The Application of Organic Phosphate Nucleating Agents in Polypropylene with Different Molecular Weights
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Specimen Preparation
2.3. Characterization
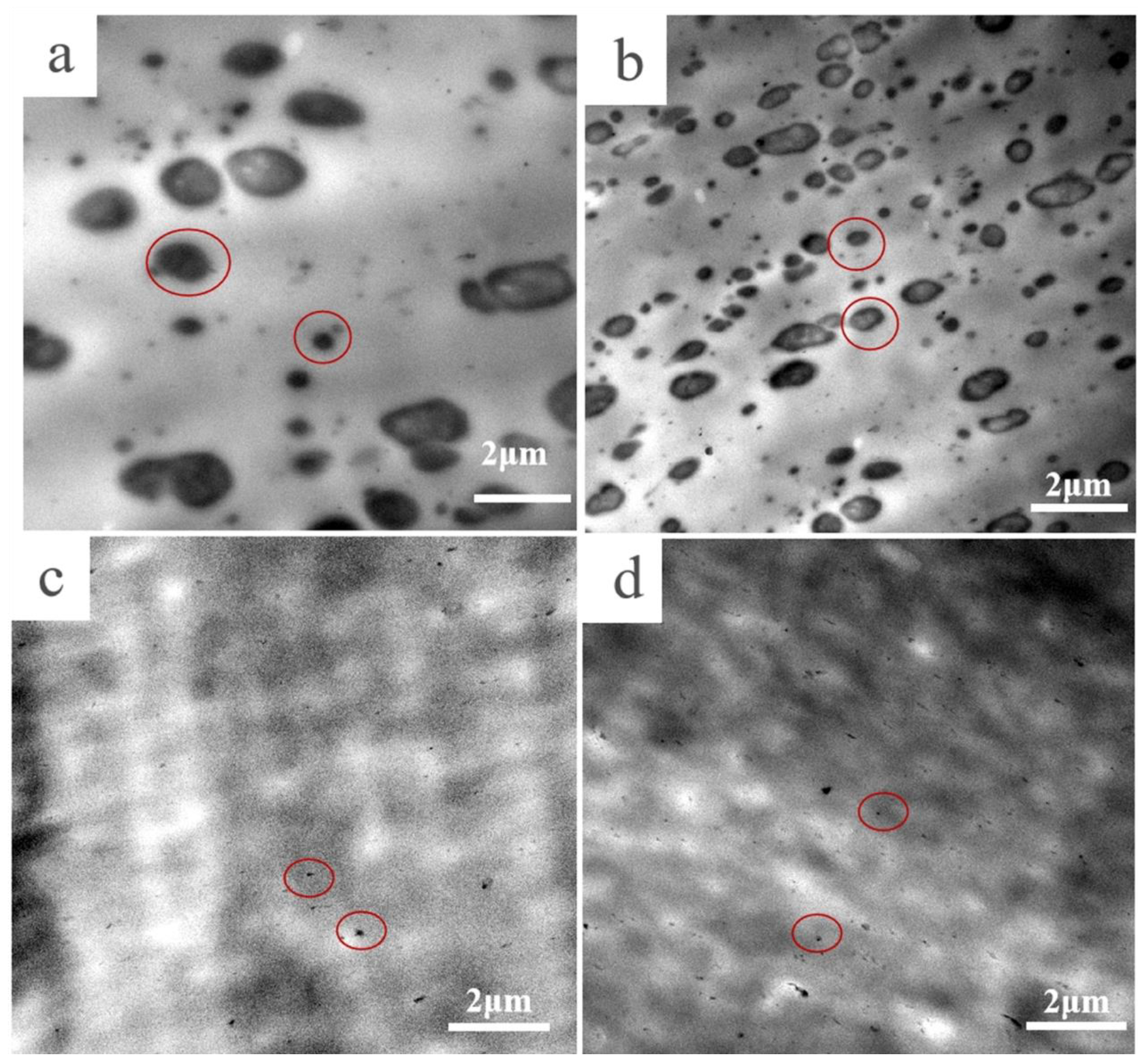
2.3.1. Morphological Characterizations
2.3.2. Melt Flow Rate (MFR)
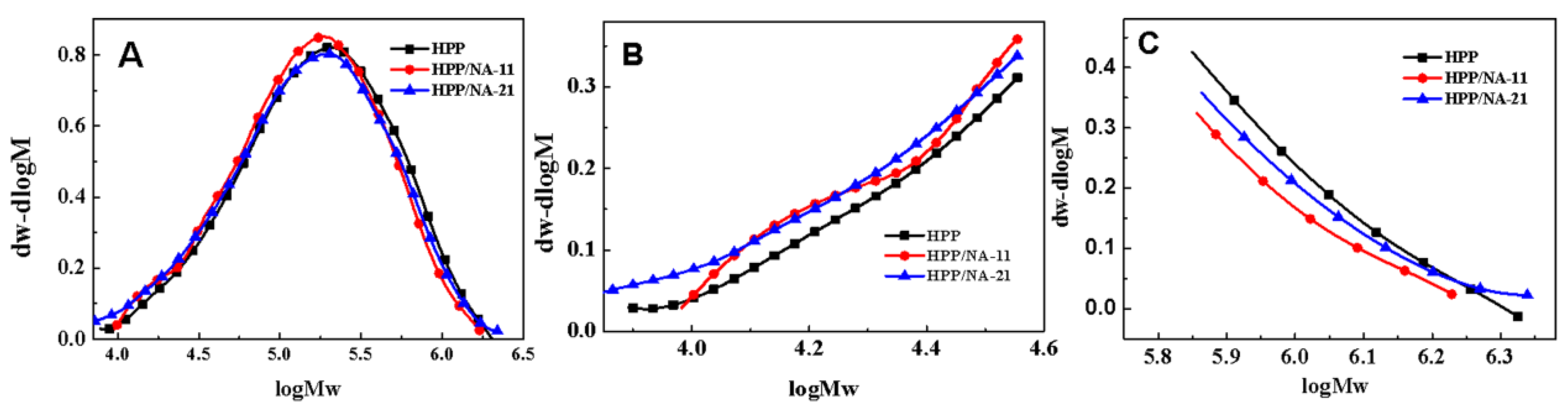
2.3.3. Gel Permeation Chromatograph (GPC)
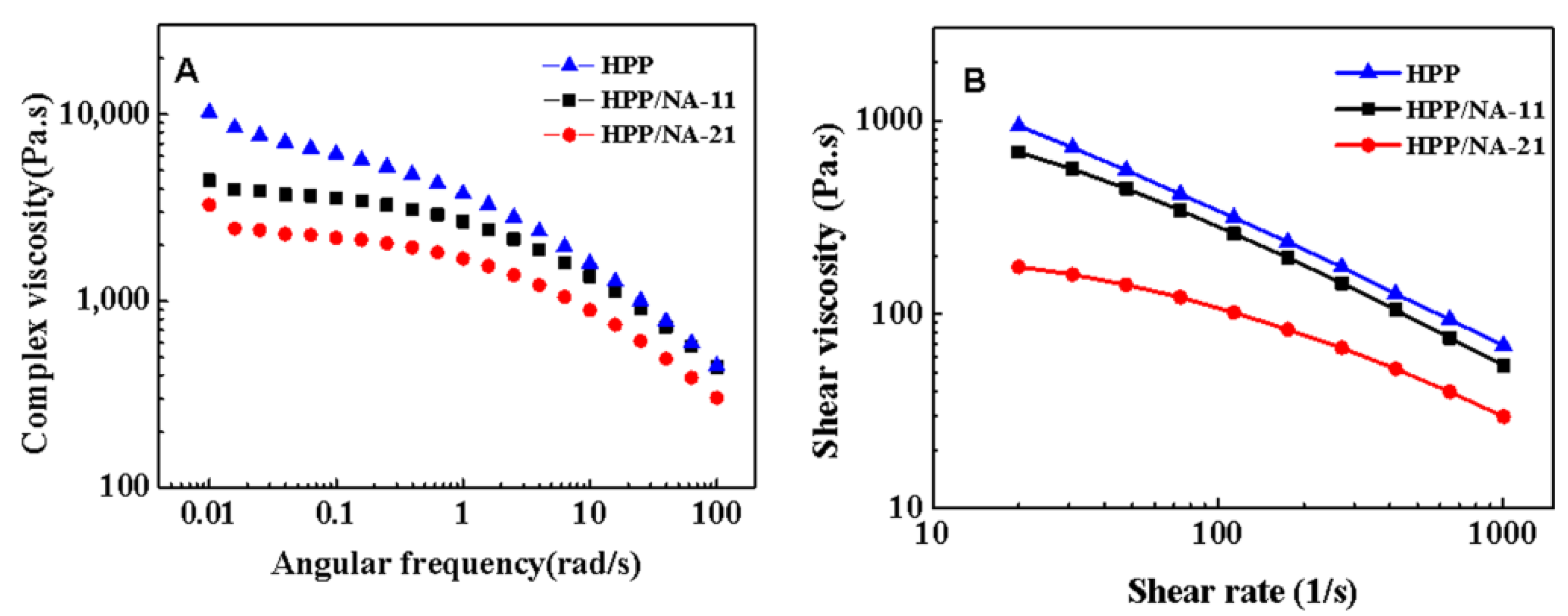
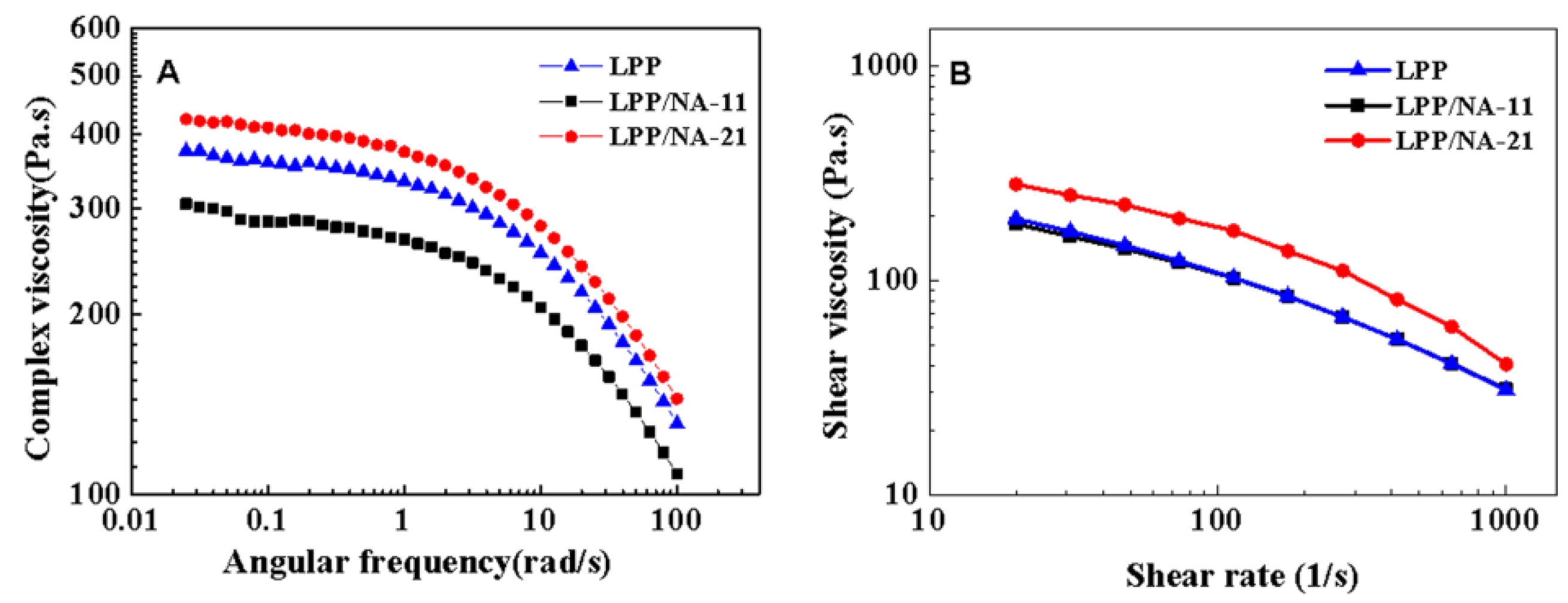
2.3.4. Rheological Analysis
Rotational Rheometer
Capillary Rheology
2.3.5. Differential Scanning Calorimeter
3. Results and Discussion
3.1. Morphological Study
3.2. Change of Molecular Weight
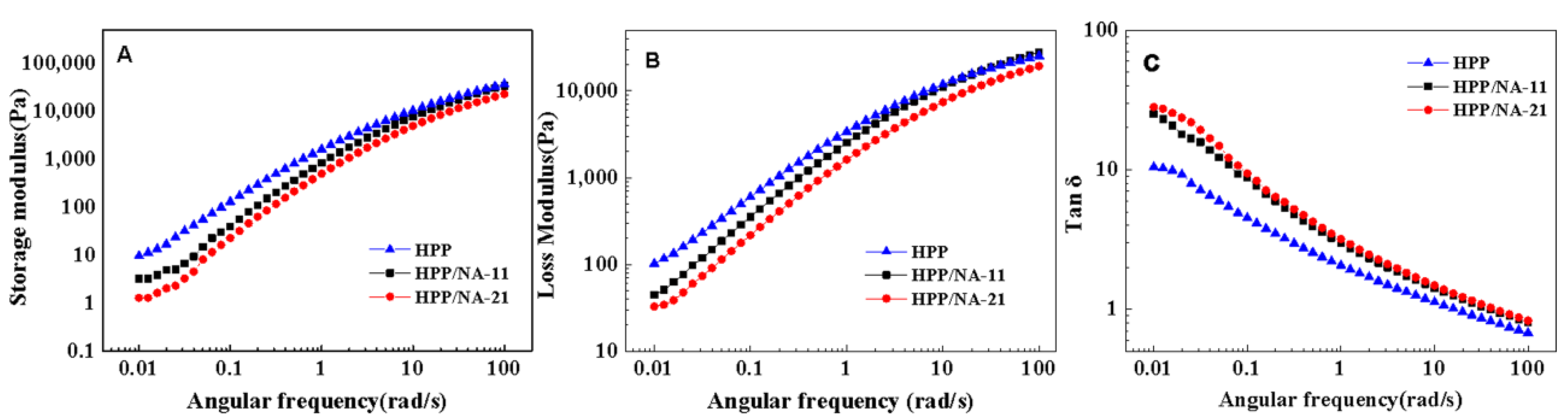
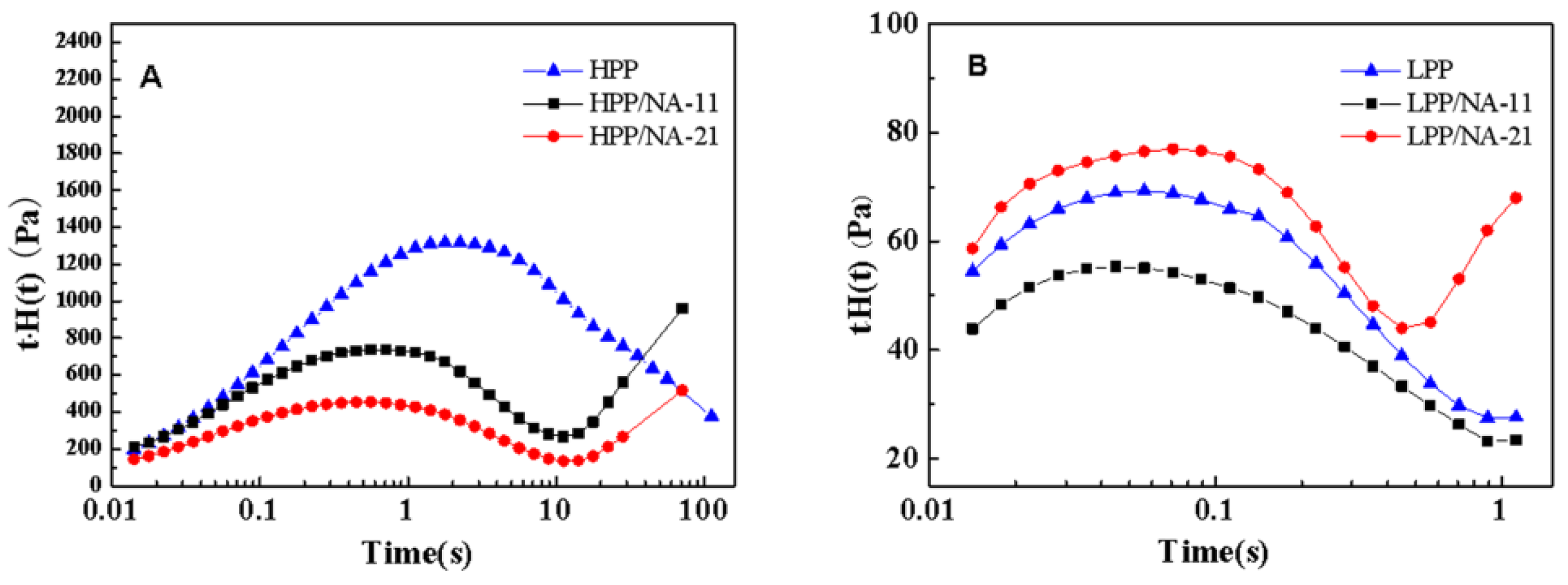
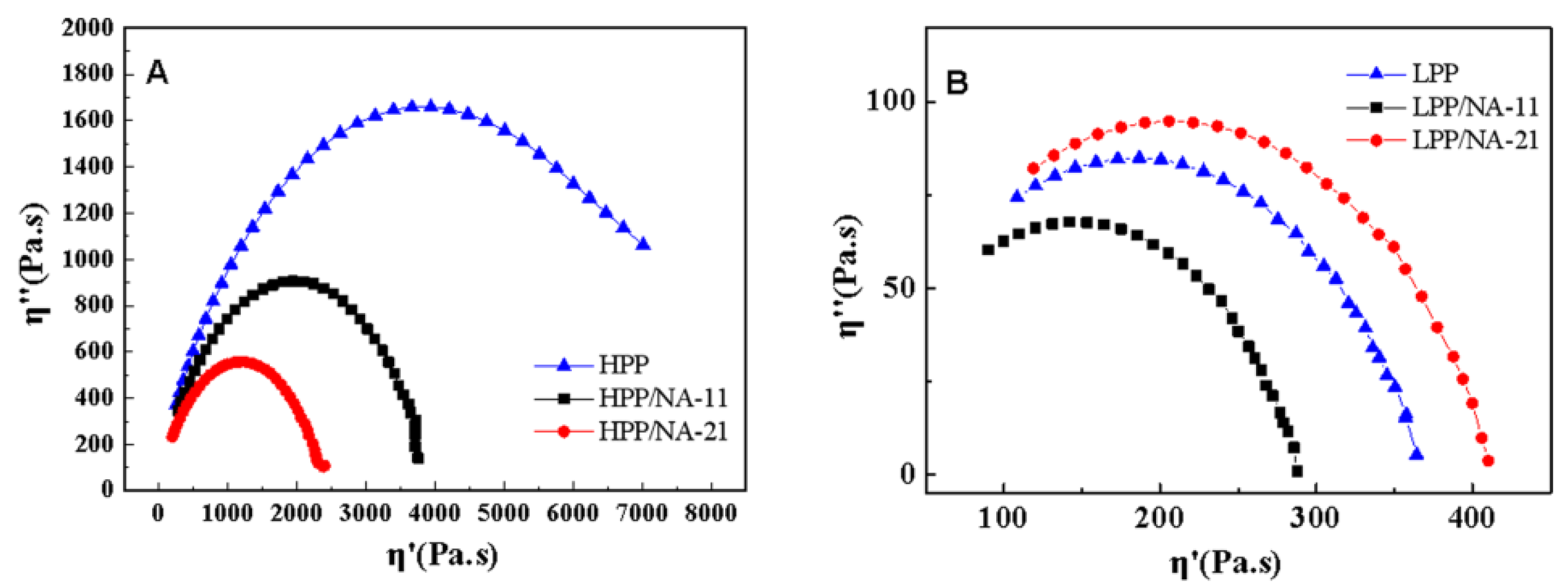
3.3. Rheological Behaviors
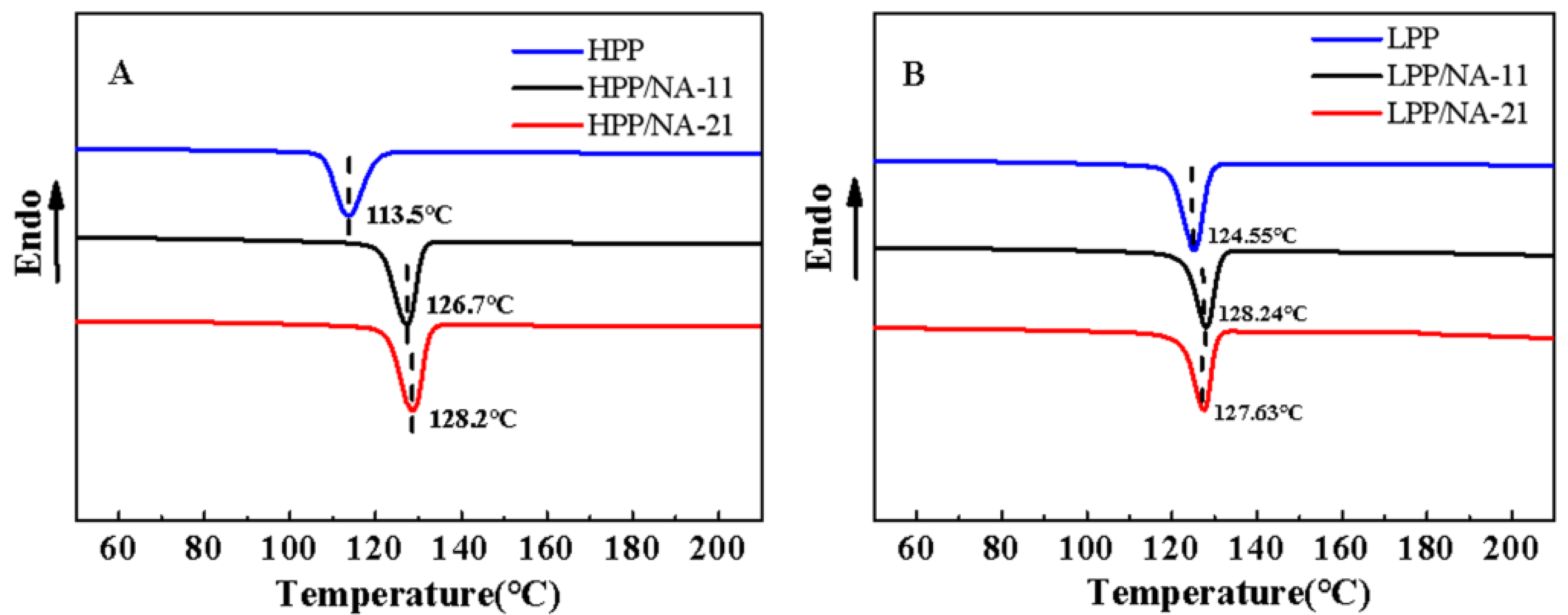
3.4. Thermal Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Markus, B.; Sandra, G.; Doris, H.; Hans, W.S.; Magnus, K.; Paul, S.; Klaus, S.; Dietmar, M.; Kurt, H. “Designer” Nucleating Agents for Polypropylene. Macromolecules 2005, 38, 3688–3695. [Google Scholar]
- Zoe, V.Q.J.; Carlos, A.; Blanca, E.C.; José, M.B.H. Effect of Sorbitol Templates on the Preferential Crystallographic Growth of Isotactic Polypropylene Wax. Crystals 2018, 8, 59. [Google Scholar]
- Roberto, S.; Marcel, S.; Christian, H. Simulation of crystallization of isotactic polypropylene with different shear regimes. Thermochim. Acta 2018, 10, 44–54. [Google Scholar]
- Li, J.; Long, L.J.; He, W.-T.; Zhang, K. Crystallization Behavior and Mechanical Properties of Nanosilica-Reinforced Isotactic Polypropylene Composites. Intern. Polym. Process. 2015, 30, 542–547. [Google Scholar] [CrossRef]
- Sowinski, P.; Piorkowska, E.; Boyer, S.; Haudin, J. Nucleation of crystallization of isotactic polypropylene in the gamma form under high pressure in nonisothermal conditions. Eur. Polym. J. 2016, 85, 564–574. [Google Scholar] [CrossRef]
- Bartosz, S.; Grzegorz, L.G. Improvement of Strength Parameters of Cement Matrix with the Addition of Siliceous Fly Ash by Using Nanometric C-S-H Seeds. Energies 2020, 13, 6734. [Google Scholar]
- Jiang, X.; Zhao, S.; Meng, X.; Xin, Z. Effect of the Metal Phenylphosphonates on the Nonisothermal Crystallization and Performance of Isotactic Polypropylene. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 161–173. [Google Scholar] [CrossRef]
- Hou, W.M.; Liu, G.; Zhou, J.J. The influence of crystal structures of nucleating agents on the crystallization behaviors of isotactic polypropylene. Colloid Polym. Sci. 2006, 285, 11–17. [Google Scholar] [CrossRef]
- Carolus, H.R.M.; Wilsens, L.G.D.H.; Enrico, M.T.; Daniel, H.M.; Gijs, K.; Nils, L.; Ketie, S.; Gerrit, W.M.P.; Sanjay, R. Effect of Self-Assembly of Oxalamide Based Organic Compounds on Melt Behavior, Nucleation, and Crystallization of Isotactic Polypropylene. Macromolecules 2018, 51, 4882–4895. [Google Scholar]
- An, Y.J.; Wang, S.H.; Li, R.; Shi, D.Z.; Gao, Y.X.; Song, L. Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene. ePolymers 2019, 19, 32–39. [Google Scholar] [CrossRef]
- Zhu, P.W.; Edward, G. Distribution of shish-kebab structure of isotactic polypropylene under shear in the presence of nucleatingagent. Macromolecules 2004, 37, 2658–2660. [Google Scholar] [CrossRef]
- Zhao, S.C.; Yu, X.; Gong, H.Z. The crystallization behavior of isotactic polypropylene induced by a novel antinucleating agent and its inhibition mechanism of nucleatione. Ind. Eng. Chem. Res. 2015, 54, 7650–7657. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, R.; Chen, Y.; Zhang, C.; Zhang, R.; Wang, X.; Kong, R.; Chen, Q. Using a Self-Assemblable Nucleating Agent to Tailor Crystallization Behavior, Crystal Morphology, Polymorphic Crystalline Structure, and Biodegradability of Poly(1,4-butyleneadipate). Ind. Eng. Chem. Res. 2017, 56, 7910–7919. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Huang, Y.J.; Liao, X.; Yang, Q. The molecular structure characteristics of long chain branched polypropylene and its effects on non-isothermal crystallization and mechanical properties. Polymer 2013, 54, 1455–1462. [Google Scholar] [CrossRef]
- Mubarak, Y.; Harkin-Jones, E.M.A.; Martin, P.J.; Ahmad, M. Modeling of non-isothermal crystallization kinetics of isotactic polypropylene. Polymer 2001, 42, 3171–3182. [Google Scholar] [CrossRef]
- Drongelen, M.; Erp, T.B.V.; Peters, G.W.M. Quantification of non-isothermal, multi-phase crystallization of isotactic polypropylene: The influence of cooling rate and pressure. Polymer 2012, 53, 4758–4769. [Google Scholar] [CrossRef]
- Deshmukh, Y.S.; Wilsens, C.H.R.M.; Leoné, N.; Portale, G.; Harings, J.A.W.; Rastogi, S. Melt-Miscible Oxalamide BasedNucleating Agent and Their Nucleation Efficiency in Isotactic Polypropylene. Ind. Eng. Chem. Res. 2016, 55, 11756–11766. [Google Scholar] [CrossRef]
- Asteasuain, M.; Sarmoria, C.; Brandolin, A. Controlled rheology of polypropylene: Modeling of molecular weight distributions. J. Appl. Polym. Sci. 2010, 88, 1676–1685. [Google Scholar] [CrossRef]
- Sugimoto, M.; Masubuchi, Y.; Takimoto, J. Melt rheology of polypropylene containing small amounts of high molecular weight chain. I. Shear flow. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2692–2704. [Google Scholar] [CrossRef]
- Abbasi, F.; Tavakoli, A.; Razavi Aghjeh, M.K. Rheology, morphology, and mechanical properties of reactive compatibilized polypropylene/polystyrene blends via Friedel–Crafts alkylation reaction in the presence of clay. J. Vinyl Addit. Technol. 2018, 24, 18–26. [Google Scholar] [CrossRef]
- Ardakani, F.; Jahani, Y.; Morshedian, J. The impact of viscoelastic behavior and viscosity ratio on the phase behavior and morphology of polypropylene/polybutene blends. J. Vinyl Addit. Technol. 2015, 21, 94–101. [Google Scholar] [CrossRef]
- Ivonne, O.N.; Milad, K.; Mohammad, A.; Uttandaraman, S. Morphology Evolution, Molecular Simulation, Electrical Properties, and Rheology of Carbon Nanotube/Polypropylene/Polystyrene Blend Nanocomposites: Effect of Molecular Interaction between Styrene-Butadiene Block Copolymer and Carbon Nanotube. Polymers 2021, 13, 230. [Google Scholar]
- Bendjaouahdou, C.; Bensaad, S. Properties of polypropylene/(natural rubber)/organo montmorillonite nanocomposites prepared by melt blending. J. Vinyl Addit. Technil. 2011, 17, 48–57. [Google Scholar] [CrossRef]
- Kilic, A.; Shim, E.; Yeom, B.Y.; Pourdeyhimi, B. Effect of DMDBS (3:2,4-bis(3,4-dimethyldibenzylidene) sorbitol) and NA11(sodium 2,2-methylene-bis (4,6-di-tertbutylphenyl)-phosphate) on electret properties of polypropylene Filaments. J. Appl. Polym. Sci. 2013, 130, 2068–2075. [Google Scholar] [CrossRef]
- Long, L.J.; He, W.T.; Zhang, M.M.; Zhang, K.; Qin, S.H.; Yu, J. Nucleation effects of sodium and ammonium salts of 2, 2′-methylene-bis-(4, 6-di-t-butylphenylene) phosphate in isotactic Polypropylene. Polym. Eng. Sci. 2015, 55, 22–28. [Google Scholar] [CrossRef]
- Long, L.J.; He, W.T.; Li, J.; Xiang, Y.S. Joint effects of molecular structure and crystal morphology of organophosphate monovalent salts on nucleated isotactic poly(propylene). J. Polym. Res. 2016, 23, 206. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.H.; He, W.T.; Xiang, Y.S. The effect of hybrid nucleating agent with silica sol as the supporter on the crystallization behavior and mechanical properties of isotactic polypropylene. J. Polym. Eng. 2015, 35, 565–573. [Google Scholar] [CrossRef]
- Huang, W.J.; Wentao, H.H.; Jie, Y. Synthesis, Characterization and Application of 2, 2′- methylene- bis (4, 6-di-tert-butyl-phenyl) Phosphate Sodium. Adv. Mater. Res. 2013, 834–836, 119–123. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zhou, P.Z.; Li, Y. The influences of α/β compound nucleating agents based on octamethylenedicarboxylic dibenzoylhydrazide on crystallization and melting behavior of isotactic polypropylene. Polym. Adv. Technol. 2019, 30, 1777–1788. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Mao, J.J.; Zhou, P.Z. The relation between chemical structure of dicarboxylic dihydrazide compounds and nucleation effect in isotactic polypropylene. J. Therm. Analy. Calorim. 2021, 145, 2379–2387. [Google Scholar] [CrossRef]
- Wang, B.; Utzeri, R.; Castellano, M.; Stagnaro, P.; MÃller, A.J.; Cavallo, D. Heterogeneous Nucleation and Self-Nucleation of Isotactic Polypropylene Microdroplets in Immiscible Blends: From Nucleation to Growth-Dominated Crystallization. Macromolecules 2020, 53, 5980–5991. [Google Scholar] [CrossRef]
- Zhang, Y.F.; He, B.; Zhou, H.H.; Guo, L.H. Isothermal Crystallization of Isotactic Polypropylene Nucleated with a Novel Aromatic Heterocyclic Phosphate Nucleating Agent. J. Macromol. Sci. Part B 2017, 56, 811–820. [Google Scholar] [CrossRef]
- Vladimirov, V.; Betchev, C.; Vassiliou, A. Dynamic mechanical and morphological studies of isotactic polypropylene/fumed silica nanocomposites with enhanced gas barrier properties. Compos. Sci. Technol. 2006, 66, 2935–2944. [Google Scholar] [CrossRef]
- González-González, V.A.; Neira-Velázquez, G.; Angulo-Sánchez, J.L. Polypropylene chain scissions and molecular weight changes in multiple extrusion. Polym. Degrad. Stab. 1998, 60, 33–42. [Google Scholar] [CrossRef]
- Bueche, F. Mechanical degradation of high polymers. J. Appl. Polym. Sci. 1960, 4, 101–106. [Google Scholar] [CrossRef]
- Hassani, A.J. Preparation and characterization of polyamide 6 nanocomposites using MWCNTs based on bimetallic Co-Mo/MgO catalyst. Express Polym. Lett. 2014, 8, 177–186. [Google Scholar] [CrossRef]
- Lazár, M.; HrcKová, L.; Borsig, E. Course of degradation and build-up reactions in isotactic polypropylene during peroxide decomposition. J. Appl. Polym. Sci. 2015, 78, 886–893. [Google Scholar] [CrossRef]
- Tzoganakis, C.; Vlachopoulos, J.; Hamielec, A.E. Production of controlled-rheology polypropylene resins by peroxide promoted degradation during extrusion. Polym. Eng. Sci. 2010, 28, 170–180. [Google Scholar] [CrossRef]
- Bremner, T.; Rudin, A.; Cook, D.G. Melt flow index values and molecular weight distributions of commercial thermoplastics. J. Appl. Polym. Sci. 1990, 41, 1617–1627. [Google Scholar] [CrossRef]
- Gui, Z.Y.; Wang, H.R.; Gao, Y. Morphology and melt rheology of biodegradable poly(lactic acid)/poly(butylene succinate adipate) blends: Effect of blend compositions. Iran. Polym. J. 2012, 21, 81–89. [Google Scholar] [CrossRef]
- Wang, Y. Role of surfactant molecular weight on morphology and properties of functionalized graphite oxide filled polypropylene nanocomposites. Express Polym. Lett. 2014, 8, 881–894. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, T.; Mu, M.; Winey, K. Relation between the viscoelastic and flammability properties of polymer nanocomposites. Polymer 2008, 49, 4358–4368. [Google Scholar] [CrossRef]



| Samples | MFI (g/10 min) | Mw (g/mol) | Mn (g/mol) | Mz (g/mol) | Mw/Mn |
|---|---|---|---|---|---|
| HPP | 2.7 | 273,381 | 91,056 | 565,908 | 3.00 |
| HPP/NA-11 | 5.9 | 239,676 | 84,364 | 493,107 | 2.84 |
| HPP/NA-21 | 5.3 | 258,599 | 97,818 | 518,524 | 2.64 |
| LPP | 65.8 | 151,948 | 53,939 | 326,923 | 2.81 |
| LPP/NA-11 | 72.4 | 144,440 | 47,976 | 314,034 | 3.01 |
| LPP/NA-21 | 59.2 | 149,623 | 52,078 | 322,823 | 2.87 |
| Sample | Tc/°C | Tm/°C | Hm/(J‧g−1) | Xc(%) |
|---|---|---|---|---|
| HPP | 113.50 | 163.93 | 92.97 | 44.48 |
| HPP/NA-11 | 126.70 | 166.16 | 99.91 | 47.80 |
| HPP/NA-21 | 128.20 | 165.25 | 106.90 | 51.15 |
| LPP | 124.55 | 160.94 | 91.47 | 43.77 |
| LPP/NA-11 | 128.24 | 161.59 | 95.75 | 45.81 |
| LPP/NA-21 | 127.63 | 160.26 | 97.48 | 46.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liang, Z.; Gao, C.; Luo, S.; Huang, S.; Zhang, D.; Qin, S. The Application of Organic Phosphate Nucleating Agents in Polypropylene with Different Molecular Weights. Crystals 2021, 11, 1543. https://doi.org/10.3390/cryst11121543
Li J, Liang Z, Gao C, Luo S, Huang S, Zhang D, Qin S. The Application of Organic Phosphate Nucleating Agents in Polypropylene with Different Molecular Weights. Crystals. 2021; 11(12):1543. https://doi.org/10.3390/cryst11121543
Chicago/Turabian StyleLi, Juan, Zhaohua Liang, Chengtao Gao, Shanshan Luo, Shaowen Huang, Daohai Zhang, and Shuhao Qin. 2021. "The Application of Organic Phosphate Nucleating Agents in Polypropylene with Different Molecular Weights" Crystals 11, no. 12: 1543. https://doi.org/10.3390/cryst11121543






