Low-Dimensional CsPbBr3@CoBr2 Super-Nanowire Structure for Perovskite/PMMA Composite with Highly Blue Emissive Performance
Abstract
:1. Introduction
2. Materials and Methods
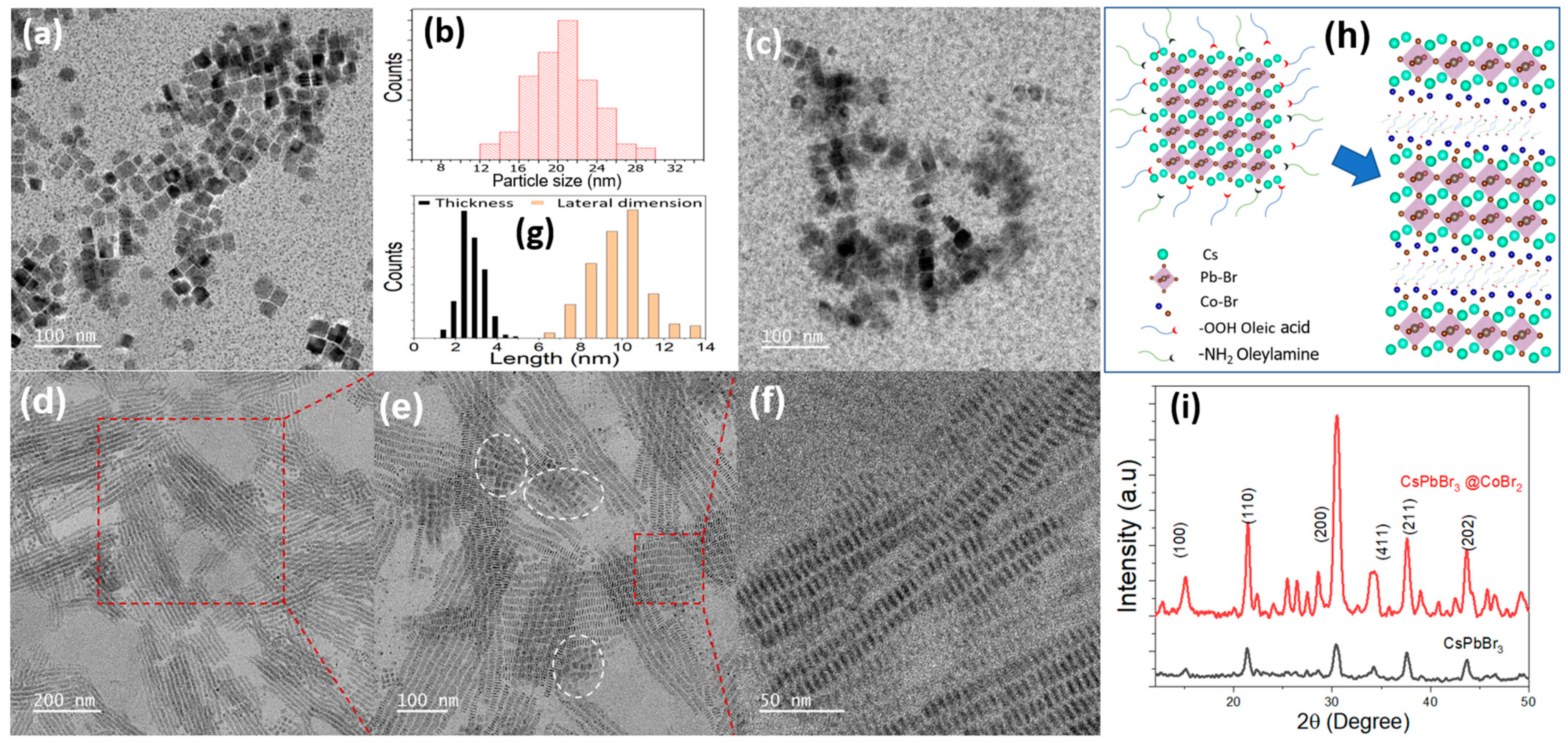
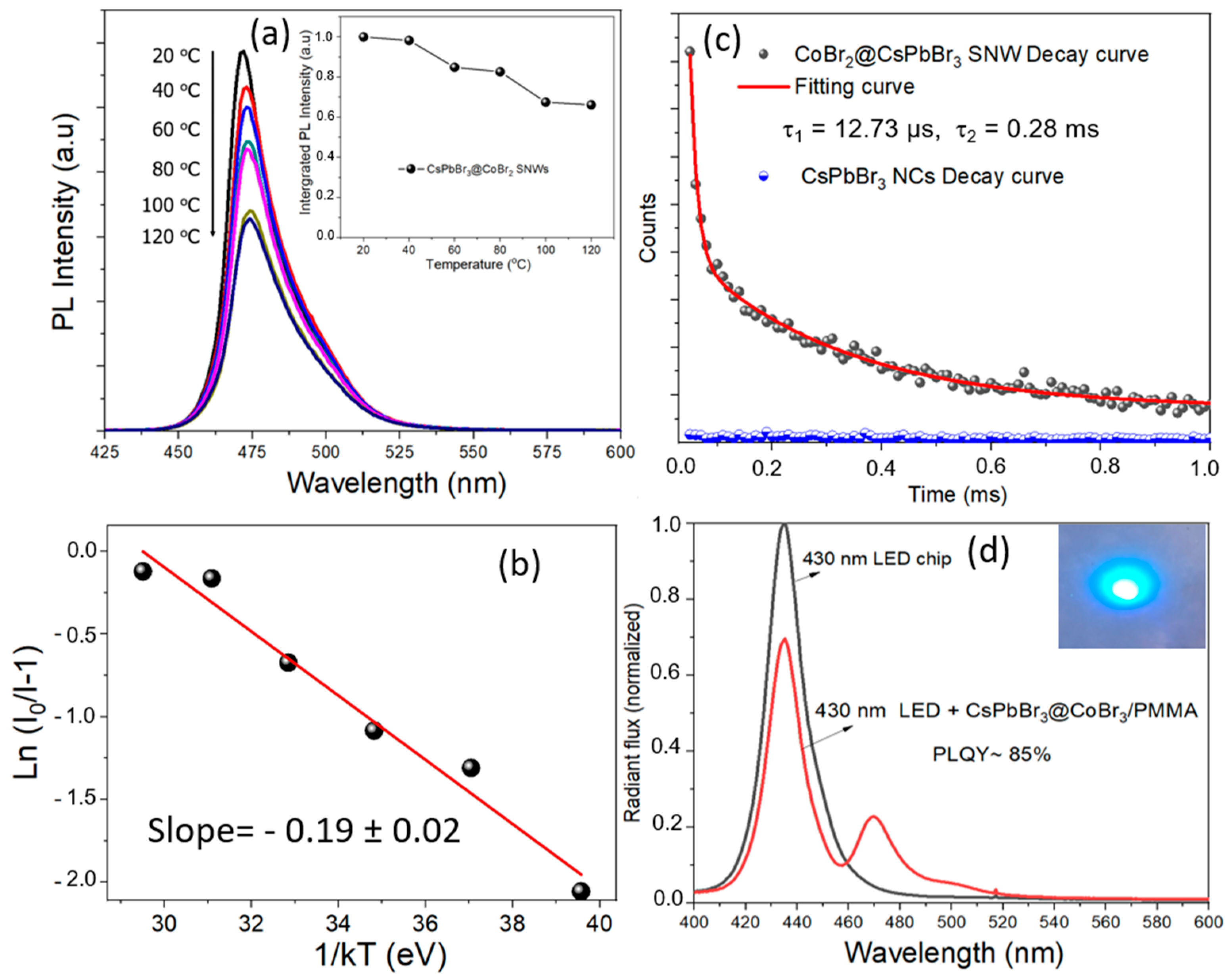
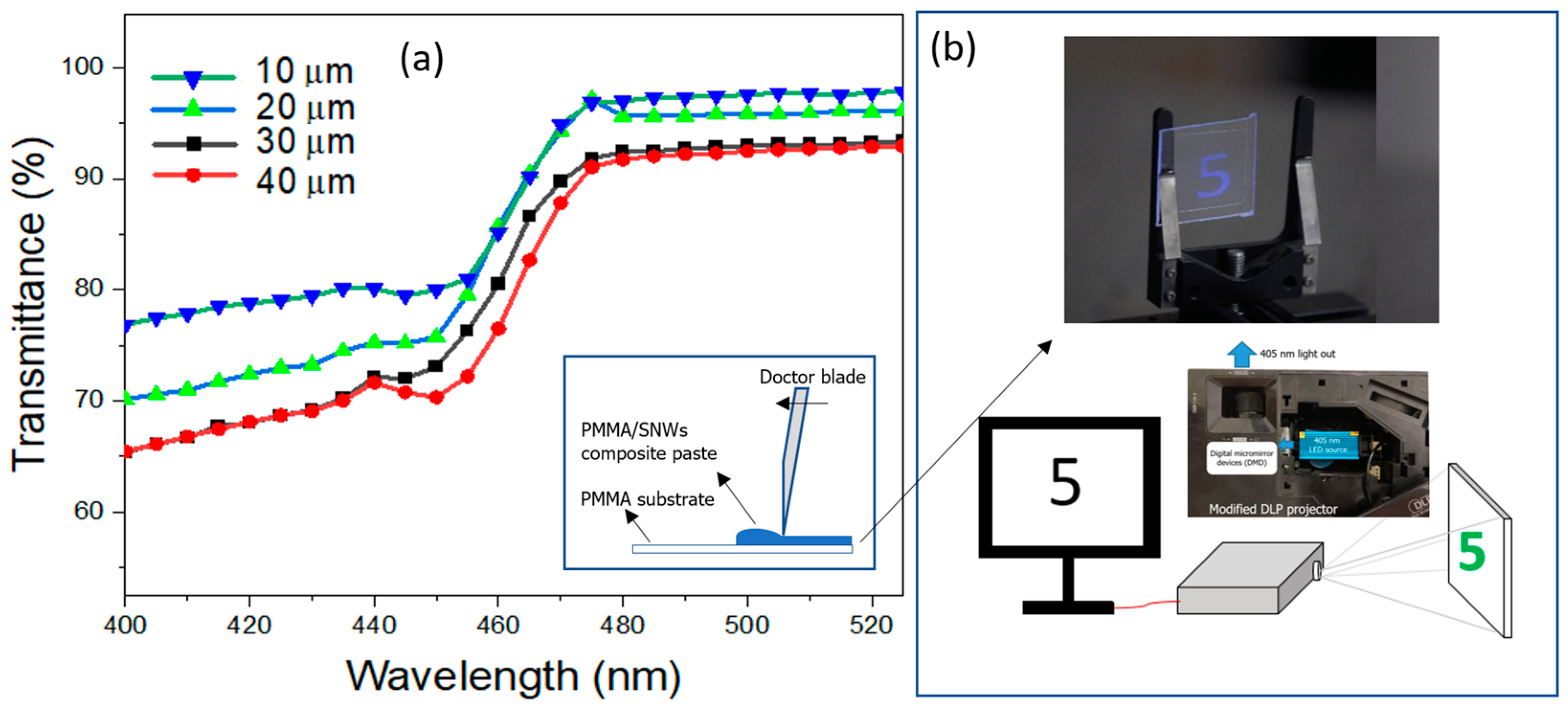
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yun, S.; Kirakosyan, A.; Yoon, S.; Choi, J. Scalable Synthesis of Exfoliated Organometal Halide Perovskite Nanocrystals by Ligand-Assisted Ball Milling. ACS Sustain. Chem. Eng. 2018, 6, 3733–3738. [Google Scholar] [CrossRef]
- Zhihai, W.; Jiao, W.; Yanni, S.; Jun, W.; Yafei, H.; Pan, W.; Nengping, W.; Zhenfu, Z. Air-stable all-inorganic perovskite quantum dot inks for multicolor patterns and white LEDs. J. Mater. Sci. 2019, 54, 6917–6929. [Google Scholar] [CrossRef]
- Adhiraki, G.; Thapa, S.; Zhu, H.; Zhu, P. Synthesis of CsPbBr3 and Transformation into Cs4PbBr6 Crystals for White Light Emission with High CRI and Tunable CCT. J. Phys. Chem. 2019, 123, 12023–12028. [Google Scholar]
- Yong, Z.J.; Guo, S.Q.; Ma, J.P.; Zhang, J.Y.; Li, Z.Y.; Chen, Y.M.; Zhang, B.B.; Zhou, Y.; Shu, Y.; Gu, J.L.; et al. Doping-Enhanced Short-Range Order of Perovskite Nanocrystals for Near-Unity Violet Luminescence Quantum Yield. J. Am. Chem. Soc. 2018, 140, 9942–9951. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M.; et al. Stabilizing Cesium Lead Halide Perovskite Lattice through Mn(II) Substitution for Air-Stable Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zou, Y.; Bourelle, S.A.; Zhai, T.; Wu, T.; Tan, Y.; Li, Y.; Li, J.; Duhm, S.; Song, T.; et al. Suppressing defect states in CsPbBr3 perovskite via magnesium substitution for efficient all-inorganic light-emitting diodes. Nanoscale Horiz. 2019, 4, 924–932. [Google Scholar] [CrossRef]
- Liu, S.; Shao, G.; Ding, L.; Liu, J.; Xiang, W.; Liang, X. Sn-doped CsPbBr3 QDs glasses with excellent stability and optical properties for WLED. Chem. Eng. J. 2019, 361, 937–944. [Google Scholar] [CrossRef]
- Boote, B.W.; Andaraarachchi, H.P.; Rosales, B.A.; BlomeFernandez, R.; Zhu, F.; Reichert, M.D.; Santra, K.; Li, J.; Petrich, J.W.; Vela, J.; et al. Unveiling the Photo-And Thermal-Stability of Cesium Lead Halide Perovskite Nanocrystals. ChemPhysChem 2019, 20, 2647–2656. [Google Scholar] [CrossRef]
- Abib, M.; Li, A.; Yang, H.; Wang, M.; Chen, T.; Xu, E.; Jiang, Y. Direct deposition of Sn-doped CsPbBr3 perovskite for efficient solar cell application. RSC Adv. 2021, 11, 3380–3389. [Google Scholar] [CrossRef]
- Li, A.; Zhou, Z.; Vashishtha, P.; Halpert, J.E. The Future Is Blue (LEDs): Why Chemistry Is the Key to Perovskite Displays. Chem. Mater. 2019, 31, 6003–6032. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Shi, Z.; Xia, Y.; Wei, Q.; Chen, Y.; Xing, G.; Huang, W. Super air stable quasi-2D organic-inorganic hybrid perovskites for visible light-emitting diodes. Opt. Express 2018, 26, A66–A74. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Qin, C.; Cui, M.; He, T.; Liu, K.; Huang, Y.; Luo, M.; Zhang, L.; Xu, H.; Li, S.; et al. Spectra stable blue perovskite light-emitting diodes. Nat. Commun. 2019, 10, 1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Liu, H.; Chun, F.; Deng, W.; Luo, C.; Zhu, Z.; Yang, M.; Li, Y.; Li, W.; Yan, W.; et al. Aqueous Phase Exfoliating Quasi-2D CsPbBr3 Nanosheets with Ultrahigh Intrinsic Water Stability. Small 2019, 15, 1901994. [Google Scholar] [CrossRef]
- Thawarkar, S.; Rana, P.; Narayan, R.; Surya, S. Ni-Doped CsPbBr3 Perovskite: Synthesis of Highly Stable Nanocubes. Langmuir 2019, 35, 17150–17155. [Google Scholar] [CrossRef]
- Yang, H.; Yin, W.; Dong, W.; Gao, L.; Tan, C.; Li, W.; Zhang, X.; Zhang, J. Enhancing the light-emitting performance and stability in CsPbBr3 perovskite quantum dots via simultaneous doping and surface passivation. J. Mater. Chem. C 2020, 8, 14439–14445. [Google Scholar] [CrossRef]
- Sidhik, S.; Pasaran, A.; Esparza, D.; Luke, T.; Carriles, R.; Rosa, E. Improving the Optoelectronic Properties of Mesoporous TiO2 by Cobalt Doping for High-Performance Hysteresis-free Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 3571–3580. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Khandy, S.; Islam, I.; Tomar, R. Optical, electrochemical and photocatalytic properties of cobalt doped CsPbCl3 nanostructures: A one-pot synthesis approach. Sci. Rep. 2021, 11, 16473. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Li, J.; Wang, L.; Xing, K.; Yuan, X.; Zhao, J.; Gao, X.; Li, H. Enhancing luminescence of intrinsic and Mn doped CsPbCl3 perovskite nanocrystals through Co2+ doping. Mater. Res. Bull. 2020, 121, 110608. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, S.; Xu, Z.; Qiao, B.; Song, P.; Gao, D.; Xu, X. Shape-Controlled Synthesis of All-Inorganic CsPbBr3 PerovskiteNanocrystals with Bright Blue Emission. ACS Appl. Mater. Interfaces 2016, 8, 28824–28830. [Google Scholar] [CrossRef]
- Kubicki, D.J.; Prochowicz, D.; Pinon, A.; Stevanato, G.; Hofstetter, A.; Zakeeruddin, S.M.; Grätzel, M.; Emsley, L. Doping and phase segregation in Mn2+-and Co2+-doped lead halide perovskites from 133Cs and 1H NMR relaxation enhancement. J. Mater. Chem. A 2019, 7, 2326–2333. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Chen, D.; Xu, H.; Zhao, W.; Sun, J.; Ji, Z. Red-emitting CaLa4(SiO4)3O: Eu3+ phosphor with superior thermal stability and high quantum efficiency for warm w-LEDs. J. Alloys Compd. 2017, 695, 311e318. [Google Scholar] [CrossRef]
- Tran, M.T.; Nguyen, T.; Quang, N.; Nguyen, D.H.; Thu, L.; Do, Q.T.; Pham, T.H. Excellent thermal stability and high quantum efficiency orange-red-emitting AlPO4: Eu3+ phosphors for WLED application. J. Alloys Compd. 2021, 853, 156941. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Luu, T.N.; Nguyen, D.H.; Duong, T.T. Comparative Study on Backlighting Unit Using CsPbBr3 Nanocrystals/KSFM Phosphor + Blue LED and Commercial WLED in Liquid Crystal Display. J. Electron. Mater. 2021, 50, 1827–1834. [Google Scholar] [CrossRef]
- Tran, T.D.; Nguyen, D.H.; Pham, T.H.; Nguyen, D.C.; Duong, T.T. Achieving High Luminescent Performance K2SiF6: Mn4+ Phosphor by Co-precipitation Process with Controlling the Reaction Temperature. J. Electron. Mater. 2018, 47, 4634. [Google Scholar] [CrossRef]
- He, J.; He, Z.; Towers, A.; Zhan, T.; Chen, H.; Zhou, L.; Zang, C.; Chen, R.; Sun, T.; Gesquiere, A.; et al. Ligand assisted swelling–deswelling microencapsulation (LASDM) for stable, color tunable perovskite–polymer composites. Nanoscale Adv. 2020, 2, 2034. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, X.-V.; Tran, B.-D.; Nguyen, D.-C.; Nguyen, T.; Nguyen, M.-V.; Nguyen, C.-N.-H.; Duong, T.-T. Low-Dimensional CsPbBr3@CoBr2 Super-Nanowire Structure for Perovskite/PMMA Composite with Highly Blue Emissive Performance. Crystals 2021, 11, 1564. https://doi.org/10.3390/cryst11121564
Pham X-V, Tran B-D, Nguyen D-C, Nguyen T, Nguyen M-V, Nguyen C-N-H, Duong T-T. Low-Dimensional CsPbBr3@CoBr2 Super-Nanowire Structure for Perovskite/PMMA Composite with Highly Blue Emissive Performance. Crystals. 2021; 11(12):1564. https://doi.org/10.3390/cryst11121564
Chicago/Turabian StylePham, Xuan-Viet, Ba-Duc Tran, Duy-Cuong Nguyen, Tu Nguyen, Minh-Vuong Nguyen, Cao-Ngoc-Hong Nguyen, and Thanh-Tung Duong. 2021. "Low-Dimensional CsPbBr3@CoBr2 Super-Nanowire Structure for Perovskite/PMMA Composite with Highly Blue Emissive Performance" Crystals 11, no. 12: 1564. https://doi.org/10.3390/cryst11121564





