Efficient Ag-Doped Perovskite Solar Cells Fabricated in Ambient Air
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Characterization Techniques
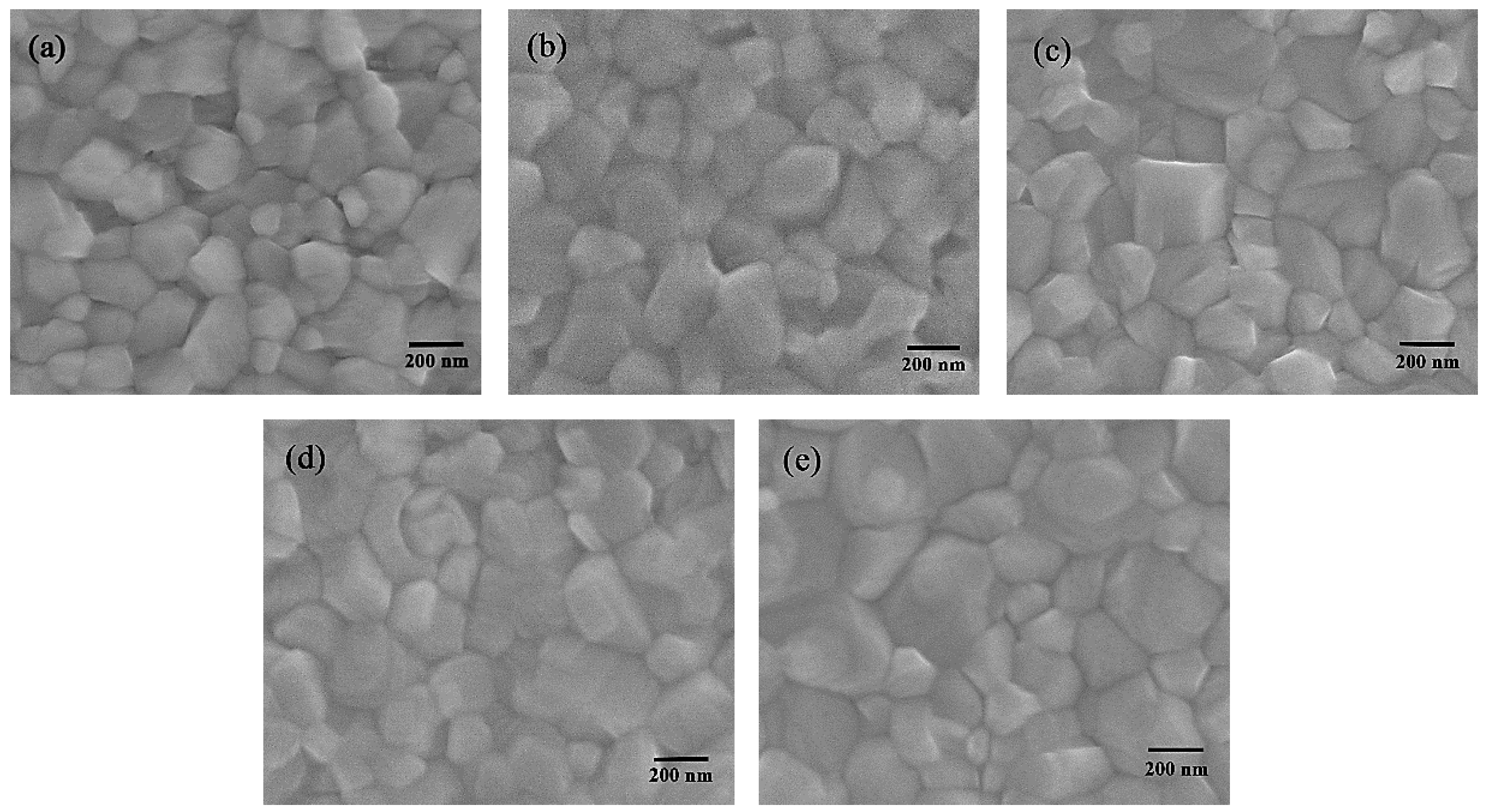
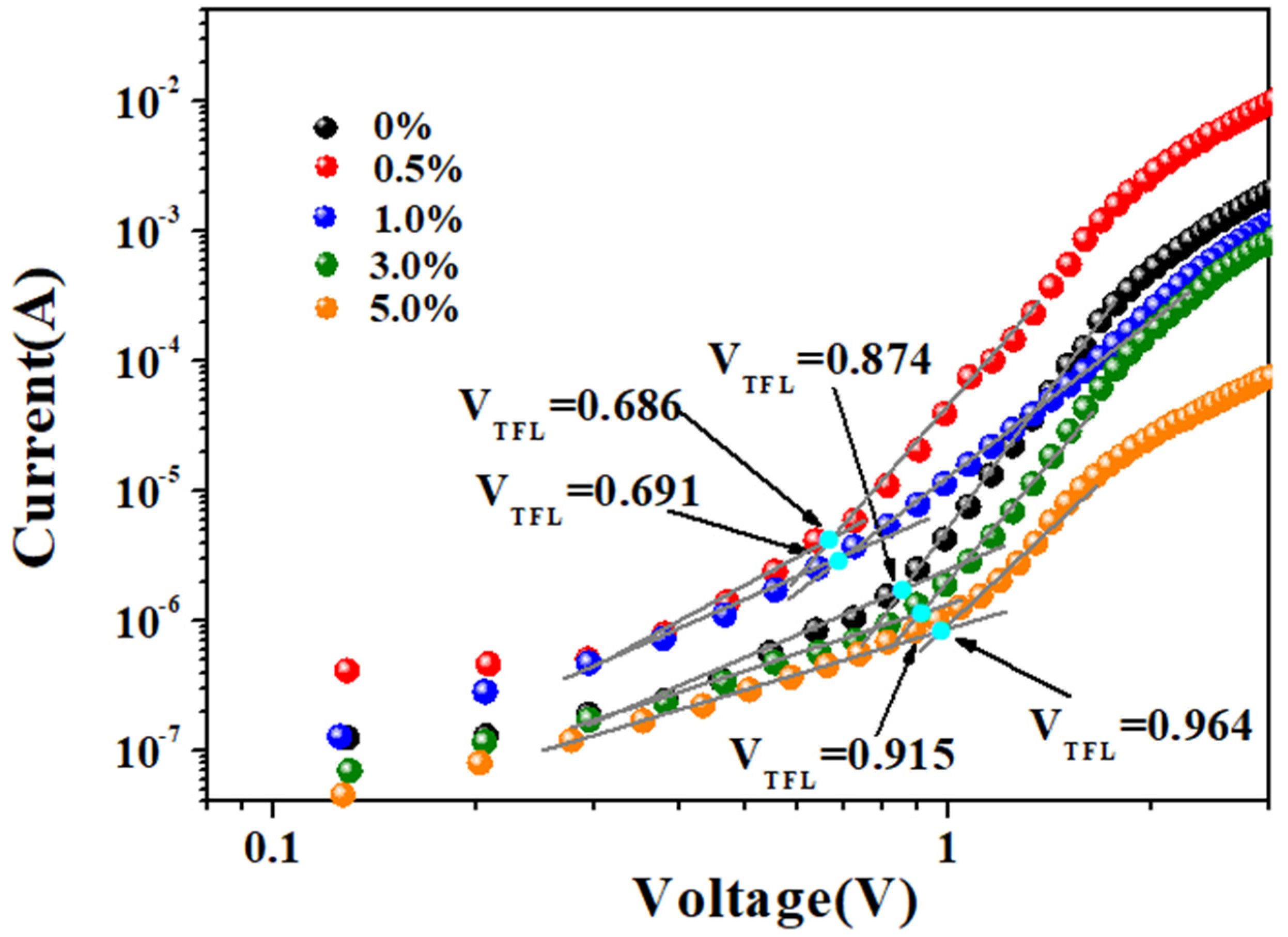
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, M.; Choi, I.W.; Go, E.M.; Cho, Y.; Kim, M.; Lee, B.; Jeong, S.; Jo, Y.; Choi, H.W.; Lee, J.; et al. Stable perovskite solar cells with efficiency exceeding 24.8% and 0.3-V voltage loss. Science 2020, 369, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Zhang, P.; Zhang, W. High intrinsic carrier mobility and photon absorption in the perovskite CH3NH3PbI3. Phys. Chem. Chem. Phys. 2015, 17, 11516–11520. [Google Scholar] [CrossRef]
- Kim, S.H.; Saeed, M.A.; Lee, S.Y.; Shim, J.W. Investigating the Indoor Performance of Planar Heterojunction Based Organic Photovoltaics. IEEE J. Photovoltaics 2021, 11, 997–1003. [Google Scholar] [CrossRef]
- Li, M.; Gao, D.; Zhang, B.; Xu, S.; Zhuang, X.; Wang, C.; Yang, L.; Ma, X.; Zheng, S.; Song, H.; et al. Multifunctional Reductive Molecular Modulator toward Efficient and Stable Perovskite Solar Cells. Sol. RRL 2021, 5, 2100320. [Google Scholar] [CrossRef]
- Dar, M.I.; Jacopin, G.; Meloni, S.; Mattoni, A.; Arora, N.; Boziki, A.; Zakeeruddin, S.M.; Rothlisberger, U.; Grätzel, M. Origin of unusual bandgap shift and dual emission in organic-inorganic lead halide perovskites. Sci. Adv. 2016, 2, e1601156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, C.; Zheng, X.; Chen, B.; Wei, H.; Huang, J. Spontaneous Passivation of Hybrid Perovskite by Sodium Ions from Glass Substrates: Mysterious Enhancement of Device Efficiency Revealed. ACS Energy Lett. 2017, 2, 1400–1406. [Google Scholar] [CrossRef]
- Li, Y.; Sun, W.; Yan, W.; Ye, S.; Rao, H.; Peng, H.; Zhao, Z.; Bian, Z.; Liu, Z.; Zhou, H.; et al. 50% Sn-Based Planar Perovskite Solar Cell with Power Conversion Efficiency up to 13.6%. Adv. Energy Mater. 2016, 6, 1601353. [Google Scholar] [CrossRef]
- Navas, J.; Sánchez-Coronilla, A.; Gallardo, J.J.; Hernández, N.C.; Pinero, J.C.; Alcántara, R.; Fernández-Lorenzo, C.; De los Santos, D.M.; Aguilar, T.; Martín-Calleja, J. New insights into organic–inorganic hybrid perovskite CH3NH3PbI3 nanoparticles. An experimental and theoretical study of doping in Pb2+ sites with Sn2+, Sr2+, Cd2+ and Ca2+. Nanoscale 2015, 7, 6216–6229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shang, M.-H.; Zheng, X.; Zeng, Z.; Chen, R.; Zhang, Y.; Zhu, Y. Ba2+ Doped CH3NH3PbI3 to Tune the Energy State and Improve the Performance of Perovskite Solar Cells. Electrochimica Acta 2017, 254, 165–171. [Google Scholar] [CrossRef]
- Jahandar, M.; Heo, J.H.; Song, C.E.; Kong, K.; Shin, W.S.; Lee, J.C.; Im, S.H.; Moon, S.J. Highly efficient metal halide substituted CH3NH3I(PbI2)1−X(CuBr2)X planar perovskite solar cells. Nano Energy 2016, 27, 330–339. [Google Scholar] [CrossRef]
- Zhao, W.; Yao, Z.; Yu, F.; Yang, D.; Liu, S. Alkali Metal Doping for Improved CH3NH3PbI3 Perovskite Solar Cells. Adv. Sci. 2017, 5, 1700131. [Google Scholar] [CrossRef] [Green Version]
- Nayak, P.K.; Sendner, M.; Wenger, B.; Wang, Z.; Sharma, K.; Ramadan, A.J.; Lovrinčić, R.; Pucci, A.; Madhu, P.K.; Snaith, H.J. Impact of Bi3+ Heterovalent Doping in Organic–Inorganic Metal Halide Perovskite Crystals. J. Am. Chem. Soc. 2018, 140, 574–577. [Google Scholar] [CrossRef]
- Abdelhady, A.L.; Saidaminov, M.I.; Murali, B.; Adinolfi, V.; Voznyy, O.; Katsiev, K.; Alarousu, E.; Comin, R.; Dursun, I.; Sinatra, L.; et al. Heterovalent Dopant Incorporation for Bandgap and Type Engineering of Perovskite Crystals. J. Phys. Chem. Lett. 2016, 7, 295–301. [Google Scholar] [CrossRef]
- Shahbazi, S.; Tsai, C.-M.; Narra, S.; Wang, C.-Y.; Shiu, H.-S.; Afshar, S.; Taghavinia, N.; Diau, E.W.-G. Ag Doping of Organometal Lead Halide Perovskites: Morphology Modification and p-Type Character. J. Phys. Chem. C 2017, 121, 3673–3679. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Pazoki, M.; Philippe, B.; Dar, M.I.; Alsari, M.; Sadhanala, A.; Divitini, G.; Imani, R.; Lilliu, S.; Kullgren, J.; et al. Dedoping of Lead Halide Perovskites Incorporating Monovalent Cations. ACS Nano 2018, 12, 7301–7311. [Google Scholar] [CrossRef] [PubMed]
- Si, F.J.; Tang, F.L.; Xue, H.T.; Liu, J.B. Electronic and optical properties of CH3NH3PbPb1-xAgxI3 from the first-principles calculations. J. Renew. Sustain. Energy 2018, 10, 033504. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.-Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.-P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Zhao, Y.; Zhang, X.W.; Yang, X.L.; Chen, Y.; Chu, Z.; Ye, Q.F.; Li, X.X.; Yin, Z.G.; You, J.B. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 1, 460–466. [Google Scholar] [CrossRef]
- Guo, Q.; Li, C.; Qiao, W.; Ma, S.; Wang, F.; Zhang, B.; Hu, L.; Dai, S.; Tan, Z. The growth of a CH3NH3PbI3 thin film using simplified close space sublimation for efficient and large dimensional perovskite solar cells. Energy Environ. Sci. 2016, 9, 1486–1494. [Google Scholar] [CrossRef]
- Gao, H.; Bao, C.; Li, F.; Yu, T.; Yang, J.; Zhu, W.; Zhou, X.; Fu, G.; Zou, Z. Nucleation and Crystal Growth of Organic–Inorganic Lead Halide Perovskites under Different Relative Humidity. ACS Appl. Mater. Interfaces 2015, 7, 9110–9117. [Google Scholar] [CrossRef]
- Singh, T.; Miyasaka, T. Stabilizing the Efficiency Beyond 20% with a Mixed Cation Perovskite Solar Cell Fabricated in Ambient Air under Controlled Humidity. Adv. Energy Mater. 2017, 8, 1700677. [Google Scholar] [CrossRef]
- Yang, F.; Kapil, G.; Zhang, P.; Hu, Z.; Kamarudin, M.A.; Ma, T.; Hayase, S. Dependence of Acetate-Based Antisolvents for High Humidity Fabrication of CH3NH3PbI3 Perovskite Devices in Ambient Atmosphere. ACS Appl. Mater. Interfaces 2018, 10, 16482–16489. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Hao, H.; Li, J.; Shi, L.; Zhong, T.; Zhang, C.; Dong, J.; Xing, J.; Liu, H.; Zhang, Z. Light Trapping Effect in Perovskite Solar Cells by the Addition of Ag Nanoparticles, Using Textured Substrates. Nanomaterials 2018, 8, 815. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Yang, Y.; Hong, Z.; Song, T.-B.; Meng, L.; Liu, Y.; Jiang, C.; Zhou, H.; Chang, W.-H.; Li, G. Moisture assisted perovskite film growth for high performance solar cells. Appl. Phys. Lett. 2014, 105, 183902. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Ye, Z.; Sarvari, H.; Park, S.M.; Abtahi, A.; Graham, K.; Zhao, Y.; Wang, Y.; Chen, Z.D.; Li, S. Humidity-insensitive fabrication of efficient perovskite solar cells in ambient air. J. Power Sources 2018, 412, 359–365. [Google Scholar] [CrossRef]
- Pascoe, A.R.; Meyer, S.; Huang, W.; Li, W.; Benesperi, I.; Duffy, N.W.; Spiccia, L.; Bach, U.; Cheng, Y.-B. Enhancing the Optoelectronic Performance of Perovskite Solar Cells via a Textured CH3NH3PbI3Morphology. Adv. Funct. Mater. 2016, 26, 1278–1285. [Google Scholar] [CrossRef]
- Lo, M.-F.; Guan, Z.-Q.; Ng, T.-W.; Chan, C.-Y.; Lee, C.-S. Electronic Structures and Photoconversion Mechanism in Perovskite/Fullerene Heterojunctions. Adv. Funct. Mater. 2014, 25, 1213–1218. [Google Scholar] [CrossRef]
- Luo, D.; Yang, W.; Wang, Z.; Sadhanala, A.; Hu, Q.; Su, R.; Shivanna, R.; Trindade, G.F.; Watts, J.F.; Xu, Z.; et al. Enhanced photovoltage for inverted planar heterojunction perovskite solar cells. Science 2018, 360, 1442–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poglitsch, A.; Weber, D. Dynamic disorder in methylammoniumtrihalogenoplumbates (II) observed by millimeter-wave spectroscopy. J. Chem. Phys. 1987, 87, 6373–6378. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Ren, X.; Zhu, X.; Yang, Z.; Li, C.; Liu, S. Hysteresis-Suppressed High-Efficiency Flexible Perovskite Solar Cells Using Solid-State Ionic-Liquids for Effective Electron Transport. Adv. Mater. 2016, 28, 5206–5213. [Google Scholar] [CrossRef] [PubMed]
- Bube, R.H. Trap density determination by space-charge-limited currents. J. App. Phys. 1962, 33, 1733–1737. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths >175 μm in solution-grown CH3NH3PbI3single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Ag Concentration | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) |
|---|---|---|---|---|
| 0% | 0.973 | 21.08 | 0.556 | 11.40 |
| 0.5% | 0.997 | 22.04 | 0.608 | 13.35 |
| 1.0% | 1.035 | 22.08 | 0.624 | 14.26 |
| 3.0% | 0.974 | 21.88 | 0.522 | 11.12 |
| 5.0% | 0.994 | 21.13 | 0.511 | 10.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Wang, Z.; Hao, H.; Wang, G.; Gao, H.; Wang, J.; Pan, B.; Qi, Q. Efficient Ag-Doped Perovskite Solar Cells Fabricated in Ambient Air. Crystals 2021, 11, 1521. https://doi.org/10.3390/cryst11121521
Hao J, Wang Z, Hao H, Wang G, Gao H, Wang J, Pan B, Qi Q. Efficient Ag-Doped Perovskite Solar Cells Fabricated in Ambient Air. Crystals. 2021; 11(12):1521. https://doi.org/10.3390/cryst11121521
Chicago/Turabian StyleHao, Jiabin, Zeming Wang, Huiying Hao, Guanlei Wang, Hongcheng Gao, Jianyu Wang, Bing Pan, and Qiang Qi. 2021. "Efficient Ag-Doped Perovskite Solar Cells Fabricated in Ambient Air" Crystals 11, no. 12: 1521. https://doi.org/10.3390/cryst11121521





