y.; Pan, J.; Zhang, Y.; Chang, Y.; Yang, X.; Yang, B.; Mao, X.; Wang, Z.; Gao, B.; Lu, X. Effects of small molecules water that may retard kidney stone formation. Int. Urol. Nephrol. 2018, 50, 225–230. [Google Scholar] [CrossRef]


| Physicochemical Parameter | Units | OW1 | OW2 |
|---|---|---|---|
| Solid residue at 180 °C | mg L−1 | 174.1 | 162 |
| Specific electric conductivity at 20 °C | μS cm−1 | 276.3 | 251 |
| pH at the spring temperature | - | 7.56 | 7.9 |
| Ca++ | mg L−1 | 57.36 | 35.9 |
| Na+ | mg L−1 | 4.13 | 2.0 |
| Mg++ | mg L−1 | 3.23 | 12.6 |
| K+ | mg L−1 | 0.35 | 0.5 |
| HCO3− | mg L−1 | 182.1 | 148 |
| SO4− | mg L−1 | 6.75 | 19.3 |
| NO3− | mg L−1 | 1.10 | 4.4 |
| Resting Time after Mixing | Deionized Water | OW1 | OW2 | |
|---|---|---|---|---|
| Filtered Precipitate Weight/mg | 3 min | 9.96 ± 0.13 | 10.9 ± 0.2 | 12.1 ± 0.2 |
| 24 h | 10.19 ± 0.15 | 11.2 ± 0.3 | 13.7 ± 0.4 |
| Resting Time after Mixing | Number of Analyzed Crystals | Distribution Range of Crystal Area/μm2 | Mean Crystal Area/μm2 | ||
|---|---|---|---|---|---|
| min | max | ||||
| OW1 | 1 h | 738 | 9 | 175 | 63 |
| 24 h | 1032 | 11 | 218 | 84 | |
| OW2 | 1 h | 72 | 11 | 62 | 25 |
| 24 h | 991 | 8 | 338 | 64 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, M.; Barone, B.; Di Domenico, D.; Esposito, R.; Fabozzi, A.; D’Errico, G.; Prezioso, D. Correlation between Ion Composition of Oligomineral Water and Calcium Oxalate Crystal Formation. Crystals 2021, 11, 1507. https://doi.org/10.3390/cryst11121507
Rossi M, Barone B, Di Domenico D, Esposito R, Fabozzi A, D’Errico G, Prezioso D. Correlation between Ion Composition of Oligomineral Water and Calcium Oxalate Crystal Formation. Crystals. 2021; 11(12):1507. https://doi.org/10.3390/cryst11121507
Chicago/Turabian StyleRossi, Manuela, Biagio Barone, Dante Di Domenico, Rodolfo Esposito, Antonio Fabozzi, Gerardino D’Errico, and Domenico Prezioso. 2021. "Correlation between Ion Composition of Oligomineral Water and Calcium Oxalate Crystal Formation" Crystals 11, no. 12: 1507. https://doi.org/10.3390/cryst11121507







